Introduction
Glioblastoma multiforme (GBM) is the most common and
aggressive type of primary brain malignancy in adults. The
prognosis of GBM is generally poor, with a median survival time of
14.6 months (1). GBM is currently
treated with surgical resection, radiotherapy and adjuvant
temozolomide (TMZ) chemotherapy. Although these treatment regimens
have evolved over the years, the improvements have not translated
into marked increases in patient survival. GBM is characterized by
chemoresistance, and the clinical success of TMZ treatment is
limited (2). Previous studies have
demonstrated that >90% of recurrent gliomas do not respond to
the second cycle of TMZ (3,4). In
addition, TMZ may induce C>T/G>A transition mutations when
DNA mismatch repair is deficient, which can potentially promote
tumor progression (5,6). Therefore, there is an urgent
requirement for the development of novel effective agents in the
treatment of GBM.
Saponins, which are glycosides present in numerous
plants, have been reported to exhibit marked cytotoxicity against
various types of cancer (7,8). In
particular, saponins derived from the rhizomes of Anemone
taipaiensis (Ranunculaceae) exhibit potent cytotoxicity toward
the human hepatocellular carcinoma cell line HepG2 and the human
promyelocytic leukemia cell line HL-60 (9). Furthermore, our previous studies
demonstrated that saponin 1 and saponin B, derived from A.
taipaiensis, effectively inhibited brain tumor progression
(10,11). Following these results, the present
study aimed to determine the potential anti-glioma activities of
other saponins derived from A. taipaiensis. In the present
study, the effects of A. taipaiensis-derived saponin 6
(3β-O-{α-L-rhamnopyranosyl-(1→2)-[β-D-gluco
pyranosyl-(1→4)]-α-L-arabinopyranosyl} oleanolic acid) on the
viability and apoptosis of human U87 malignant glioblastoma (U87
MG) cells were evaluated. In addition, the molecular mechanisms
underlying these effects were explored.
Materials and methods
Chemical reagents
Saponin 6 (>98% purity) was provided by Professor
Tang (Institute of Materia Medica, School of Pharmacy, Fourth
Military Medical University, Xi'an, China). The purity of saponin 6
was determined using a Dionex P680 high pressure liquid
chromatography pump (Dionex; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) equipped with a UV 70 UV/Vis detector at 206 nm,
and a YMC-Pack R&D ODS-A column (YMC Co., Ltd., Kyoto, Japan).
Saponin 6 was dissolved in dimethyl sulfoxide (DMSO; Thermo Fisher
Scientific, Inc.) and diluted with water to generate the 111.61
µM stock solution, containing no more than 0.1% DMSO. The
stock solution was stored in aliquots at −20°C. Prior to
experimentation, the stock solution was thawed, sterilized by
filtration through a 0.22-µm sterile filter (EMD Millipore,
Billerica, MA, USA), and diluted in Dulbecco's modified Eagle
medium (DMEM) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) containing 0.1% DMSO. DMEM
containing 0.1% DMSO was used as the vehicle control in subsequent
experiments.
Cell culture
The human U87 MG cell line and the murine HT-22
hippocampal neuronal cell line were purchased from the American
Type Culture Collection (Manassas, VA, USA). The cells were
cultured in DMEM supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator
containing 5% CO2.
Cell viability
Cell viability was determined using the Cell
Counting kit 8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) (12,13). Briefly, U87 MG and HT-22 cells were
seeded in 96-well plates (2×104 cells/well) and
incubated overnight. Subsequently, the cells were treated with
saponin 6 at various concentrations (vehicle control, 1.79, 3.57,
7.14 and 14.28 µM) for 24 or 48 h. The optical density was
measured at 450 nm using a Model 680 microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Cell morphology and terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL) assay
DNA fragmentation was assessed using TUNEL staining
(Roche Molecular Diagnostics, Pleasanton, CA, USA). Briefly, U87 MG
cells were seeded in 24-well plates (1×105 cells/well)
and incubated overnight. Subsequently, the cells were treated with
2.83 or 5.66 µM saponin 6 for 24 h, washed with
phosphate-buffered saline (PBS), and fixed in 4% paraformaldehyde
at 4°C for 10 min. Following two PBS washes, the cells were
permeabilized by incubating with 0.1% sodium citrate/0.1% Triton
X-100 for 20 min. The cells were then washed again and incubated
with the TUNEL reaction mixture at 37°C for 60 min. Cell nuclei
were counterstained with Hoechst 33342 (Beyotime Institute of
Biotechnology, Haimen, China). Cell morphology was examined under a
fluorescence microscope (FV-1000; Olympus Corporation, Tokyo,
Japan). Images of the cells were acquired using a Q-imaging video
camera (QImaging, Surrey, BC, Canada).
Quantitation of apoptosis by flow
cytometry
Cell apoptosis was determined by flow cytometry
using Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide
(PI) double staining. Briefly, U87 MG cells were seeded in 24-well
plates (2×105 cells/well) and incubated overnight.
Subsequently, the cells were treated with vehicle only, 2.83
µM or 5.66 µM saponin 6 for 24 h. Following the
treatment, the cells were harvested by trypsinization, washed with
cold PBS, and double-stained with Annexin V-FITC and PI (Immunotech
S.A., Marseilles, France) at room temperature for 15 min. The cells
were immediately subjected to flow cytometric analysis using a
FACSCalibur™ system (BD Biosciences, Franklin Lakes, NJ, USA). Data
were processed using ModFit software version 3.0 (Verity Software
House, Topsham, ME, USA) and presented in a quadrantal diagram.
Ultrastructural studies by transmission
electron microscopy (TEM)
U87 MG cells were seeded in culture dishes
(2×105 cells/ml) and incubated overnight. Subsequently,
the cells were treated with vehicle only, 2.83 or 5.66 µM
saponin 6 for 24 h. The cells were harvested by trypsinization and
fixed with 2.5% glutaraldehyde (PBS, pH 7.4) at 4°C overnight. The
cells were then post-fixed in 1% buffered osmium tetroxide at room
temperature for 2 h, dehydrated through a graded series of ethanol
solutions, and embedded in Poly/Bed (Polysciences, Warrington, PA,
USA) for 24 h at 60°C. Ultrathin sections (70–90 nm) were cut on an
ultramicrotome and double-stained with uranyl acetate and lead
citrate. The cell TEM micrographs were acquired using a JEM-2000EX
electron microscope (JEOL, Ltd., Tokyo, Japan).
Cell cycle analysis
U87 MG cells were seeded in 6-well plates
(2×105 cells/ml) and incubated overnight. Subsequently,
the cells were treated with vehicle only, 2.85 µM or 5.66
µM saponin 6 for 24 h. Following the treatment, the cells
were collected by trypsinization, washed in cold PBS, and fixed in
75% ethanol at 4°C overnight. The fixed cells were subsequently
washed with cold PBS and incubated with 50 µg/ml PI solution
containing 50 µg/ml RNase A (Sigma-Aldrich, St. Louis, MO,
USA) at room temperature for 30 min in the dark. Cell cycle
analysis was performed using a flow cytometer (FACSCalibur™; BD
Biosciences) and ModFit software (Verity Software House).
Western blot analysis
U87 MG cells were treated with vehicle only, 2.83
µM or 5.66 µM saponin 6 for 24 h as previously
described. The cells were then lysed in radioimmunoprecipitation
assay buffer [150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1%
sodium dodecyl sulfate (SDS), 50 mM Tris-HCl, pH 8.0] supplemented
with a complete protease inhibitor cocktail (Roche Diagnostics,
Basel, Switzerland) at 4°C for 30 min. The cell lysates were
centrifuged at 15,000 × g for 15 min, and the supernatants were
collected. The protein concentrations were determined using a
Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific,
Inc.). Protein samples (25 µg) were then separated by 12%
SDS-polyacrylamide gel electrophoresis and transferred to
polyvinylidene fluoride membranes (EMD Millipore). Subsequent to
blocking the membranes in 5% nonfat dry milk containing 0.1%
Tween-20 (Sigma-Aldrich) for 2 h, the membranes were incubated with
polyclonal rabbit anti-Fas (1:500; cat. no. BS1461; Bioworld
Technology, Inc., St. Louis Park, MN, USA), polyclonal rabbit
anti-Fas ligand (FasL; 1:500; cat. no. BS1122; Bioworld Technology,
Inc.), poly-clonal rabbit anti-caspase-3 (1:1,000; cat. no.
ab90437; Abcam, Cambridge, MA, USA), monoclonal rabbit
anti-caspase-8 (1:800; cat. no. 9496; Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-caspase-9 (1:500; cat. no. BS1388;
Bioworld Technology, Inc.), polyclonal rabbit anti-B-cell lymphoma
2 (Bcl-2; 1:500; cat. no. BS1511; Bioworld Technology, Inc.),
polyclonal rabbit anti-Bcl-2-associated X protein (Bax; 1:500; cat.
no. BS2538; Bioworld Technology, Inc.), and mouse monoclonal
anti-β-actin (1:1,000; cat. no. sc-47778; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) antibodies at 4°C overnight. The membranes
were subsequently washed three times with 0.1% Tween-20 in PBS (10
min each wash) and incubated with the secondary antibodies bovine
anti-mouse IgG-horseradish peroxidase-conjugated (HRP) (cat. no.
sc-2371) and bovine anti-rabbit IgG-HRP (cat. no. sc-2370) (both
1:2,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. Immunoreactive bands were visualized using the
enhanced chemiluminescence detection system (GE Healthcare,
Uppsala, Sweden). Data were normalized to β-actin using ImageJ
version 1.47 software (imagej.nih.gov/ij/).
Statistical analysis
All data are presented as the mean ± standard
deviation calculated from at least three independent experiments.
Data were analyzed using SPSS 20.0 (IBM SPSS, Armonk, NY, USA) or
GraphPad Prism 5.01 (GraphPad Software, Inc., La Jolla, CA, USA)
software. Differences between groups were analyzed using Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Saponin 6 exerts cytotoxic effects
against U87 MG cells
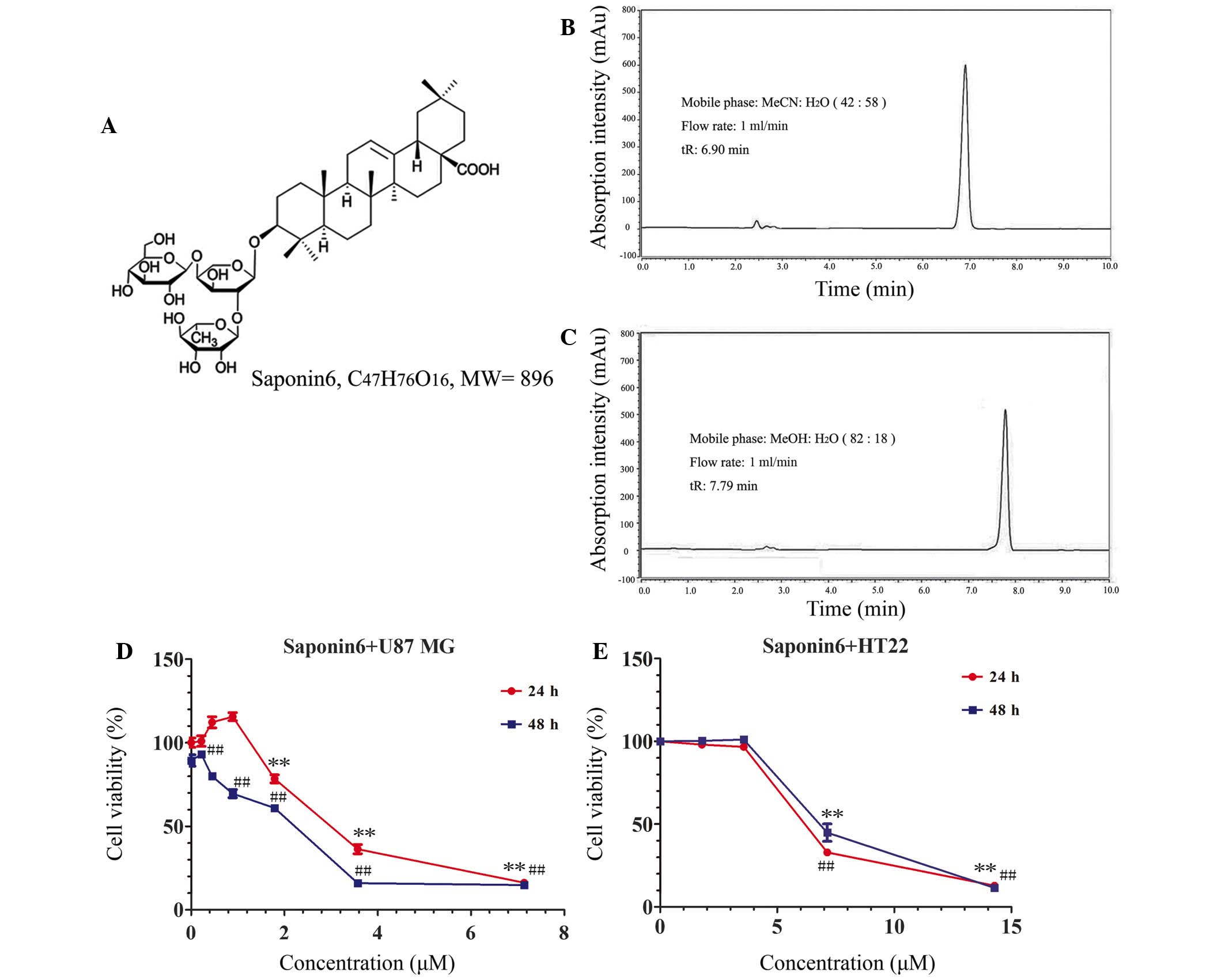
The chemical structure and high-performance liquid
chromatography (HPLC) chromatographs of saponin 6 are presented in
Fig. 1A–C. Saponin 6 was >98%
pure, as assessed by HPLC. The effects of saponin 6 were initially
determined on the cell viability of human U87 MG cells and murine
HT-22 hippocampal neuronal cells using the CCK-8 assay. Treatment
with saponin 6 significantly decreased U87 MG cell viability in a
time- and dose-dependent manner (Fig.
1D). The half maximal inhibitory concentration
(IC50) of saponin 6 was calculated as 2.83 µM
following a 48 h treatment. However, low concentrations of saponin
6 (<3.57 µM) did not significantly affect the viability
of HT-22 cells following treatment for 48 h. The IC50
value of saponin 6 against HT-22 cells was 6.24 µM, which
was more than two-fold higher than the value against U87 MG cells
(Fig. 1E). These results indicate
that saponin 6 may exhibit selective cytotoxicity against
glioblastoma cells.
Saponin 6 causes DNA fragmentation,
alters nuclear morphology, and induces apoptosis in U87 MG
cells
To further investigate saponin 6-induced U87 MG cell
death, the present study assessed the effects of saponin 6 on DNA
fragmentation, nuclear morphology and cell apoptosis using TUNEL
staining, TEM and flow cytometry, respectively. TUNEL staining
detected fragmented DNA, and the TEM micrographs revealed the loss
of cellular microvilli and the presence of condensed, fractured and
marginalized chromatin following a 24 h treatment with saponin 6.
In addition, these effects increased with the increasing
concentration of saponin 6 (Fig.
2A). Flow cytometric analysis using Annexin V-FITC/PI double
staining revealed that saponin 6 significantly increased the number
of early and late apoptotic cells in a dose-dependent manner
(Fig. 2B and C). In particular,
~70% of cells underwent apoptosis (early or late stage) following a
24 h treatment with 5.66 µM saponin 6 (Fig. 2D). Furthermore, Hoechst 33342
staining revealed the presence of pyknotic nuclei and apoptotic
bodies in TUNEL-positive cells (Fig.
2E), further confirming the activation of apoptosis. Although
treatment with saponin 6 also increased cell necrosis, this
accounted for only a small fraction of cell death induced by
saponin 6 (Fig. 2B and C). These
results indicate that saponin 6 may induce U87 MG cell death,
predominantly via the promotion of cell apoptosis.
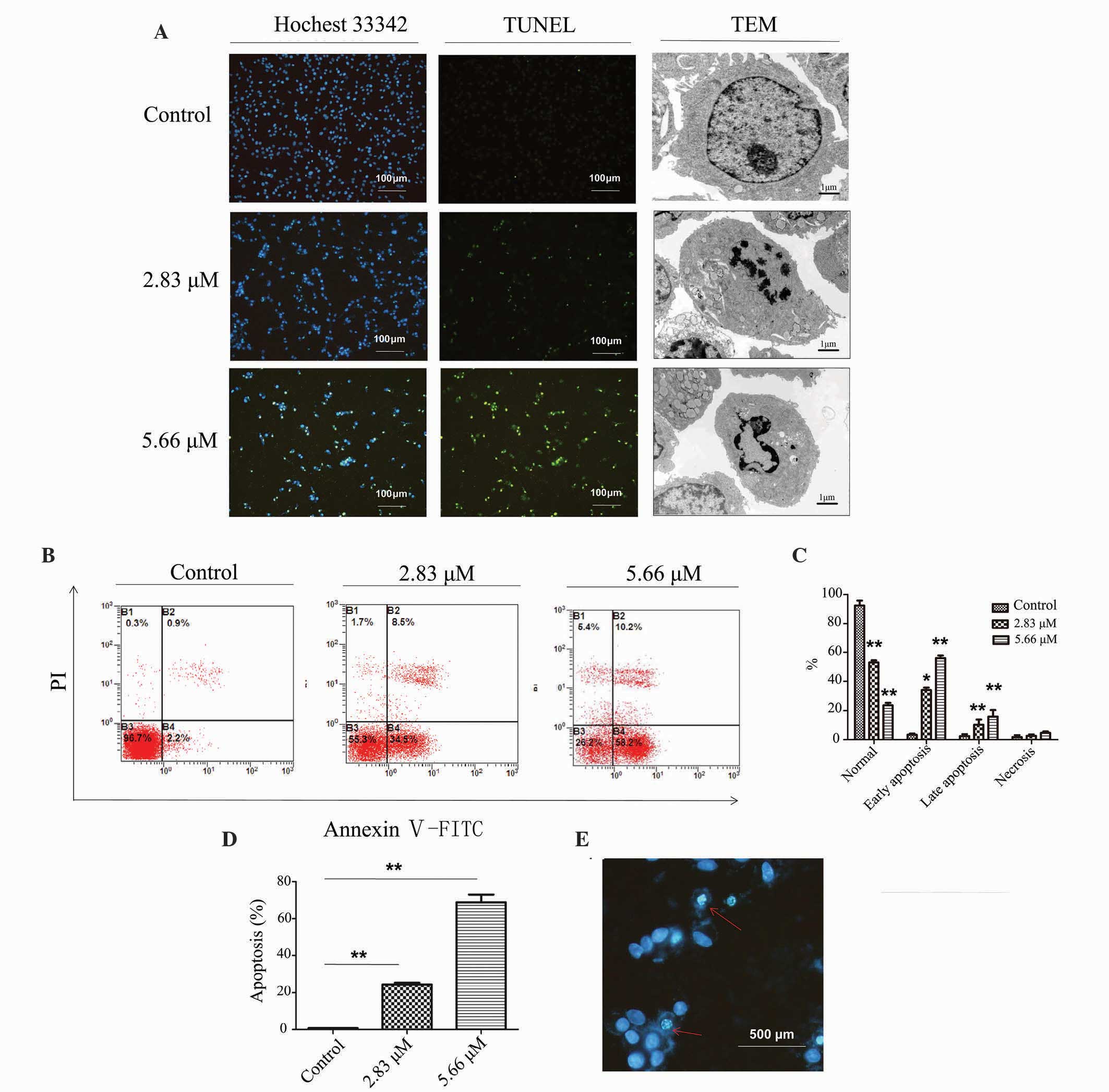 | Figure 2Saponin 6 causes DNA fragmentation,
alters cell morphology, and induces apoptosis in human U87 MG
malignant glioblastoma cells. Cells were treated with vehicle
control, 2.83 µM or 5.66 µM saponin 6 for 24 h. (A)
DNA fragmentation and subcellular morphological alterations were
examined by terminal deoxynucleotidyl transferase-mediated dUTP
nick end labeling (TUNEL) staining and transmission electron
microscopy, respectively. Annexin V-FITC/PI (B) quadrantal and (C)
bar diagrams. (D) Cell apoptosis was determined by flow cytometry
using Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide
(PI) double staining. (E) Fluorescence imaging with Hoechst 33342
staining. The red arrows indicate nuclear morphological changes
characteristic of apoptosis, including chromatin condensation,
boundary aggregation and splitting, and DNA fragmentation. B3,
normal cells; B4, early apoptotic cells; B2, late apoptotic cells;
B1, necrotic cells. Data are presented as the mean ± standard
deviation; n=3. *P<0.05 and **P<0.01
vs. the vehicle control group. |
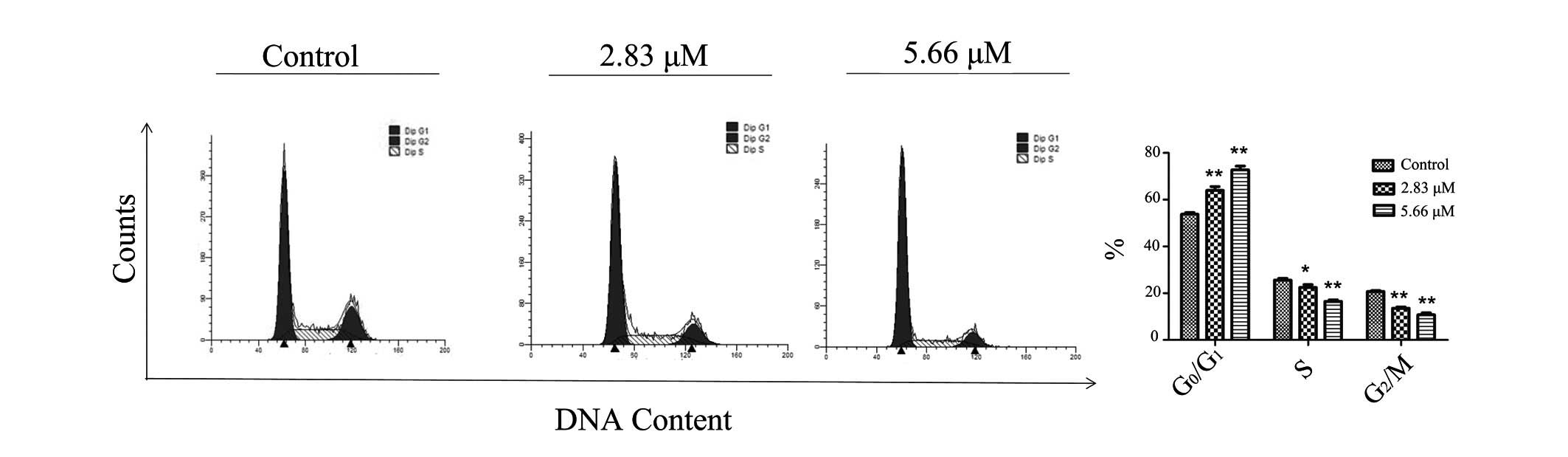
Saponin 6 induces
G0/G1 cell cycle arrest in U87 MG cells
To fully characterize the effects of saponin 6, cell
cycle progression of the U87 MG cells was determined by flow
cytometry using PI staining. Treatment with saponin 6 significantly
increased the percentage of cells at G0/G1
phase, and decreased the percentage of cells at S phase in a
concentration-dependent manner (Fig.
3). These results indicate that saponin 6 may induce
G0/G1 arrest in U87 MG cells.
Saponin 6 activates both extrinsic and
intrinsic apoptotic pathways in U87 MG cells
To explore the signaling pathways underlying saponin
6-induced cell apoptosis in U87 MG cells, the effects of saponin 6
were determined on the protein expression levels of: The Fas death
receptor and its ligand FasL; proteins involved in the extrinsic
(death receptor) apoptotic pathway; Bcl-2 family proteins,
including Bax (pro-apoptotic) and Bcl-2 (anti-apoptotic); and key
regulators of the intrinsic (mitochondrial) apoptotic pathway.
Treatment with saponin 6 for 24 h significantly increased the
expression levels of Fas and FasL, and decreased Bcl-2 expression
in a dose-dependent manner; however, there were no significant
effects on Bax expression (Fig.
4A). These results suggest that saponin 6 may induce U87 MG
cell apoptosis via activation of both the extrinsic and intrinsic
apoptotic pathways. In addition, the protein expression levels of
cleaved caspase-8 (an initiator caspase in the extrinsic pathway),
cleaved caspase-9 (an initiator caspase in the intrinsic pathway),
and cleaved caspase-3 (an effector caspase in both the extrinsic
and intrinsic pathways) were detected. Treatment with saponin 6
significantly increased the expression levels of cleaved caspase-8,
-9 and -3 in a concentration-dependent manner (Fig. 4B–C). These results are in agreement
with the conclusion that saponin 6 may activate both the extrinsic
and intrinsic apoptotic pathways in U87 MG cells.
Discussion
Saponins are natural glycosides that consist of a
triterpene or steroid aglycone, with one or more sugar chains.
Although these are primarily found in plants, saponins are also
produced by certain marine organisms (14). Due to the great variability of
their structures, saponins exert diverse biological functions,
including anticancer (15),
anti-inflammatory (16),
antidiabetic (17), and
cardioprotective (18) properties.
In particular, numerous saponins have recently been reported to
inhibit glioma cell proliferation in vitro (19–22)
and suppress glioblastoma progression in vivo (21). Our previous studies demonstrated
that saponin B and saponin 1 derived from A. taipaiensis
exhibited significant in vitro and in vivo
anti-glioma activity (10,11). In addition, our previous
preliminary structure-activity relationship studies demonstrated
that the cytotoxicity of these A. taipaiensis-derived
saponins is markedly affected by the length of their
oligosaccharide chain at C-3. The greatest potency was observed in
compounds with a chain of intermediate length (15). Therefore, the present study
hypothesized that saponin 6 derived from A. taipaiensis,
which has an oligosaccharide chain of intermediate length, may
possess potent antiglioma activity. The results of the present
study demonstrated that saponin 6 induced U87 MG cell death in a
concentration- and time-dependent manner. Furthermore, saponin 6
had an IC50 value of 2.83 µM following a 48 h
treatment. Although saponin 6 also exerted cytotoxic effects
against noncancerous HT-22 hippocampal neuronal cells, its potency
toward HT-22 cells was much lower than that toward U87 MG cells,
thus indicating differential cytotoxicity against cancer cells.
Future studies on the in vivo efficacy and safety of saponin
6 for the treatment of GBM are warranted.
Dysregulated cell apoptosis may lead to malignant
transformation, tumor progression and resistance to anticancer
drugs (23). Molecules targeting
apoptotic pathways are being actively developed for the treatment
of cancer (24). To further
investigate saponin 6-induced U87 MG cell death, cell apoptosis was
assessed by flow cytometry using Annexin V-FITC/PI double staining.
The results revealed that saponin 6 significantly induced U87 MG
cell apoptosis in a dose-dependent manner. In addition, the
majority of apoptotic cells were in the early stage of apoptosis
following treatment with saponin 6 for 24 h. A TUNEL assay and
ultrastructural TEM study of saponin 6-treated cells detected DNA
fragmentation and other nuclear morphological changes typical of
apoptosis, including condensed, fractured and marginalized
chromatin. Furthermore, Hoechst 33342 staining revealed the
presence of pyknotic nuclei and apoptotic bodies in TUNEL-positive
cells. These results provide evidence to suggest that saponin 6 may
induce apoptosis in U87 MG cells. In addition, increased levels of
cell necrosis were detected following saponin 6 treatment; however,
this accounted for only a very small fraction of cell death caused
by saponin 6. Therefore, saponin 6-induced U87 MG cell death was
predominantly attributed to apoptosis. Furthermore, cell cycle
distribution was analyzed by flow cytometry, which revealed that
saponin 6 induced cell cycle arrest at G0/G1
phase, suggesting that saponin 6 may inhibit DNA synthesis in U87
MG cells.
Apoptosis can be triggered by extrinsic (death
receptor) and intrinsic (mitochondrial) pathways. The extrinsic
apoptotic pathway is initiated by death ligand binding to death
receptors, such as tumor necrosis factor receptor 1 and Fas
(25). The intrinsic apoptotic
pathway, which is characterized by permeabilization of the
mitochondria, release of cytochrome c into the cytoplasm and
activation of caspases, is under the tight regulation of Bcl-2
family proteins (26). Fas is
expressed in the majority of glioma tissues, but not in normal
brain tissues (25). It has
previously been reported that the Fas pathway has a pivotal role in
the tumorigenesis and progression of glioma (27). To investigate the apoptotic
pathways involved in saponin 6-induced U87 MG cell apoptosis, the
protein expression levels of Fas, its ligand FasL, and the Bcl-2
family proteins Bax (pro-apoptotic) and Bcl-2 (anti-apoptotic) were
detected by western blot analysis. The results demonstrated that
the protein expression levels of Fas and FasL were significantly
increased following saponin 6 treatment. Furthermore, the protein
expression levels of Bcl-2 were decreased following treatment with
saponin 6, whereas the protein expression levels of Bax remained
similar. These results suggested that both the extrinsic and
intrinsic pathways may be involved in saponin 6-induced U87 MG cell
apoptosis. In addition, the present study examined the activation
of caspase-8 and -9, the initiator caspases for the extrinsic and
intrinsic pathways, respectively, and caspase-3, an effector
caspase involved in both pathways (28). The results demonstrated that the
expression levels of cleaved caspase-8, -9 and -3 were
significantly increased following treatment with saponin 6. These
results provide further evidence to suggest that saponin 6 is able
to induce U87 MG cell apoptosis via both the extrinsic and
intrinsic apoptotic pathways.
In conclusion, the present study demonstrated that
saponin 6 derived from A. taipaiensis induced U87 MG cell
death by inducing cell apoptosis via activation of both the
intrinsic and extrinsic apoptotic pathways. Therefore, saponin 6
may have therapeutic potential for the treatment of GBM, and future
studies regarding the in vivo efficacy and safety of saponin
6 are warranted.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81372457
and 81274029).
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Magaña-Maldonado R, Manoutcharian K,
Hernández-Pedro NY, Rangel-López E, Pérez-De la Cruz V,
Rodríguez-Balderas C, Sotelo J and Pineda B: Concomitant treatment
with pertussis toxin plus temozolomide increases the survival of
rats bearing intracerebral RG2 glioma. J Cancer Res Clin Oncol.
140:291–301. 2014. View Article : Google Scholar
|
|
3
|
Oliva CR, Nozell SE, Diers A, McClugage SG
III, Sarkaria JN, Markert JM, Darley-Usmar VM, Bailey SM, Gillespie
GY, Landar A and Griguer CE: Acquisition of temozolomide
chemo-resistance in gliomas leads to remodeling of mitochondrial
electron transport chain. J Biol Chem. 285:39759–39767. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Azevedo H and Moreira-Filho CA:
Topological robustness analysis of protein interaction networks
reveals key targets for overcoming chemotherapy resistance in
glioma. Sci Rep. 5:168302015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bodell WJ, Gaikwad NW, Miller D and Berger
MS: Formation of DNA adducts and induction of lacI mutations in Big
Blue Rat-2 cells treated with temozolomide: Implications for the
treatment of low-grade adult and pediatric brain tumors. Cancer
Epidemiol Biomarkers Prev. 12:545–551. 2003.PubMed/NCBI
|
|
6
|
Johnson BE, Mazor T, Hong C, Barnes M,
Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et
al: Mutational analysis reveals the origin and therapy-driven
evolution of recurrent glioma. Science. 343:189–193. 2014.
View Article : Google Scholar :
|
|
7
|
Man S, Gao W, Zhang Y, Huang L and Liu C:
Chemical study and medical application of saponins as anti-cancer
agents. Fitoterapia. 81:703–714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Son MK, Jung KH, Hong SW, Lee HS, Zheng
HM, Choi MJ, Seo JH, Suh JK and Hong SS: SB365, Pulsatilla saponin
D suppresses the proliferation of human colon cancer cells and
induces apoptosis by modulating the AKT/mTOR signalling pathway.
Food Chem. 136:26–33. 2013. View Article : Google Scholar
|
|
9
|
Wang XY, Chen XL, Tang HF, Gao H, Tian XR
and Zhang PH: Cytotoxic triterpenoid saponins from the rhizomes of
Anemone taipaiensis. Planta Med. 77:1550–1554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Tang H, Zhang Y, Li J, Li B, Gao
Z, Wang X, Cheng G and Fei Z: Saponin B, a novel cytostatic
compound purified from Anemone taipaiensis, induces apoptosis in a
human glioblastoma cell line. Int J Mol Med. 32:1077–1084.
2013.PubMed/NCBI
|
|
11
|
Li J, Tang H, Zhang Y, Tang C, Li B, Wang
Y, Gao Z, Luo P, Yin A, Wang X, et al: Saponin 1 induces apoptosis
and suppresses NF-κB-mediated survival signaling in glioblastoma
multiforme (GBM). PloS One. 8:e812582013. View Article : Google Scholar
|
|
12
|
Wang K, Hu X, Du C, Tu S, Zhang F and Xie
X: Angiotensin-(1–7) suppresses the number and function of the
circulating fibrocytes by upregulating endothelial nitric oxide
synthase expression. Mol Cell Biochem. 365:19–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maekawa Y, Yagi K, Nonomura A, Kuraoku R,
Nishiura E, Uchibori E and Takeuchi K: A tetrazolium-based
colorimetric assay for metabolic activity of stored blood
platelets. Thromb Res. 109:307–314. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Osbourn A, Goss RJ and Field RA: The
saponins: Polar isoprenoids with important and diverse biological
activities. Nat Prod Rep. 28:1261–1268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian X, Tang H, Lin H, Cheng G, Wang S and
Zhang X: Saponins: The potential chemotherapeutic agents in
pursuing new anti-glioblastoma drugs. Mini Rev Med Chem.
13:1709–1724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Costa F, Yendo AC, Fleck JD, Gosmann G
and Fett-Neto AG: Immunoadjuvant and anti-inflammatory plant
saponins: Characteristics and biotechnological approaches towards
sustainable production. Mini Rev Med Chem. 11:857–880. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uzayisenga R, Ayeka PA and Wang Y:
Anti-diabetic potential of Panax notoginseng saponins (PNS): A
review. Phytother Res. 28:510–516. 2014. View Article : Google Scholar
|
|
18
|
Yang X, Xiong X, Wang H and Wang J:
Protective effects of Panax notoginseng saponins on cardiovascular
diseases: A comprehensive overview of experimental studies. Evid
Based Complement. Alternat Med. 2014:2048402014.
|
|
19
|
Wu N, Wu GC, Hu R, Li M and Feng H:
Ginsenoside Rh2 inhibits glioma cell proliferation by targeting
microRNA-128. Acta Pharmacol Sin. 32:345–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou J, Cheng G, Cheng G, Tang HF and
Zhang X: Novaeguinoside II inhibits cell proliferation and induces
apoptosis of human brain glioblastoma U87MG cells through the
mitochondrial pathway. Brain Res. 1372:22–28. 2011. View Article : Google Scholar
|
|
21
|
Lv L, Zheng L, Dong D, Xu L, Yin L, Xu Y,
Qi Y, Han X and Peng J: Dioscin, a natural steroid saponin, induces
apoptosis and DNA damage through reactive oxygen species: A
potential new drug for treatment of glioblastoma multiforme. Food
Chem Toxicol. 59:657–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang R, Xiao X, Wang PY, Wang L, Guan Q,
Du C and Wang XJ: Stimulation of autophagic activity in human
glioma cells by anti-proliferative ardipusilloside I isolated from
Ardisia pusilla. Life Sci. 110:15–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Millimouno FM, Dong J, Yang L, Li J and Li
X: Targeting apoptosis pathways in cancer and perspectives with
natural compounds from mother nature. Cancer Prev Res (Phila).
7:1081–1107. 2014. View Article : Google Scholar
|
|
25
|
Thorburn A: Death receptor-induced cell
killing. Cell Signal. 16:139–144. 2004. View Article : Google Scholar
|
|
26
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parney IF, Hao C and Petruk KC: Glioma
immunology and immunotherapy. Neurosurgery. 46:778–791; discussion
791–792. 2000.PubMed/NCBI
|
|
28
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|