Introduction
Thyroid cancer represents the most common endocrine
malignancy, and its incidence has continued to rise worldwide over
the past few decades (1). The
estimated incidence rate for thyroid cancer is ~1.7% of total
cancer diagnoses worldwide, and the mortality rate has improved in
China during recent years (2).
Thyroid cancer accounts for 5–10% of cancers in women (3), and is classified into four major
histological groups: Papillary thyroid carcinoma (PTC), follicular
thyroid carcinoma, poorly differentiated carcinoma and
undifferentiated anaplastic carcinoma (4). PTC is the most common type of thyroid
cancer and accounts for ~80% of all thyroid cancers (5). Although the majority of patients with
PTC display a good prognosis, patients with locoregional recurrence
and distant metastases frequently have a poor clinical prognosis
following treatment with standard therapies (6,7).
Therefore, understanding the molecular mechanisms underlying the
progression of thyroid cancer, and the development of novel
targeted therapies are important, in order to improve the prognosis
for patients with thyroid cancer.
Recently, aberrant expression of microRNAs (miRNAs)
has been demonstrated in various types of human cancer, including
thyroid cancer (8). miRNAs belong
to a class of non-coding small RNA molecules, ~19–25 nucleotides
long (9). miRNAs regulate target
mRNA translation and stability by binding to their 3′-untranslated
regions (3′-UTRs) (10,11). Previous studies have suggested that
miRNAs have essential roles in biological processes, including cell
cycle progression, cell proliferation, migration, invasion,
apoptosis, differentiation and development (12,13).
Certain miRNAs may function as tumor suppressor
genes or oncogenes in the development of tumors (14). miRNAs can exert tumour-suppressing
and tumour-promoting activities, thus indicating that
miRNA-targeting therapeutic strategies may be promising in the
treatment of cancer (15). The
identification of miRNA targets is important to understand the
function of miRNAs in tumorigenesis and progression, and miRNA have
also been suggested as a potential target for cancer therapy.
Downregulation of miR-126 has been verified in
various types of cancer (16–19).
However, to the best of our knowledge, there are currently no
studies regarding miR-126 expression in human thyroid cancer. In
the present study, the expression and function of miR-126 was
determined in human thyroid cancer. The results demonstrated that
miR-126 was downregulated in human thyroid cancer tissues and cell
lines. Conversely, upregulation of miR-126 decreased cell
proliferation, migration and invasion by directly targeting C-X-C
chemokine receptor type 4 (CXCR4). These finding have therapeutic
implications and may be exploited for the future treatment of human
thyroid cancer.
Materials and methods
Clinical specimens
The present study was approved by the ethics
committee of the Chinese Research Academy of Environmental
Sciences' Protection of Human Subjects (Beijing, China). A total of
20 pairs of human PTC tissues and matched normal adjacent tissues
(NATs) were collected from patients (9 males and 11 females; age,
27–77 years old) diagnosed with PTC and had undergone surgery at
hospitals associated with Anhui Medical University (Anhui, China).
Written informed consent was provided by all patients in the
present study. Tissues were immediately snap-frozen in liquid
nitrogen following surgery and were stored at −80°C.
Cell culture
The human PTC cell lines, TPC-1 and HTH83, were
purchased from the Shanghai Institute of Biochemistry and Cell
Biology (Shanghai, China). TPC-1 and HTH83 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS), 100 IU/ml penicillin and 100 mg/ml streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) in a 5% CO2 cell
incubator at 37°C.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
PTC tissues and NATs were homogenized, then RNA was
isolated from tissues and PCT cells using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. RT was performed using the Reverse
Transcription kit (Tiangen Biotech Co., Ltd., Beijing, China). The
incubation protocol for RT 70°C for 5 min, 0°C for 2 min, 42°C for
50 min and 95°C for 5 min. The expression levels of miR-126 were
quantified using the All-in-One™ miRNA qRT-PCR Detection kit
(GeneCopoeia, Inc., Rockville, MD, USA) and ABI Prism 7500
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Primers were purchased from Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). The reaction system contained 10 µl
2XAll-in-One qPCR Mix, 2 µl forward primer, 2 µl
reverse primer, 2 µl cDNA and 4 µl double distilled
water. The thermocycling conditions of the reaction were as
follows: 95°C for 10 min; then 40 cycles of 95°C for 10 sec, 55°C
for 20 sec and 72°C for 10 sec. U6 small RNA was used as an
internal control with relative RNA levels calculated using the
2−ΔΔCq method (20).
Each sample was analyzed in triplicate.
Cell transfection
Mature miR-126 mimics, miRNA negative control (NC)
mimics and the luciferase reporter plasmid were purchased from
Shanghai GenePharma Co., Ltd. (Shanghai, China). TPC-1 and HTH83
cells were seeded onto a 6-well plate and were cultured with
antibiotic-free DMEM. Cells were transfected with miR-126 mimics,
NC mimics or the luciferase reporter plasmid using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) when cell density
reached 30–40%, according to the manufacturer's protocols.
Cell proliferation assay
The proliferation of PTC cells was determined using
a 3-(4,5-dimethyl-2-thiazoyl)-2,5-di-phenyl-2H-tetrazolium bromide
(MTT) assay according to the manufacturer's protocol. Cells were
seeded onto 96-well plates at a density of 3×103
cells/well 24 h post-transfection with miR-126 or NC mimics. At
various time points post-transfection, 20 µl MTT (5 mg/ml;
Sigma-Aldrich, St. Louis, MO, USA) solution was added to each well
and incubated at 37°C for 4 h. Following removal of the MTT
solution, the formazan precipitates were dissolved in 200 µl
dimethyl sulfoxide. The absorbance was measured at 490 nm using an
xMark enzyme-linked immunosorbent assay reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). All experiments were
performed in triplicate. Suppression rate was calculated using the
following formula: Suppression rate = (1 −
ODmiR-126/ODmiR-NC) × 100%; where OD refers
to optical density.
Cell migration assay
The migration potential of TPC-1 and HTH-83 cells
was determined using Transwell chambers with an 8-µm pore
polycarbonate membrane (Costar; Corning Incorporated, Corning, NY,
USA). A total of 5×104 cells, transfected with miR-126
or NC mimics, in 200 µl DMEM without serum were added to the
upper chamber. A total of 0.5 ml DMEM supplemented with 10% FBS was
added to the lower chamber. Following a 12 h incubation at 37°C,
the TPC-1 and HTH-83 cells that had not migrated through the pores
were carefully removed with cotton wool. The inserts were then
fixed with 100% methanol, stained with 0.5% crystal violet
(Beyotime Institute of Biotechnology, Haimen, China) and were
counted under an inverted microscope (CKX41; Olympus Corporation,
Tokyo, Japan).
Cell invasion assay
The invasion potential of TPC-1 and HTH-83 cells was
determined using Transwell chambers with an 8-µm pore
polycarbonate membrane (Costar) coated with Matrigel (BD
Bioscience, San Jose, CA, USA). A total of 5×104 cells,
transfected with miR-126 or NC mimics, in 200 µl DMEM
without serum were added to the upper chamber. A total of 0.5 ml
DMEM supplemented with 10% FBS was added to the lower chamber.
Following a 24 h incubation at 37°C, the TPC-1 and HTH-83 cells
that had not invaded through the pores were carefully removed with
cotton wool. The inserts were then fixed with 100% methanol,
stained with 0.5% crystal violet (Beyotime Institute of
Biotechnology) and were counted under an inverted microscope
(CKX41; Olympus Corporation).
Western blotting
A total of 72 h post-transfection, total cellular
proteins was extracted from the cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). The protein concentration was measured using the
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. Equal
amounts of protein (20 µg) were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, and were then
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MD, USA). The membranes were blocked with 5% non-fat dry
milk for 2 h, and were then incubated overnight at 4°C with the
primary antibodies, according to the manufacturer's protocols.
Subsequently, the membranes were incubated with goat anti-rabbit
(1:5,000 dilution; cat. no. sc-2054; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) or goat anti-mouse (1:5,000 dilution; cat.
no. sc-2055; Santa Cruz Biotechnology, Inc.) horseradish-peroxidase
conjugated antibodies fro 2 h at room temperature. The primary
antibodies used were as follows: Rabbit anti-human CXCR4 (1:1,000
dilution; cat. no. sc-9046; Santa Cruz Biotechnology, Inc.) and
mouse anti-human β-actin (1:1,000 dilution; cat. no. sc-130300;
Santa Cruz Biotechnology, Inc.). Blots were visualized using
enhanced chemiluminescence reagents (EMD Millipore). β-actin was
used as a loading control. The intensity of the bands was
determined with Image Lab software (Bio-Rad Laboratories,
Inc.).
Luciferase assay
To determine whether CXCR4 was a direct target of
miR-126, a luciferase activity assay was conducted.
pMIR-CXCR4-3′UTR wild-type and pMIR-CXCR4-3′UTR mutant plasmids
were obtained from GenePharma Co., Ltd. TPC-1 and HTH-83 cells were
transfected with 0.5 µg wild-type or mutant plasmids, and 40
nmol miR-126 or NC mimics in a 12-well plate using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). A total of 48 h
post-transfection, firefly and Renilla luciferase activities
were measured using the Dual-Luciferase Reporter Assay system
(Promega Corporation, Manheim, Germany) with the xMark microplate
reader. Firefly luciferase activity was normalized to
Renilla luciferase activity. Each sample was analyzed in
triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation
and compared using Student's t test and analysis of variance.
Results were analyzed using SPSS software (version 19; IBM SPSS,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-126 expression in PTC tissues and
cell lines
To detect the expression levels of miR-126 in 20 PTC
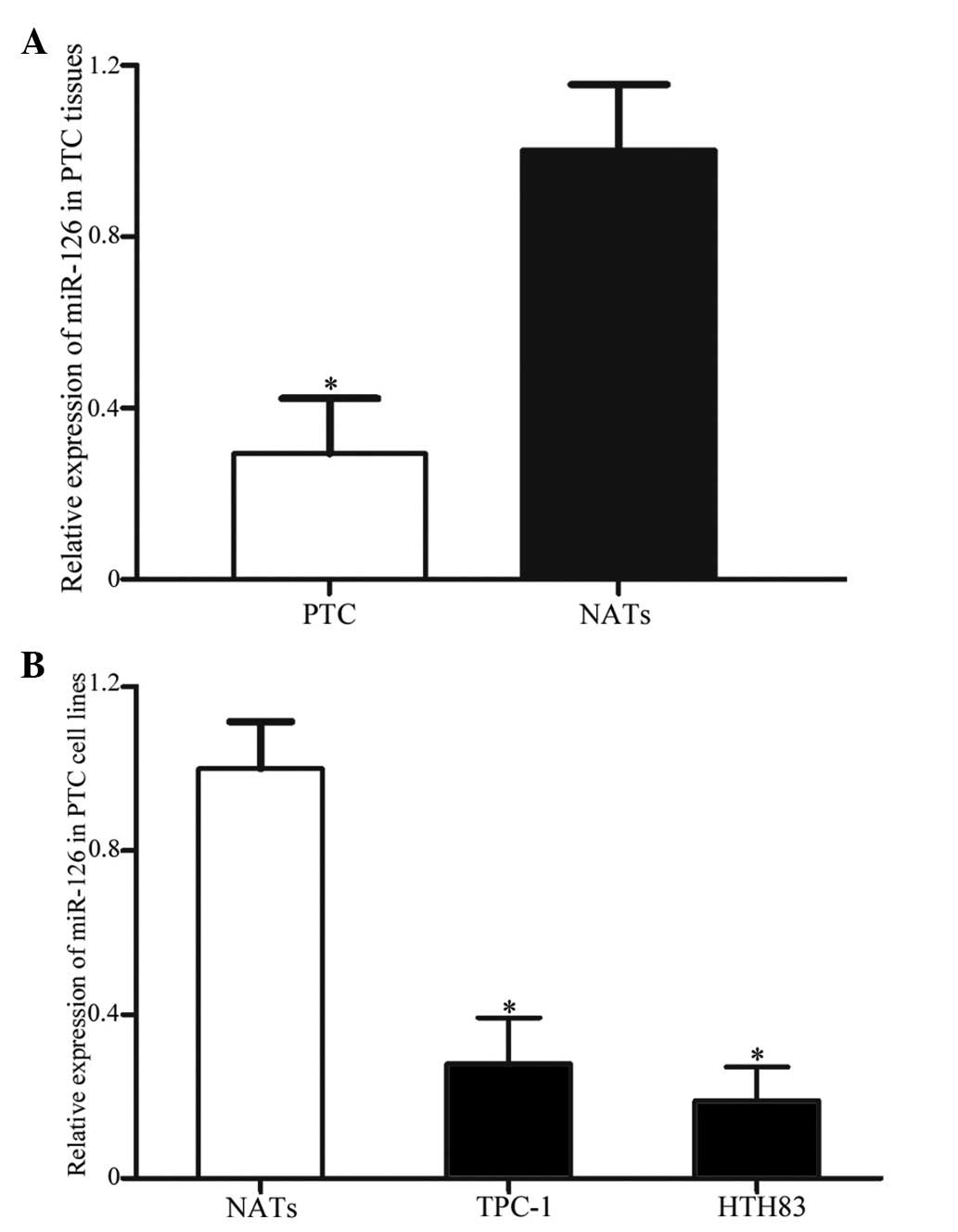
tissues and matched NATs, RT-qPCR was performed. As presented in
Fig. 1A, miR-126 was significantly
downregulated in PTC tissues compared with in the matched NATs
(P=0.011). In addition, the expression levels of miR-126 were
compared between TPC-1 and HTH83 cell lines, and NATs. As presented
in Fig. 1B, miR-126 was also
downregulated in the TPC-1 and HTH83 cell lines compared with in
the NATs (P=0.019).
miR-126 expression in TPC-1 and HTH83
cell lines post-transfection with miR-126 mimics
To determine the expression levels of miR-126 in PTC
cell lines post-transfection with miR-126 mimics, RT-qPCR was
conducted. A total of 120 h post-transfection, miR-126 expression
was significantly upregulated in TPC-1 and HTH83 cell lines
(Fig. 2; P=0.001).
miR-126 suppresses proliferation of TPC-1
and HTH83 cells
To verify the effects of miR-126 on cell
proliferation, an MTT assay was conducted. As shown in Fig. 3, upregulation of miR-126
significantly inhibited cell proliferation. MTT assays revealed
that 144 h post-transfection, the suppression rate of miR-126
reached 28.91±4.6% in TPC-1 cells (P=0.017) and 31.43±3.2% in HTH83
cells (P=0.008).
miR-126 inhibits cell migration and
invasion of TPC-1 and HTH83 cells
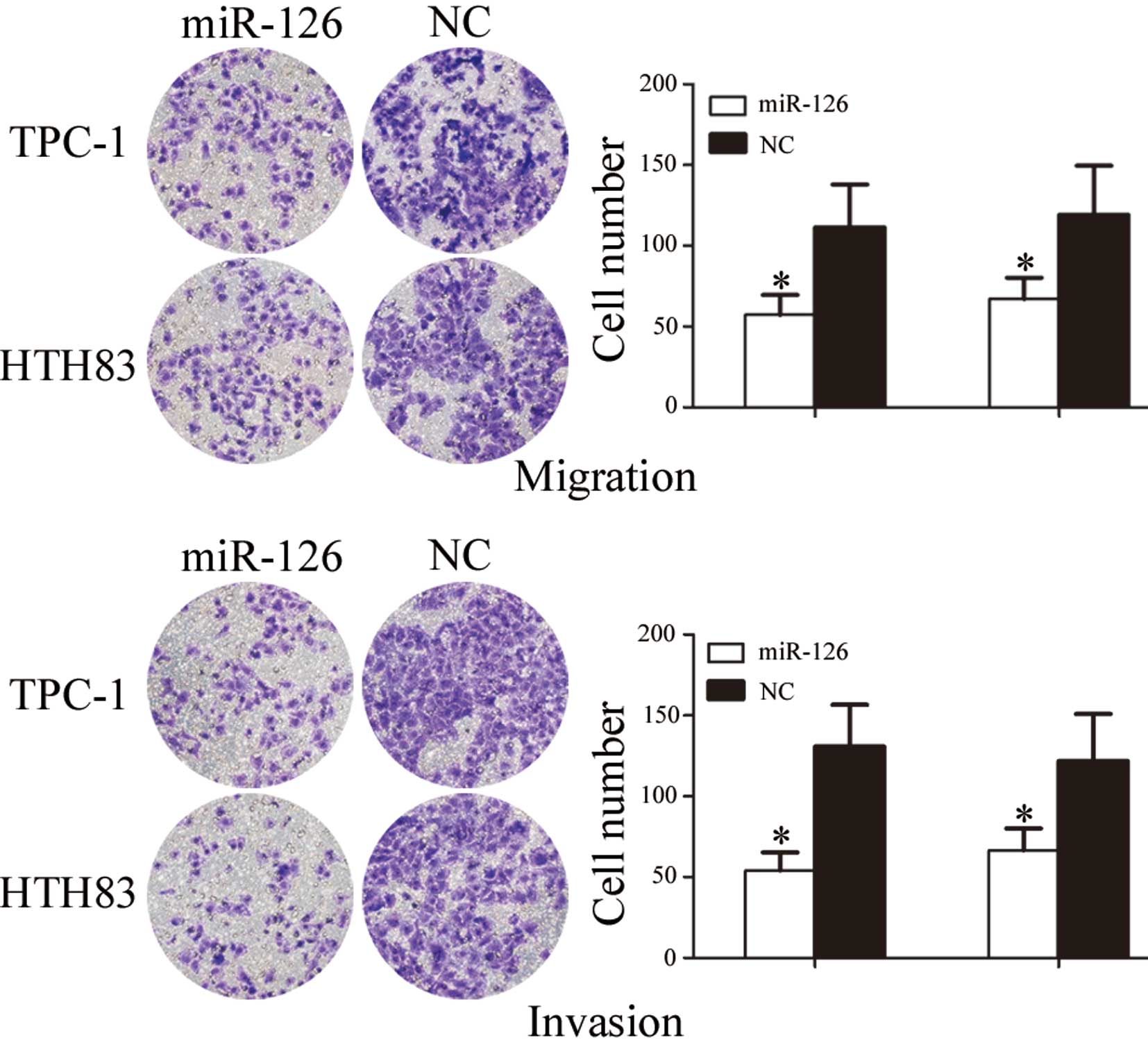
To investigate the role of miR-126 in cell
migration, a migration assay was performed. As presented in
Fig. 4, the number of migrated
TPC-1 and HTH83 cells transfected with miR-126 was significantly
downregulated compared with the NC groups (P=0.024 and P=0.039,
respectively).
To investigate the role of miR-126 on cell invasion,
an invasion assay was performed. As shown in Fig. 4, the number of invasive TPC-1 and
HTH83 cells transfected with miR-126 was significantly
downregulated compared with in the NC groups (P=0.019 and P=0.032,
respectively). These results indicate that miR-126 may decrease the
migration and invasion of TPC-1 and HTH83 cells.
CXCR4 is downregulated post-transfection
of TPC-1 and HTH83 cells with miR-126
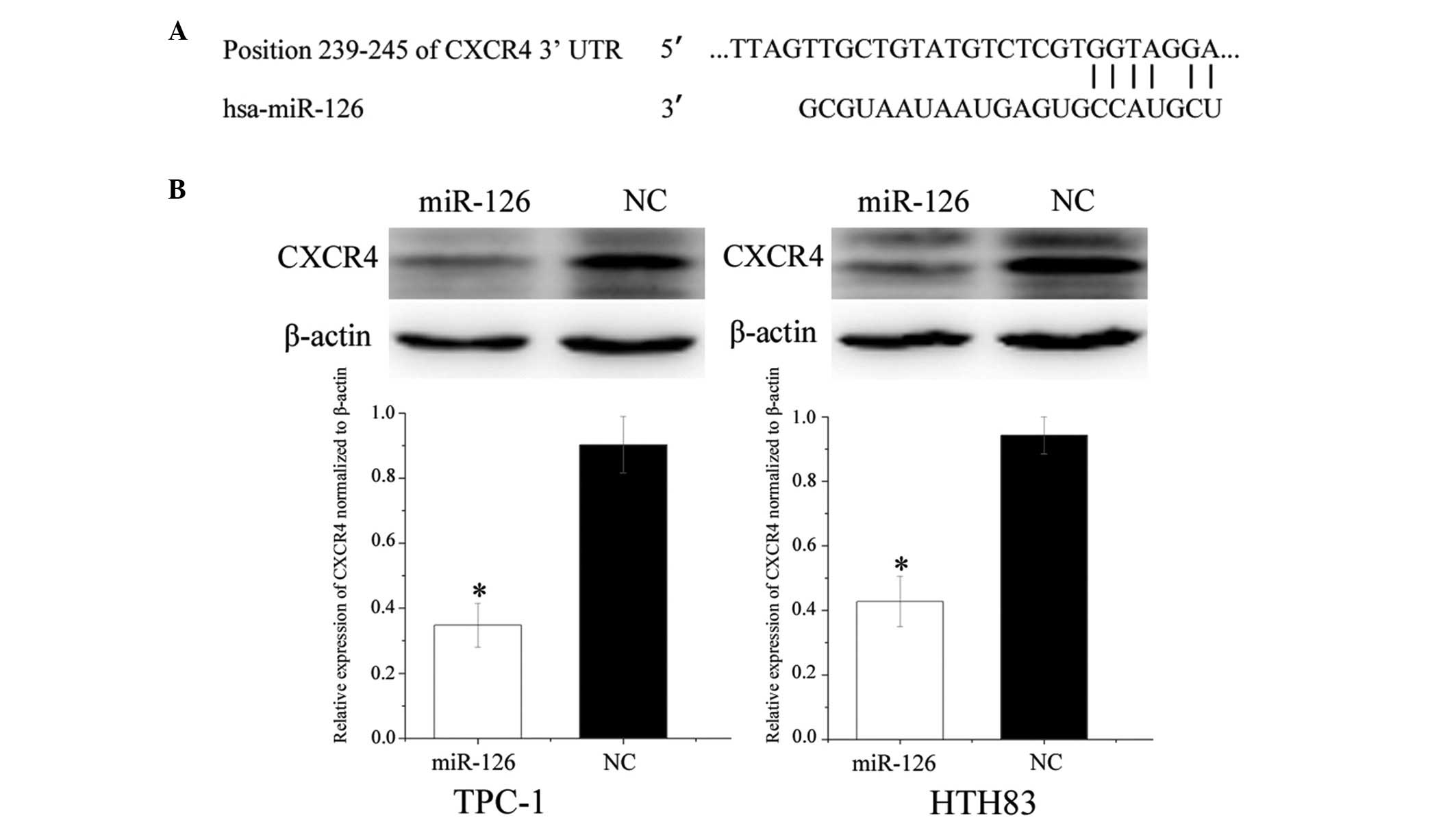
To identify targets of miR-126, TargetScan
(http://www.targetscan.org/) was used.
CXCR4 was predicted to be a target gene of miR-126 (Fig. 5A). To verify whether miR-126
directly targeted CXCR4, western blotting was performed, in order
to determine whether CXCR4 was downregulated post-transfection of
TPC-1 and HTH83 cells with miR-126. As presented in Fig. 5B, CXCR4 was significantly
downregulated in the TPC-1 and HTH83 cells post-transfection with
miR-126 (P=0.022 and P=0.036, respectively).
CXCR4 is a direct target gene of
miR-126
Luciferase assays indicated that miR-126
significantly inhibited wild-type CXCR4, but not mutated CXCR4,
luciferase activity in TPC-1 (P=0.026) and HTH83 cells (P=0.018;
Fig. 6). These results suggest
that CXCR4 is a direct target gene of miR-126 in vitro.
Discussion
miR-126 is derived from the epidermal growth
factor-like domain 7 genes, and is located on chromosome 9q34.3
(21,22). Previous studies have suggested that
miR-126 expression differs between normal tissues and tumor tissues
(17,19,23).
It has been reported to be upregulated in highly vascularized
tissues, including the heart, liver and lungs (24,25).
However, downregulated miR-126 expression has been detected in
various types of cancer, including lung cancer (16), gastric cancer (17), leukemia (18), breast cancer (19), colon cancer (26), cervical cancer (27), bladder cancer (28) and prostate cancer (29). To the best of our knowledge, there
are no studies regarding the expression of miR-126 in thyroid
cancer. The present study demonstrated that miR-126 was
significantly downregulated in human thyroid cancer tissues and PTC
cell lines, thus suggesting that miR-126 may have an important role
in thyroid cancer.
Recently, an increasing number of studies have
focused on the function of miR-126 in various types of cancer. In
bladder cancer, restoration of miR-126 expression attenuated the
invasive potential of bladder cancer cells through its ability to
target ADAM metalloproteinase domain 9 (28). In lung cancer, Liu et al
(16) reported that miR-126
inhibited cancer cell proliferation and induced cell cycle arrest
by directly targeting vascular endothelial growth factor-A. In
gastric cancer, Liu et al (17) revealed that miR-126 inhibited
cancer cell growth and motility, with Crk as a direct target. In
breast cancer, Zhang et al (30) demonstrated that upregulation of
miR-126 suppressed cancer cell metastasis and induced cell cycle
arrest via the negative regulation of insulin receptor substrate 1
(31). However, to the best of our
knowledge, there are no studies regarding the function of miR-126
in thyroid cancer. The present study revealed that miR-126
inhibited thyroid cancer cell proliferation, migration and
invasion. These results suggested that miR-126 may be used for the
development of novel therapeutic approaches for the treatment of
thyroid caner.
Identification of the target genes of miR-126 is
essential to improve understanding regarding its role in
tumorigenesis, and is important for defining novel therapeutic
targets. In the present study, a molecular link between miR-126 and
CXCR4 was identified in thyroid cancer. Firstly, TargetScan
predicted that CXCR4 was a direct target gene of miR-126. Secondly,
as determined by western blotting, upregulation of miR-126
decreased the expression levels of CXCR4 protein in thyroid cancer
cell lines. Finally, a luciferase activity assay demonstrated that
miR-126 directly targeted CXCR4 3′-UTR, as predicted by TargetScan.
These findings suggested that miR-126 may negatively regulate CXCR4
expression in vitro, and may have a tumor-suppressive role
in thyroid cancer tumorigenesis and progression.
The processes that induce and stimulate tumor cell
migration, invasion and metastasis are very complex, and are
modulated by chemokines and their receptors (32). Chemokines are a superfamily of
small, structurally related proteins that bind to and interact with
G-protein-coupled receptors, resulting in cytoskeletal
rearrangement, firm adhe sion to endothelial cells and directional
migration (33). Chemokine
receptors are transmembrane proteins, which interact with chemokine
ligands, resulting in G-protein-coupled signal transduction that
leads to chemotaxis or directional movement along a chemical
gradient (34). CXCR4 is the
G-protein-coupled receptor for the chemokine C-X-C motif chemokine
ligand 12 (35). CXCR4 is known to
be highly expressed in various types of human cancer, and is
associated with several biological and pathological processes
(36).
High expression of CXCR4 has been detected in
various types of tumor, including thyroid cancer (37). CXCR4 expression has also been
reported to be correlated with tumor infiltration degree, tumor
size, pathological indicators of tumor aggressiveness and lymph
node metastasis (34,38,39).
In a previous study, knockdown of CXCR4 using a CXCR4-short hairpin
RNA interfering vector significantly inhibited thyroid cancer cell
proliferation, adhesion and migration (40). These findings suggested that CXCR4
may have an oncogenic role, and may be considered a potentially
novel therapeutic target for the suppression of tumor metastasis.
The present study demonstrated that miR-126 inhibited CXCR4
expression, in order to regulate thyroid cancer cell migration and
invasion. Therefore, miR-126 may be investigated as a predictive
value for the early detection of tumor metastasis, and as a
therapeutic target for the suppression of thyroid cancer
invasion.
In conclusion, the present study demonstrated that
miR-126 was downregulated in thyroid cancer. In addition, miR-126
was revealed to inhibit thyroid cancer cell proliferation,
migration and invasion by targeting CXCR4. Therefore, miR-126 may
be investigated as a target therapy for the suppression of thyroid
cancer invasion. Further work is required to address whether the
potential of miR-126 may be fully realized in thyroid cancer
treatment.
References
|
1
|
Xing M, Haugen BR and Schlumberger M:
Progress in molecular-based management of differentiated thyroid
cancer. Lancet. 381:1058–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang C, Lu S, Jiang J, Jia X, Dong X and
Bu P: Hsa-microRNA-101 suppresses migration and invasion by
targeting Rac1 in thyroid cancer cells. Oncol Lett. 8:1815–1821.
2014.PubMed/NCBI
|
|
3
|
Zhang X, Li M, Zuo K, Li D, Ye M, Ding L,
Cai H, Fu D, Fan Y and Lv Z: Upregulated miR-155 in papillary
thyroid carcinoma promotes tumor growth by targeting APC and
activating Wnt/β-catenin signaling. J Clin Endocrinol Metab.
98:E1305–E1313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hartmann C, Mueller W and von Deimling A:
Pathology and molecular genetics of oligodendroglial tumors. J Mol
Med (Berl). 82:638–655. 2004. View Article : Google Scholar
|
|
5
|
Geraldo MV, Fuziwara CS, Friguglieti CU,
Costa RB, Kulcsar MA, Yamashita AS and Kimura ET: MicroRNAs
miR-146-5p and let-7f as prognostic tools for aggressive papillary
thyroid carcinoma: A case report. Arq Bras Endocrinol Metabol.
56:552–557. 2012. View Article : Google Scholar
|
|
6
|
Vasko VV and Saji M: Molecular mechanisms
involved in differentiated thyroid cancer invasion and metastasis.
Curr Opin Oncol. 19:11–17. 2007. View Article : Google Scholar
|
|
7
|
Yang Q, Ji M, Guan H, Shi B and Hou P:
Shikonin inhibits thyroid cancer cell growth and invasiveness
through targeting major signaling pathways. J Clin Endocrinol
Metab. 98:E1909–E1917. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Li D, Li M, Ye M, Ding L, Cai H,
Fu D and Lv Z: MicroRNA-146a targets PRKCE to modulate papillary
thyroid tumor development. Int J Cancer. 134:257–267. 2014.
View Article : Google Scholar
|
|
9
|
Lee JC, Gundara JS, Glover A, Serpell J
and Sidhu SB: MicroRNA expression profiles in the management of
papillary thyroid cancer. Oncologist. 19:1141–1147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rigoutsos I: New tricks for animal
microRNAs: Targeting of amino acid coding regions at conserved and
nonconserved sites. Cancer Res. 69:3245–3248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li D, Jian W, Wei C, Song H, Gu Y, Luo Y
and Fang L: Down-regulation of miR-181b promotes apoptosis by
targeting CYLD in thyroid papillary cancer. Int J Clin Exp Pathol.
7:7672–7680. 2014.
|
|
14
|
Wu D, Zhou Y, Pan H, Qu P and Zhou J:
MicroRNA99a inhibits cell proliferation, colony formation ability,
migration and invasion by targeting fibroblast growth factor
receptor 3 in prostate cancer. Mol Med Rep. 11:1469–1475. 2015.
|
|
15
|
Pallante P, Battista S, Pierantoni GM and
Fusco A: Deregulation of microRNA expression in thyroid neoplasias.
Nat Rev Endocrinol. 10:88–101. 2014. View Article : Google Scholar
|
|
16
|
Liu B, Peng XC, Zheng XL, Wang J and Qin
YW: MiR-126 restoration down-regulate VEGF and inhibit the growth
of lung cancer cell lines in vitro and in vivo. Lung Cancer.
66:169–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu LY, Wang W, Zhao LY, Guo B, Yang J,
Zhao XG, Hou N, Ni L, Wang AY, Song TS, et al: Mir-126 inhibits
growth of SGC-7901 cells by synergistically targeting the oncogenes
PI3KR2 and Crk, and the tumor suppressor PLK2. Int J Oncol.
45:1257–1265. 2014.PubMed/NCBI
|
|
18
|
Akbari Moqadam F, Boer JM, Lange-Turenhout
EA, Pieters R and den Boer ML: Altered expression of miR-24,
miR-126 and miR-365 does not affect viability of childhood
TCF3-rearranged leukemia cells. Leukemia. 28:1008–1014. 2014.
View Article : Google Scholar
|
|
19
|
Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu
T, Bai Y, Shen Y, Yuan W, Jing Q and Qin Y: Endothelial-specific
intron-derived miR-126 is down-regulated in human breast cancer and
targets both VEGFA and PIK3R2. Mol Cell Biochem. 351:157–164. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
MiR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saito Y, Friedman JM, Chihara Y, Egger G,
Chuang JC and Liang G: Epigenetic therapy upregulates the tumor
suppressor microRNA-126 and its host gene EGFL7 in human cancer
cells. Biochem Biophys Res Commun. 379:726–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng R, Chen X, Yu Y, Su L, Yu B, Li J,
Cai Q, Yan M, Liu B and Zhu Z: MiR-126 functions as a tumour
suppressor in human gastric cancer. Cancer Lett. 298:50–63. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meister J and Schmidt MH: MiR-126 and
miR-126*: New players in cancer. Scientific World Journal.
10:2090–2100. 2010. View Article : Google Scholar
|
|
26
|
Li N, Tang A, Huang S, Li Z, Li X, Shen S,
Ma J and Wang X: MiR-126 suppresses colon cancer cell proliferation
and invasion via inhibiting RhoA/ROCK signaling pathway. Mol Cell
Biochem. 380:107–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu Q, Liu SL, Wang H, Shi G, Yang P and
Chen XL: MiR-126 suppresses the proliferation of cervical cancer
cells and alters cell sensitivity to the chemotherapeutic drug
bleomycin. Asian Pac J Cancer Prev. 14:6569–6572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia AY, Castillo-Martin M, Bonal DM,
Sánchez-Carbayo M, Silva JM and Cordon-Cardo C: MicroRNA-126
inhibits invasion in bladder cancer via regulation of ADAM9. Br J
Cancer. 110:2945–2954. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun X, Liu Z, Yang Z, Xiao L, Wang F, He
Y, Su P, Wang J and Jing B: Association of microRNA-126 expression
with clinico-pathological features and the risk of biochemical
recurrence in prostate cancer patients undergoing radical
prostatectomy. Diagn Pathol. 8:2082013. View Article : Google Scholar
|
|
30
|
Zhang J, Du YY, Lin YF, Chen YT, Yang L,
Wang HJ and Ma D: The cell growth suppressor, mir-126, targets
IRS-1. Biochem Biophys Res Commun. 377:136–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y,
Li C, Chong M, Ibrahim T, Mercatali L, et al: MiR-126 and miR-126*
repress recruitment of mesenchymal stem cells and inflammatory
monocytes to inhibit breast cancer metastasis. Nat Cell Biol.
15:284–294. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yarden Y: The EGFR family and its ligands
in human cancer. signalling mechanisms and therapeutic
opportunities. Eur J Cancer. 37(Suppl 4): S3–S8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Balkwill FR: The chemokine system and
cancer. J Pathol. 226:148–157. 2012. View Article : Google Scholar
|
|
34
|
Wang N, Luo HJ, Yin GB, Dong CR, Xu M,
Chen GG and Liu ZM: Overexpression of HIF-2α, TWIST, and CXCR4 is
associated with lymph node metastasis in papillary thyroid
carcinoma. Clin Dev Immunol. 2013:5894232013. View Article : Google Scholar
|
|
35
|
Oberlin E, Amara A, Bachelerie F, Bessia
C, Virelizier JL, Arenzana-Seisdedos F, Schwartz O, Heard JM,
Clark-Lewis I, Legler DF, et al: The CXC chemokine SDF-1 is the
ligand for LESTR/fusin and prevents infection by
T-cell-line-adapted HIV-1. Nature. 382:833–835. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He X, Wei Q, Zhang X, Xiao J, Jin X, Zhu
Y, Cui B and Ning G: Immunohistochemical expression of CXCR4 in
thyroid carcinomas and thyroid benign lesions. Pathol Res Pract.
206:712–715. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Castellone MD, Guarino V, De Falco V,
Carlomagno F, Basolo F, Faviana P, Kruhoffer M, Orntoft T, Russell
JP, Rothstein JL, et al: Functional expression of the CXCR4
chemokine receptor is induced by RET/PTC oncogenes and is a common
event in human papillary thyroid carcinomas. Oncogene.
23:5958–5967. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Torregrossa L, Giannini R, Borrelli N,
Sensi E, Melillo RM, Leocata P, Materazzi G, Miccoli P, Santoro M
and Basolo F: CXCR4 expression correlates with the degree of tumor
infiltration and BRAF status in papillary thyroid carcinomas. Mod
Pathol. 25:46–55. 2012. View Article : Google Scholar
|
|
39
|
Wagner PL, Moo TA, Arora N, Liu YF,
Zarnegar R, Scognamiglio T and Fahey TJ III: The chemokine
receptors CXCR4 and CCR7 are associated with tumor size and
pathologic indicators of tumor aggressiveness in papillary thyroid
carcinoma. Ann Surg Oncol. 15:2833–2841. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guo S, Xiao D, Liu H, Zheng X, Liu L and
Liu S: Interfering with CXCR4 expression inhibits proliferation,
adhesion and migration of breast cancer MDA-MB-231 cells. Oncol
Lett. 8:1557–1562. 2014.PubMed/NCBI
|