Introduction
In diabetic patients, a wide range of structural
reconfiguration has been observed, such as cardiomyocyte
hypertrophy, ventricular dilation, prominent interstitial fibrosis
(1,2), diastolic and systolic dysfunction,
and left ventricular hypertrophy (3,4).
This disease process is termed diabetic cardiomyopathy (DC), which
is a heart muscle-specific disease without other vascular pathology
(5,6). Investigation revealed that patients
with diabetes mellitus (DM) were more likely to suffer from
coronary artery disease, hypertension and mortality following
myocardial infarction (7,8), while DC was one of the most common
serious cardiovascular complications of DM (9). Cellular metabolic abnormalities and
defections of organelles were shown to participate in the pathology
of DC (10–14), which is a chronic and complex
process associated with impaired calcium homeostasis, increased
lipid uptake, myocardial insulin resistance, glucotoxicity,
increased oxidative stress and activation of the renin-angiotensin
system (15). Furthermore, the
pathological role of endoplasmic reticulum (ER) stress in DC has
been noted in a number of studies (16–18).
The endoplasmic reticulum (ER) is central in lipid
synthesis, calcium homeostasis, and the folding and maturation of
membrane and secretory proteins (19). The normal functions of the ER can
be disturbed by various conditions, such as glucotoxicity,
ER-Ca2+ disequilibrium, ischemia and hypoxia, free
radicals, hyperhomocysteine, increased protein synthesis and gene
mutation, which results in ER stress (ERS) (20–25).
ERS occurs in a number of pathological conditions, such as diabetic
kidney disease (26). ERS is known
to be involved in a number of complex homeostatic signaling
pathways among which the unfolded protein response (UPR) is most
commonly recognized (27). The
expression of glucose-regulated protein 78 (Grp78) can be activated
by the UPR, which is known to be a safeguard for normal function of
the ER. Grp78 is an ER resident protein, which reacts to
accumulated proteins. Moderate ER stress can relieve injury
triggered by stress, while severe and chronic ERS result in
apoptosis and induce a number of diseases. Apoptotic processes can
be initiated by caspase-12-dependent pathways and CHOP-dependent
pathways (28,29), which are ER-specific pathways.
Recently, a number of studies have demonstrated the crucial role of
ER stress in the development of DC (30,31).
Therefore, it was hypothesized that downregulating ER stress in
diabetic rats could prevent the development of DC.
Hydrogen sulfide (H2S) is a toxic gas
with a pungent rotten egg smell. It is produced naturally in
mammalian tissues and exhibits various biological and physiological
effects (32–35). A large number of experiments showed
that H2S had the anti-ER stress, anti-apoptosis,
anti-inflammatory and anti-oxidant effects (36–39).
In the nervous system, H2S acts as a neuroprotectant and
may exhibit pharmacological effects in patients with Parkinson's
disease and Alzheimer's disease (40–45).
A recent study found that H2S had antidepressant-like
and anxiolytic-like effects (46).
In the cardiovascular system, H2S was shown to relax
smooth muscles, regulate blood pressure (47–49)
and prevent atherosclerosis (39,50–52),
which resulted in the prevention of ischemia-reperfusion injury in
myocardial cells (53–55). In DM, H2S has been shown
to improve insulin resistance and protect β-cells in the pancreas
(56,57). A previous study has also shown that
H2S acts as a potent inhibitor of fibrosis in the heart
of diabetic rats (58). Thus,
these studies suggested that H2S may exhibit a
cardioprotective effect in the pathophysiology of DC. However,
whether H2S can prevent the pathological process of DC
by suppressing ERS has not yet been demonstrated. Thus, the present
study aimed to demonstrate whether H2S exhibits
protective effects on the myocardium of streptozotocin
(STZ)-induced diabetic rats by suppressing ERS.
Materials and methods
Animals
Fifty adult male Sprague-Dawley (SD) rats, weighing
280–300 g, were purchased from the SJA Lab Animal Center of
Changsha (Changsha, China). The animals were maintained in
accordance with institutional policies, and all experiments were
performed with approval of the University of China Committee on the
Use and Care of Animals of University of South China (Hengyang,
China).
Drugs and reagents
Sodium hydrosulfide (NaHS) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Streptozotocin (STZ) was
purchased from MP Biomedicals Company (Santa Ana, CA, USA).
Specific monoclonal anti-GRP78 antibody was purchased from
Epitomics Inc. (Burlingame, UK). Specific monoclonal anti-CHOP
antibodies were purchased from Proteintech Group, Inc. (Chicago,
IL, USA). Specific monoclonal anti-caspase-12 antibodies were
obtained from Sigma-Aldrich. Anti-rabbit and anti-rat IgG secondary
antibodies were purchased from Proteintech Group, Inc. Cell lysis
buffer for western blot analysis, Enhanced Chemiluminescence
Reagent kit, Bicinchoninic acid assay (BCA) Protein Assay kit and
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) Gel Preparation kit were purchased from Beyotime
Institute of Biotechnology (Shanghai, China).
Study design
Prior to the experiment, rats were adapted to the
experimental environment for one week. During this period, SD rats
were kept under a 12 h light/dark cycle at a constant temperature
(23±1°C) and humidity (60%). They had access to standard rat chow
and normal water ad libitum. NaHS, acting as a
H2S donor, was dissolved in stroke-physiological saline
solution (also termed ʻphysiological salineʼ; purchased from Ruji
Biological Technology Development Co., Ltd., Shanghai, China) and
filtered through a 0.2 mm device (Pall Corporation, Port
Washington, NY, USA). STZ was dissolved in sodium citrate buffer
(pH 4.4; the citric acid and trisodium citrate were purchased from
Sinpharm Chemical Reagent Co., Ltd (Shanghai, China). One week
later, DM was induced in SD rats by a single intraperitoneal (i.p.)
40 mg/kg STZ injection after an overnight fast. Instead of normal
water, 5% glucose solution (Kelun Pharmaceutical Co., Ltd., Hunan,
China) was administered to STZ-treated rats for 24 h after
injection in order to prevent death caused by hypoglycemic shock.
At 72 h following the STZ injection, blood samples were collected
from the tail vein to measure blood glucose levels. Rats with
fasting blood glucose levels >16.7 mmol/l were considered
successful DM models and were used for further investigation
(59). The normal rats who did not
accept the treatment of STZ were randomly divided into two groups:
The control group (treated with normal saline injection every day),
and the H2S-c2 group (normal rats treated with a high
concentration of NaHS, c2=100 µmol/kg; received i.p.,
daily). By contrast, the rats who accepted the treatment of STZ
were divided into three groups: the STZ group (diabetic rats group,
treated with normal saline injection every day), the
STZ+H2S-c1 group (diabetic rats treated with a low
concentration of NaHS; c1=30 µmol/kg) and the
STZ+H2S-c2 group (diabetic rats treated with a high
concentration of NaHS; c2=100 µmol/kg). The rats had free to
access to food and water during the experiment, and feeding
conditions were consistent with the acclimatization period. Eight
weeks later, rats in the five groups were weighed. Rats were
anesthetized by i.p. injection of chloral hydrate (350 mg/kg).
Thoracic cavities were opened, and the hearts of rats were lavaged
with ice-cold normal saline, then removed and weighed. Three rats
were randomly selected from the five groups, their myocardial
tissues were fixed in 10% formalin for immunohistochemical
examination. The remaining hearts were preserved at −80°C prior to
further analysis. This study including animal care was supervised
and approved by the Animal Ethics Committee of the University of
South China.
Body weight, heart weight/body weight
(HW/BW) and blood glucose assay
The body weight of the rats, and their blood glucose
levels, were measured immediately prior to the STZ injection, and
also subsequently, prior to their sacrifice. The following formula
was used to analyze HW/BW: HW/BW = (heart weight / body weight) ×
100. Tail vein blood was tested for the blood glucose levels and it
was analyzed during the acclimatization period, 72 h after STZ
injection and before the end of experiment.
Histopathology and
immunohistochemistry
Myocardium samples from rats were fixed using 4%
paraformaldehyde (Sinopharm Chemical Reagent Co.), dehydrated with
alcohol, embedded in paraffin (Sinopharm Chemical Reagent Co.) and
cut into 5 µm sections. Some of the samples were stained
using a hematoxylin and eosin (H&E) staining kit (Beyotime
Institute of Biotechnology) and observed under a microscope (Motic
BA210; Motic Medical Diagnostic Systems, Co., Ltd., Xiamen, China)
at a magnification of ×200. Several of the sections were incubated
overnight with rabbit polyclonal anti-collagen I or rabbit
polyclonal anti-collagen III. Subsequently, after washing three
times (10 min each wash) in 0.1 M Tris buffer, the sections were
flat-mounted, placed on coverslips, and images were captured using
microscopy (Motic BA210; Motic Medical Diagnostic Systems, Co.,
Ltd.). The extent of myocyte hypertrophy was measured using
H&E-stained sections; collagen components were displayed by
immunostaining (60).
SDS-PAGE and western blot analysis
Cell lysis buffer (20 mM Tris, pH 7.5; 150 mM NaCl
and 1% Triton X-100) containing protease inhibitors (sodium
pyrophosphate, β-glycerophosphate, EDTA, Na3VO4 and
leupeptin) was added to heart tissues and homogenized on the ice.
Then, lysates were centrifuged at 12,000 rpm (7,992 g) for 30 min
at 4°C to attain the supernatant. Protein concentrations were
quantified using a BCA protein assay kit. Protein (15 µg)
was used for electrophoresis using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) [the SDS-PAGE
gel preparation kit (cat. no. P0012A) was purchased from Beyotime
Institute of Biotechnology, and the concentration (%) was made up
according to the molecular weight of the proteins], and transferred
to a polyvinylidene difluoride membrane (Merck Millipore,
Billerica, MA, USA). It was then blocked in Tris-buffered saline
with Tween (TBST) (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; and 0.05%
Tween-20) containing 5% skimmed milk for 2 h. The primary
antibodies used for western blot analysis were diluted in TBST
buffer (5% skimmed milk) at the following concentrations: The
monoclonal antibodies used were: Monoclonal anti-GRP78 antibody
(1:2,000; cat. no. Ab108613, purchased from Epitomics, Inc.,
Burlingame, CA, USA), specific monoclonal mouse anti-CHOP antibody
(1:1,000; cat. no. 60304-1-Ig, purchased from ProteinTech Group,
Inc., Chicago, IL, USA), specific rabbit monoclonal anti-caspase-12
antibody (1:2,000, cat. no. SBP2325, purchased from Sigma-Aldrich,
Inc., St. Louis, MO, USA) and mouse anti-β-actin monoclonal
antibody (1:2,000; cat. no. 60008-1-Ig, purchased from ProteinTech
Group, Inc.). The secondary antibodies used were as follows:
Peroxidase-conjugated Affinipure goat anti-rabbit immunoglobulin G
(IgG)(H+L) (1:8,000; cat. no. SA00001-2, purchased from ProteinTech
Group, Inc.) and peroxidase-conjugated Affinipure goat anti-Mouse
IgG(H+L) (1:8,000, cat. no. SA00001-1, purchased from ProteinTech
Group, Inc.). Rabbit polyclonal anti-collagen I (1:400, cat no.
BA0235) and rabbit polyclonal anti-collagen III (1:400, cat no.
BA0326) were purchased from Wuhan Boster Biological Technology,
Ltd. (Wuhan, China). The membranes were incubated with primary
antibody overnight at 4°C. The following day, the membranes were
washed with TBST buffer three times (for 15 min each time), and
incubated with secondary antibody for 2 h at normal temperature.
Subsequently, the membrane was washed again. Finally, the blot was
visualized using an enhanced chemiluminescence reagent kit and the
optical density was quantified using the Molecular Imager VersaDoc
MP 5000 system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
β-actin was used to ensure that equal protein was loaded in every
sample. All values were normalized by setting the optical density
of the control group to 1.0.
Statistical analysis
Data are presented as the mean ± standard error
values. Potential differences between groups with different
treatments were determined using one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference. All analysis was performed using SPSS software package
18.0 (SPSS Software, Inc., Chicago, IL, USA).
Results
H2S has no effect on BW, HW,
HW/BW and plasma glucose concentration
The body weight of the rats prior to the STZ
injection was determined, and no significant differences among the
five groups were identified (Fig.
1). The BW, HW, HW/BW and plasma glucose concentration were
detected before the end of the experiment. The results revealed
that the BW and HW in the Control group were significantly higher
compared with those in the STZ group (P<0.001), the
STZ+H2S-c1 group (P<0.001) and the STZ+H2S-c2 group
(P<0.001). No significant differences in BW and HW were
identified among the STZ, the STZ+H2S-c1 and the
STZ+H2S-c2 groups. Blood glucose levels in the STZ,
STZ+H2S-c1 and STZ+H2S-c2 groups were
significantly higher compared with that in the Control group
(P<0.001), although no significant differences were identified
when making comparisons among the STZ, STZ+H2S-c1 and
STZ+H2S-c2 groups (Table
I).
 | Table IEffect of H2S on HW, BW,
HW/BW and BG. |
Table I
Effect of H2S on HW, BW,
HW/BW and BG.
| Parameter | Control | STZ |
STZ+H2S-c1 |
STZ+H2S-c2 |
H2S-c2 |
|---|
| BW (g) | 437.71±64.75 |
269.86±20.41b |
285.57±34.60b |
280.14±12.06b | 471.29±24.16 |
| HW (g) | 1.46±0.17 | 1.04±0.10b | 1.08±0.13b | 1.07±0.13b | 1.52±0.96 |
| HW/BW (×100%) | 0.34±0.02 | 0.39±0.04a | 0.38±0.05a | 0.38±0.05a | 0.32±0.01 |
| BG (mmol/l) | 7.26±0.58 | 28.5±2.55b | 24.5±6.96b | 25.2±5.54b | 8.12±2.06 |
H2S can reduce myocardial
fibrosis caused by STZ-induced high plasma glucose
concentrations
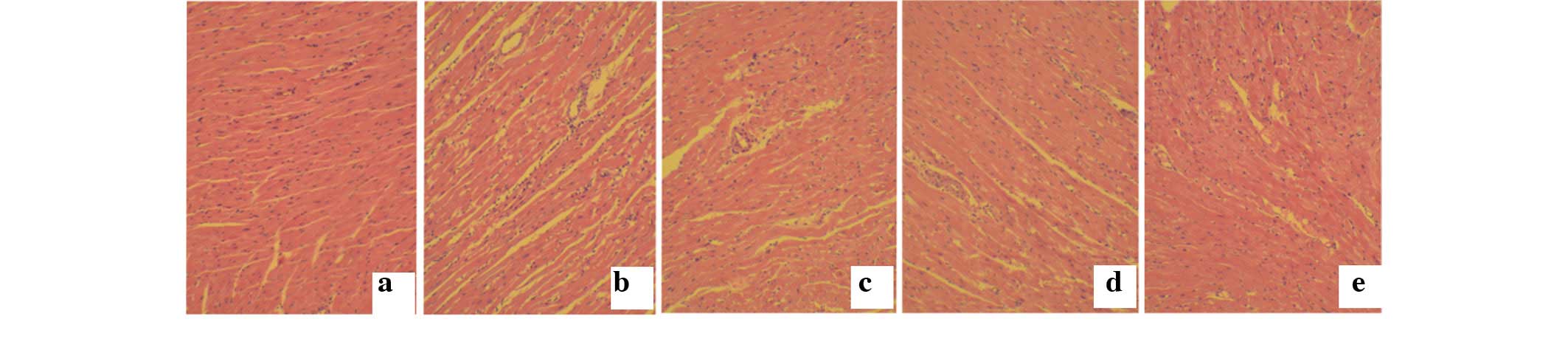
Myocardial hypertrophy was analyzed by hematoxylin
and eosin staining. The results show that the myocardial tissue
structure was tightly organized in the control and
H2S-c2 groups, whereas it was notably looser with a
less-ordered structure in the STZ group. However, these
pathological changes were improved following treatment with
H2S in the STZ+H2S-c1 and
STZ+H2S-c2 groups (Fig.
2).
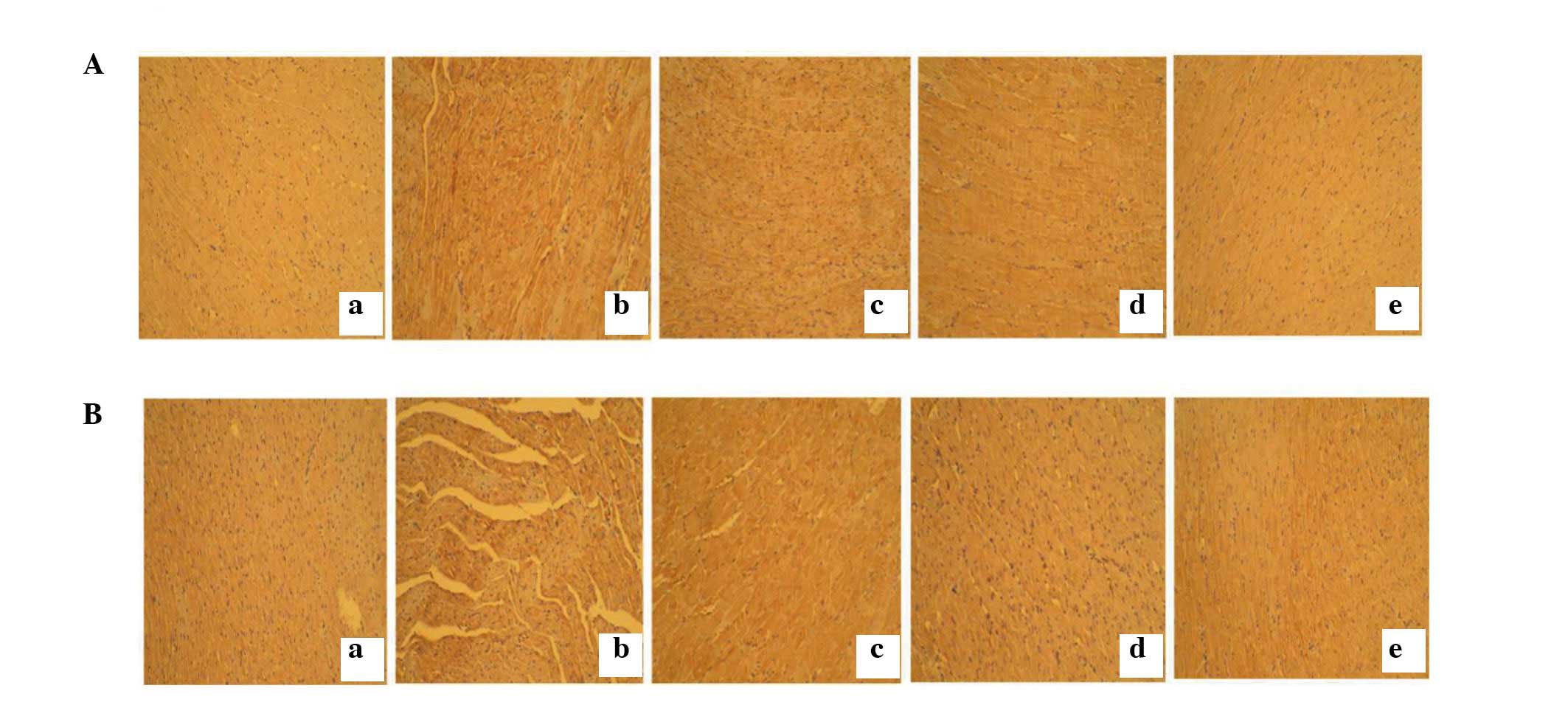
H2S can reduce myocardial
fibrosis caused by high plasma glucose concentration
Myocardial collagen fibrosis was detected by
immunostaining. Results showed that type I collagen (Fig. 3A) and type III collagen (Fig. 3B) expression was markedly
increased, accompanied by disordered arrangement in the STZ group
compared with the control group. These changes were reversed by
H2S intervention.
H2S reduces ERS caused by high
plasma glucose concentrations
High plasma glucose concentrations, caused by the
injection of STZ, significantly increased the expression of
caspase-12 (P<0.01), GRP-78 (P<0.05) and CHOP (P<0.05)
proteins, which are markers of ERS. The expression of these
proteins was shown to be decreased in STZ+H2S-c1
(caspase-12 P<0.05, GRP-78 P<0.05, CHOP P<0.05) and
STZ+H2S-c2 (caspase-12 P<0.001, GRP-78 P<0.05,
CHOP P<0.05) groups, and the expression of GRP-78 and CHOP was
not significant different between the control, H2S-c2,
STZ+H2S-c1 and STZ+H2S-c2 groups. However,
the expression of caspase-12 protein was lower in
STZ+H2S-c2 and H2S-c2 groups compared with
the STZ+H2S-c1 group (STZ+H2S-c2 P<0.05,
H2S-c2 P<0.01), and was also lower in the
H2S-c2 group compared with the control group
(P<0.05).
Discussion
DM is a group of metabolic diseases characterized by
hyperglycemia resulting from defects in insulin and/or insulin
resistance. The long-standing hyperglycemia of DM is associated
with long-term damage, dysfunction and failure of various organs,
particularly the heart (61). In
the current study, the DM model was established, and the
histological changes were evaluated using H&E staining to
identify the existence of myocardial hypertrophy and with Masson's
trichrome staining to identify the existence of myocardial
fibrosis. In order to evaluate the ER stress, western blotting was
used to test the expression of GRP-78, CHOP and caspase-12 protein.
The present study shows that H2S acts as a protective
agent in rats with DC. The key findings were as follows:
Hyperglycemia increased ERS levels, leading to myocardial
hypertrophy and myocardial fibrosis in rats. Moreover,
H2S reduced the ERS caused by hyperglycemia, thus
inhibiting myocardial hypertrophy and improving myocardial
fibrosis.
The main pathophysiological effects of DC were
myocardial fibrosis, cardiomyocyte hypertrophy and cardiac
remodeling, leading to systolic and diastolic dysfunction (62). Hyperglycemia is the primary agent
responsible for the occurrence and development of DC, which can
lead to the progression of complex and chronic processes, including
abnormal cellular metabolism and gene expression, even leading to
cell death. The death of myocytes has been considered to be an
important consequence of DC. As a type of permanent cell, myocytes
are not capable of proliferation, and a decrease in the number of
myocytes can cause systolic and diastolic dysfunction in the heart
(63). The remaining
cardiomyocytes embark upon a pathway of compensatory hypertrophy.
Apoptosis is an important pathway for cell death, and a previous
study demonstrated that myocardial apoptosis was increased notably
in an STZ-induced DM model (64).
Moreover, the effect of myocardial apoptosis caused by
hyperglycemia was also demonstrated in vitro (65,66).
The pathophysiology of apoptosis was closely associated with ERS.
New evidence demonstrated that hyperglycemia induced apoptosis by
activating ERS (67,68). In addition, in diabetic heart
tissue, the ER was found to be swollen and dilated following
ultrastructural analysis, suggesting abnormalities in the ER in a
hyperglycemic environment (69–71).
Recently, an increasing number of studies have investigated the
correlation between ER stress and DC (18,30,31).
H2S was found to be the third gaseous signaling
molecule, which is involved in neuromodulation, neuroprotection,
vasodilatation, cardioprotection and regulation of the inflammatory
response. It is known that H2S affects the development
of DM and its complications, such as protects β-cells in the
pancreas, improves insulin resistance (56,57),
prevents diabetic kidney from reconstruction (72) and alleviates myocardial damage in
DM rat (73). It is encouraging
that H2S functions as an antioxidant, and as an
anti-apoptotic and anti-ERS compound. It was hypothesized that
H2S could protect against DC by inhibiting ERS.
The UPR is activated following ERS to inhibit
protein synthesis and promote protein folding. However, following
chronic ERS, the UPR activates the apoptotic pathway (74–77).
A study by Gunn et al demonstrated that activation of the
UPR contributes to myocardial apoptosis (78). In addition, cytokines and
norepinephrine are vital in the pathophysiology of DC and were
shown to stimulate the UPR (79,80).
This suggests that ERS is involved in the occurrence and
development of DC. Grp78, a regulatory protein of ERS, is important
in assembly and folding of proteins, degradation of unfolded
protein, calcium homeostasis and control of the activation of
transmembrane ER stress sensors. Furthermore, Grp78 serves as a
master modulator for the UPR network by binding to the ERS sensors,
such as protein kinase R-like ER kinase, inositol requiring 1
(IRE1), and activating transcription factor 6 (ATF6) and inhibiting
their activation (81). These
results suggested that Grp78 is an activator of ER stress and UPR.
Previous studies have demonstrated that two specific ER-related
death pathways are involved in the apoptosis pathway. The first is
activation of the transcriptional gene for CHOP and IRE1, and ATF6
signaling has been shown to be involved in the activation of ER
stress (82,83). A previous study demonstrated that
overexpression of CHOP promotes apoptosis (84), while lack of expression of CHOP
inhibits apoptosis by downregulating ER stress (85). Furthermore, CHOP lowers the
expression of Bcl-2, which is an anti-apoptosis protein (86). The second is the activation of
caspase-12. It was demonstrated that pre-caspase-12 is hydrolyzed,
and caspase-12 is activated during ERS (87–90).
Activated caspase-12 promotes apoptosis by activating caspase-9 and
caspase-3 through a non-cytochrome c-dependent pathway. The
above information suggests that CHOP and caspase-12 are markers of
ER stress. In the present study, expression of Grp78, CHOP and
casepase-12 was increased in diabetic rats compared with controls.
Conversely, H2S decreased the expression level of these
three proteins. In addition, the present study demonstrated that
H2S suppresses the expression of caspase-12 in a
concentration-dependent manner, which suggests that the
caspase-12-dependent apoptosis pathway is very sensitive to
H2S. These findings indicated that ERS serves as an
important pathophysiological mechanism for DC, and H2S
acts as a cardioprotective agent and could reduce apoptosis by
inhibiting ERS in the diabetic heart.
There have been large numbers of studies reporting a
decrease in BW in diabetic rats compared with normal rats (91–93).
However, there is no evidence for the impact of H2S on
the HW and BW of diabetic rats. It was demonstrated that
H2S did not affect the BW or HW in diabetic rats. The
potential reasons for the decrease in BW and HW are inefficient use
of glucose, and increased consumption of fat and protein. A number
of studies have demonstrated that as H2S improves
insulin resistance, inhibits the activity of α-glucosidase and
increases hepatic glucokinase activity and glycogen storage, it can
reduce blood glucose (94–96). Conversely, the present study shows
no significant effect of H2S on blood glucose. This may
be because STZ destroys the pancreatic β-cells, leading to an
absolute lack of insulin secretion. Conversely, the present study
reveals no significant effect of H2S on blood glucose.
Possible explanations are as follows: On one hand, STZ may destroy
the pancreatic β-cells, leading to an absolute lack of insulin
secretion. Although it has the effect of improving insulin
resistance, H2S has no role in reducing blood glucose.
On the other hand, the animal models and experimental conditions
were different.
In the clinic, most diabetic patients are suffering
from DC when they go hospital, because it has gone unnoticed in
terms of their health. Therefore, there is a need to focus on the
effect of H2S on cardiomyopathy in the heart. One
shortcoming of the present study was that H2S was added
as an intervention factor at the same time as when the DM model was
established, rather than after DC had developed. In the present
study, we have confirmed the cardioprotective effects of
H2S, as it restrained DC. In a subsequent study,
diabetic rats will receive i.p. infusions of NaHS after the
occurrence of DC, by which means we will be able to investigate
clearly the therapeutic action of H2S on DC. It is
widely known that ER stress is not the only factor that impacts DC,
other mechanisms, including oxidative stress, autophagy and
inflammatory reactions are also involved. These mechanisms interact
to stimulate the development of DC. For example, active oxygen,
increased under conditions of oxidative stress, may transform gene
expression and cause the abnormal functioning of signal
transduction pathways; it may also activate ERS to adapt to
oxidative damage. Apoptosis occurs when the damage exceeds the
regulative capability of the UPR. A previous study showed that DC
is associated with suppression of cardiac autophagy, and
restoration of cardiac autophagy can prevent DC in DM (97). Future experiments may focus on
whether H2S exhibits a protective effect on DC by
affecting oxidative stress and autophagy. In addition, other
signaling pathways associated with these mechanisms are also the an
area of research.
In conclusion, the present study clearly demonstrate
that high blood sugar can promote the development of DC by
increasing the ERS. H2S was shown to inhibit
hyperglycemia-induced ERS, resulting in myocardial protection
against diabetes. This renders H2S a potential anti-DC
agent.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81270181) and the National
Natural Science Foundation of China (grant no. 81202830).
References
|
1
|
Mizushige K, Yao L, Noma T, Kiyomoto H, Yu
Y, Hosomi N, Ohmori K and Matsuo H: Alteration in left ventricular
diastolic filling and accumulation of myocardial collagen at
insulin-resistant prediabetic stage of a type II diabetic rat
model. Circulation. 101:899–907. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guan SJ, Ma ZH, Wu YL, Zhang JP, Liang F,
Weiss JW, Guo QY, Wang JY, Ji ES and Chu L: Long-term
administration of fasudil improves cardiomyopathy in
streptozotocin-induced diabetic rats. Food Chem Toxicol.
50:1874–1882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poirier P, Bogaty P, Garneau C, Marois L
and Dumesnil JG: Diastolic dysfunction in normotensive men with
well-controlled type 2 diabetes: Importance of maneuvers in
echocardiographic screening for preclinical diabetic
cardiomyopathy. Diabetes Care. 24:5–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schannwell CM, Schneppenheim M, Perings S,
Plehn G and Strauer BE: Left ventricular diastolic dysfunction as
an early manifestation of diabetic cardiomyopathy. Cardiology.
98:33–39. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Avogaro A, Vigili de Kreutzenberg S, Negut
C, Tiengo A and Scognamiglio R: Diabetic cardiomyopathy: A
metabolic perspective. Am J Cardiol. 93:13A–16A. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Picano E: Diabetic cardiomyopathy. The
importance of being earliest. J Am Coll Cardiol. 42:454–457. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abbott RD, Donahue RP, Kannel WB and
Wilson PW: The impact of diabetes on survival following myocardial
infarction in men vs women. The Framingham Study. JAMA.
260:3456–3460. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cohen-Solal A, Beauvais F and Logeart D:
Heart failure and diabetes mellitus: Epidemiology and management of
an alarming association. J Card Fail. 14:615–625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trost S and LeWinter M: Diabetic
Cardiomyopathy. Curr Treat Options Cardiovasc Med. 3:481–492. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feuvray D: Diabetic cardiomyopathy. Arch
Mal Coeur Vaiss. 97:261–265. 2004.PubMed/NCBI
|
|
11
|
Tappia PS, Asemu G, Aroutiounova N and
Dhalla NS: Defective sarcolemmal phospholipase C signaling in
diabetic cardiomyopathy. Mol Cell Biochem. 261:193–199. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dyntar D, Sergeev P, Klisic J, Ambühl P,
Schaub MC and Donath MY: High glucose alters cardiomyocyte contacts
and inhibits myofibrillar formation. J Clin Endocrinol Metab.
91:1961–1967. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ligeti L, Szenczi O, Prestia CM, Szabó C,
Horváth K, Marcsek ZL, van Stiphout RG, van Riel NA, Op den Buijs
J, Van der Vusse GJ and Ivanics T: Altered calcium handling is an
early sign of streptozotocin-induced diabetic cardiomyopathy. Int J
Mol Med. 17:1035–1043. 2006.PubMed/NCBI
|
|
14
|
Pereira L, Matthes J, Schuster I, Valdivia
HH, Herzig S, Richard S and Gómez AM: Mechanisms of [Ca2+] i
transient decrease in cardiomyopathy of db/db type 2 diabetic mice.
Diabetes. 55:608–615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bugger H and Abel ED: Rodent models of
diabetic cardiomyopathy. Dis Model Mech. 2:454–466. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Z, Zhang T, Dai H, Liu G, Wang H, Sun
Y, Zhang Y and Ge Z: Involvement of endoplasmic reticulum stress in
myocardial apoptosis of streptozocin-induced diabetic rats. J Clin
Biochem Nutr. 41:58–67. 2007. View Article : Google Scholar
|
|
17
|
Li Z, Zhang T, Dai H, Liu G, Wang H, Sun
Y, Zhang Y and Ge Z: Endoplasmic reticulum stress is involved in
myocardial apoptosis of streptozocin-induced diabetic rats. J
Endocrinol. 196:565–572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu ZW, Zhu HT, Chen KL, Dong X, Wei J,
Qiu C and Xue JH: Protein kinase RNA-like endoplasmic reticulum
kinase (PERK) signaling pathway plays a major role in reactive
oxygen species (ROS)-mediated endoplasmic reticulum stress-induced
apoptosis in diabetic cardiomyopathy. Cardiovasc Diabetol.
12:1582013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sundar Rajan S, Srinivasan V,
Balasubramanyam M and Tatu U: Endoplasmic reticulum (ER) stress
& diabetes. Indian J Med Res. 125:411–424. 2007.PubMed/NCBI
|
|
20
|
Tajiri S, Oyadomari S, Yano S, Morioka M,
Gotoh T, Hamada JI, Ushio Y and Mori M: Ischemia-induced neuronal
cell death is mediated by the endoplasmic reticulum stress pathway
involving CHOP. Cell Death Differ. 11:403–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dromparis P, Paulin R, Sutendra G, Qi AC,
Bonnet S and Michelakis ED: Uncoupling protein 2 deficiency mimics
the effects of hypoxia and endoplasmic reticulum stress on
mitochondria and triggers pseudohypoxic pulmonary vascular
remodeling and pulmonary hypertension. Circ Res. 113:126–136. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang C, Koulajian K, Schuiki I, Zhang L,
Desai T, Ivovic A, Wang P, Robson-Doucette C, Wheeler MB, Minassian
B, et al: Glucose-induced beta cell dysfunction in vivo in rats:
Link between oxidative stress and endoplasmic reticulum stress.
Diabetologia. 55:1366–1379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Back SH and Kaufman RJ: Endoplasmic
reticulum stress and type 2 diabetes. Annu Rev Biochem. 81:767–793.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Williams JA, Hou Y, Ni HM and Ding WX:
Role of intracellular calcium in proteasome inhibitor-induced
endoplasmic reticulum stress, autophagy and cell death. Pharm Res.
30:2279–2289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shinohara M, Ji C and Kaplowitz N:
Differences in betaine-homocysteine methyltransferase expression,
endoplasmic reticulum stress response and liver injury between
alcohol-fed mice and rats. Hepatology. 51:796–805. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu G, Sun Y, Li Z, Song T, Wang H, Zhang
Y and Ge Z: Apoptosis induced by endoplasmic reticulum stress
involved in diabetic kidney disease. Biochem Biophys Res Commun.
370:651–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marciniak SJ and Ron D: Endoplasmic
reticulum stress signaling in disease. Physiol Rev. 86:1133–1149.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao L and Ackerman SL: Endoplasmic
reticulum stress in health and disease. Curr Opin Cell Biol.
18:444–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu K, Wang X, Shi Q, Chen C, Tian C, Li
XL, Zhou RM, Chu YL and Dong XP: Human prion protein mutants with
deleted and inserted octarepeats undergo different pathways to
trigger cell apoptosis. J Mol Neurosci. 43:225–234. 2011.
View Article : Google Scholar
|
|
30
|
Nauntofte B and Dissing S: K+ transport
and membrane potentials in isolated rat parotid acini. Am J
Physiol. 255:C508–C518. 1988.PubMed/NCBI
|
|
31
|
Lakshmanan AP, Harima M, Suzuki K,
Soetikno V, Nagata M, Nakamura T, Takahashi T, Sone H, Kawachi H
and Watanabe K: The hyperglycemia stimulated myocardial endoplasmic
reticulum (ER) stress contributes to diabetic cardiomyopathy in the
transgenic non-obese type 2 diabetic rats: A differential role of
unfolded protein response (UPR) signaling proteins. Int J Biochem
Cell Biol. 45:438–447. 2013. View Article : Google Scholar
|
|
32
|
Kimura H: Hydrogen sulfide as a
neuromodulator. Mol Neurobiol. 26:13–19. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang R: Two's company, three's a crowd:
Can H2S be the third endogenous gaseous transmitter?
FASEB J. 16:1792–1798. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szabó C: Hydrogen sulphide and its
therapeutic potential. Nat Rev Drug Discov. 6:917–935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Łowicka E and Bełtowski J: Hydrogen
sulfide (H2S)-the third gas of interest for pharmacologists.
Pharmacol Rep. 59:4–24. 2007.
|
|
36
|
Zanardo RC, Brancaleone V, Distrutti E,
Fiorucci S, Cirino G and Wallace JL: Hydrogen sulfide is an
endogenous modulator of leukocyte-mediated inflammation. FASEB J.
20:2118–2120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rinaldi L, Gobbi G, Pambianco M, Micheloni
C, Mirandola P and Vitale M: Hydrogen sulfide prevents apoptosis of
human PMN via inhibition of p38 and caspase 3. Lab Invest.
86:391–397. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang XY, Yang CT, Zheng DD, Mo LQ, Lan AP,
Yang ZL, Hu F, Chen PX, Liao XX and Feng JQ: Hydrogen sulfide
protects H9c2 cells against doxorubicin-induced cardiotoxicity
through inhibition of endoplasmic reticulum stress. Mol Cell
Biochem. 363:419–426. 2012. View Article : Google Scholar
|
|
39
|
Chen ZF, Zhao B, Tang XY, Li W, Zhu LL,
Tang CS, Du JB and Jin HF: Hydrogen sulfide regulates vascular
endoplasmic reticulum stress in apolipoprotein E knockout mice.
Chin Med J (Engl). 124:3460–3467. 2011.
|
|
40
|
Tang XQ, Yang CT, Chen J, Yin WL, Tian SW,
Hu B, Feng JQ and Li YJ: Effect of hydrogen sulphide on
beta-amyloid-induced damage in PC12 cells. Clin Exp Pharmacol
Physiol. 35:180–186. 2008.
|
|
41
|
Hu LF, Lu M, Wu ZY, Wong PT and Bian JS:
Hydrogen sulfide inhibits rotenone-induced apoptosis via
preservation of mitochondrial function. Mol Pharmacol. 75:27–34.
2009. View Article : Google Scholar
|
|
42
|
Yin WL, He JQ, Hu B, Jiang ZS and Tang XQ:
Hydrogen sulfide inhibits MPP (+)-induced apoptosis in PC12 cells.
Life Sci. 85:269–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schreier SM, Muellner MK, Steinkellner H,
Hermann M, Esterbauer H, Exner M, Gmeiner BM, Kapiotis S and
Laggner H: Hydrogen sulfide scavenges the cytotoxic lipid oxidation
product 4-HNE. Neurotox Res. 17:249–256. 2010. View Article : Google Scholar
|
|
44
|
Tiong CX, Lu M and Bian JS: Protective
effect of hydrogen sulphide against 6-OHDA-induced cell injury in
SH-SY5Y cells involves PKC/PI3 K/Akt pathway. Br J Pharmacol.
161:467–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kida K, Yamada M, Tokuda K, Marutani E,
Kakinohana M, Kaneki M and Ichinose F: Inhaled hydrogen sulfide
prevents neurodegeneration and movement disorder in a mouse model
of Parkinson's disease. Antioxid Redox Signal. 15:343–352. 2011.
View Article : Google Scholar :
|
|
46
|
Chen WL, Xie B, Zhang C, Xu KL, Niu YY,
Tang XQ, Zhang P, Zou W, Hu B and Tian Y: Antidepressant-like and
anxiolytic-like effects of hydrogen sulfide in behavioral models of
depression and anxiety. Behav Pharmacol. 24:590–597. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao W, Zhang J, Lu Y and Wang R: The
vasorelaxant effect of H(2)S as a novel endogenous gaseous K (ATP)
channel opener. EMBO J. 20:6008–6016. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao W and Wang R: H(2)S-induced
vasorelaxation and underlying cellular and molecular mechanisms. Am
J Physiol Heart Circ Physiol. 283:H474–H480. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang G, Wu L, Jiang B, Yang W, Qi J, Cao
K, Meng Q, Mustafa AK, Mu W, Zhang S, et al: H2S as a
physiologic vasorelaxant: Hypertension in mice with deletion of
cystathionine gamma-lyase. Science. 322:587–590. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mani S, Li H, Untereiner A, Wu L, Yang G,
Austin RC, Dickhout JG, Lhoták Š, Meng QH and Wang R: Decreased
endogenous production of hydrogen sulfide accelerates
atherosclerosis. Circulation. 127:2523–2534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang H, Guo C, Wu D, Zhang A, Gu T, Wang
L and Wang C: Hydrogen sulfide inhibits the development of
atherosclerosis with suppressing CX3CR1 and CX3CL1 expression. PLoS
One. 7:e411472012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D,
Tang X, Ren Y, Tang C and Du J: Role of hydrogen sulfide in the
development of atherosclerotic lesions in apolipoprotein E knockout
mice. Arterioscler Thromb Vasc Biol. 29:173–179. 2009. View Article : Google Scholar
|
|
53
|
Calvert JW, Jha S, Gundewar S, Elrod JW,
Ramachandran A, Pattillo CB, Kevil CG and Lefer DJ: Hydrogen
sulfide mediates cardioprotection through Nrf2 signaling. Circ Res.
105:365–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li H, Ran K, Tang ZG, Li SF and Chang YT:
Effects of hydrogen sulfide preconditioning on myocardial ischemia
reperfusion injury in rats. Zhejiang Da Xue Xue Bao Yi Xue Ban.
41:559–563. 2012.In Chinese. PubMed/NCBI
|
|
55
|
Elrod JW, Calvert JW, Morrison J, Doeller
JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, et al:
Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury
by preservation of mitochondrial function. Proc Natl Acad Sci USA.
104:15560–15565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Feng X, Chen Y, Zhao J, Tang C, Jiang Z
and Geng B: Hydrogen sulfide from adipose tissue is a novel insulin
resistance regulator. Biochem Biophys Res Commun. 380:153–159.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Okamoto M, Yamaoka M and Kimura T:
Hydrogen sulfide and its effect on pancreatic beta-cells. Nihon
Rinsho. 71:175–180. 2013.In Japanese. PubMed/NCBI
|
|
58
|
El-Seweidy MM, Sadik NA and Shaker OG:
Role of sulfurous mineral water and sodium hydrosulfide as potent
inhibitors of fibrosis in the heart of diabetic rats. Arch Biochem
Biophys. 506:48–57. 2011. View Article : Google Scholar
|
|
59
|
Bhutada P, Mundhada Y, Bansod K, Bhutada
C, Tawari S, Dixit P and Mundhada D: Ameliorative effect of
quercetin on memory dysfunction in streptozotocin-induced diabetic
rats. Neurobiol Learn Mem. 94:293–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dong B, Yu QT, Dai HY, Gao YY, Zhou ZL,
Zhang L, Jiang H, Gao F, Li SY, Zhang YH, et al:
Angiotensin-converting enzyme-2 overexpression improves left
ventricular remodeling and function in a rat model of diabetic
cardiomyopathy. J Am Coll Cardiol. 59:739–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Schuster DP and Duvuuri V: Diabetes
mellitus. Clin Podiatr Med Surg. 19:79–107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Galderisi M: Diastolic dysfunction and
diabetic cardiomyopathy: Evaluation by Doppler echocardiography. J
Am Coll Cardiol. 48:1548–1551. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Adeghate E: Molecular and cellular basis
of the aetiology and management of diabetic cardiomyopathy: A short
review. Mol Cell Biochem. 261:187–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cai L and Kang YJ: Cell death and diabetic
cardiomyopathy. Cardiovasc Toxicol. 3:219–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Detaille D, Guigas B, Chauvin C, Batandier
C, Fontaine E, Wiernsperger N and Leverve X: Metformin prevents
high-glucose-induced endothelial cell death through a mitochondrial
permeability transition-dependent process. Diabetes. 54:2179–2187.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Malhotra A, Kang BP, Hashmi S and Meggs
LG: PKCepsilon inhibits the hyperglycemia-induced apoptosis signal
in adult rat ventricular myocytes. Mol Cell Biochem. 268:169–173.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cicek FA, Toy A, Tuncay E, Can B and Turan
B: Beta-blocker timolol alleviates hyperglycemia-induced cardiac
damage via inhibition of endoplasmic reticulum stress. J Bioenerg
Biomembr. 46:377–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cao Y, Hao Y, Li H, Liu Q, Gao F, Liu W
and Duan H: Role of endoplasmic reticulum stress in apoptosis of
differentiated mouse podocytes induced by high glucose. Int J Mol
Med. 33:809–816. 2014.PubMed/NCBI
|
|
69
|
Bhimji S, Godin DV and McNeill JH:
Myocardial ultrastructural changes in alloxan-induced diabetes in
rabbits. Acta Anat (Basel). 125:195–200. 1986. View Article : Google Scholar
|
|
70
|
Jackson CV, McGrath GM, Tahiliani AG,
Vadlamudi RV and McNeill JH: A functional and ultrastructural
analysis of experimental diabetic rat myocardium. Manifestation of
a cardiomyopathy. Diabetes. 34:876–883. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mulhern ML, Madson CJ, Danford A, Ikesugi
K, Kador PF and Shinohara T: The unfolded protein response in lens
epithelial cells from galactosemic rat lenses. Invest Ophthalmol
Vis Sci. 47:3951–3959. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Nikitina EK, Abrosimenkova NN and Rebrov
LB: Changes in rat skeletal muscle actin during postmortem
autolysis. Vopr Med Khim. 36:65–68. 1990.In Russian. PubMed/NCBI
|
|
73
|
Zhong X, Wang L, Wang Y, Dong S, Leng X,
Jia J, Zhao Y, Li H, Zhang X, Xu C, et al: Exogenous hydrogen
sulfide attenuates diabetic myocardial injury through cardiac
mitochondrial protection. Mol Cell Biochem. 371:187–198. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ma Y and Hendershot LM: The unfolding tale
of the unfolded protein response. Cell. 107:827–830. 2001.
View Article : Google Scholar
|
|
75
|
Wiest DL, Burkhardt JK, Hester S, Hortsch
M, Meyer DI and Argon Y: Membrane biogenesis during B cell
differentiation: Most endoplasmic reticulum proteins are expressed
coordinately. J Cell Biol. 110:1501–1511. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Iwakoshi NN, Lee AH, Vallabhajosyula P,
Otipoby KL, Rajewsky K and Glimcher LH: Plasma cell differentiation
and the unfolded protein response intersect at the transcription
factor XBP-1. Nat Immunol. 4:321–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Okada K, Minamino T, Tsukamoto Y, Liao Y,
Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani
T, et al: Prolonged endoplasmic reticulum stress in hypertrophic
and failing heart after aortic constriction: Possible contribution
of endoplasmic reticulum stress to cardiac myocyte apoptosis.
Circulation. 110:705–712. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gunn KE, Gifford NM, Mori K and Brewer JW:
A role for the unfolded protein response in optimizing antibody
secretion. Mol Immunol. 41:919–927. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cardozo AK, Ortis F, Storling J, Feng YM,
Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz
A and Eizirik DL: Cytokines downregulate the sarcoendoplasmic
reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum
Ca2+, leading to induction of endoplasmic reticulum stress in
pancreatic beta-cells. Diabetes. 54:452–461. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mao W, Iwai C, Keng PC, Vulapalli R and
Liang CS: Norepinephrine-induced oxidative stress causes PC-12 cell
apoptosis by both endoplasmic reticulum stress and mitochondrial
intrinsic pathway: Inhibition of phosphatidylinositol 3-kinase
survival pathway. Am J Physiol Cell Physiol. 290:C1373–C1384. 2006.
View Article : Google Scholar
|
|
81
|
Schröder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yoshida H, Okada T, Haze K, Yanagi H, Yura
T, Negishi M and Mori K: ATF6 activated by proteolysis binds in the
presence of NF-Y (CBF) directly to the cis-acting element
responsible for the mammalian unfolded protein response. Mol Cell
Biol. 20:6755–6767. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang XZ, Lawson B, Brewer JW, Zinszner H,
Sanjay A, Mi LJ, Boorstein R, Kreibich G, Hendershot LM and Ron D:
Signals from the stressed endoplasmic reticulum induce
C/EBP-homologous protein (CHOP/GADD153). Mol Cell Biol.
16:4273–4280. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zinszner H, Kuroda M, Wang X, Batchvarova
N, Lightfoot RT, Remotti H, Stevens JL and Ron D: CHOP is
implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Oyadomari S, Koizumi A, Takeda K, Gotoh T,
Akira S, Araki E and Mori M: Targeted disruption of the Chop gene
delays endoplasmic reticulum stress-mediated diabetes. J Clin
Invest. 109:525–532. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Moldoveanu E, Stoian I, Voinea L, Marta D
and Popescu LM: BCL-2-general considerations. Haematologia (Budap).
29:167–180. 1998.
|
|
87
|
Wang ZC, Wang JF, Li YB, Guo CX, Liu Y,
Fang F and Gong SL: Involvement of endoplasmic reticulum stress in
apoptosis of testicular cells induced by low-dose radiation. J
Huazhong University of Science and Technology Med Sci. 33:551–558.
2013.In Chinese. View Article : Google Scholar
|
|
88
|
Chang CF, Wang TM, Wang JH, Huang SC and
Lu TW: Adolescents after Pemberton's osteotomy for developmental
dysplasia of the hip displayed greater joint loading than healthy
controls in affected and unaffected limbs during gait. J Orthop
Res. 29:1034–1041. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chen L, Ren F, Zhang H, Wen T, Piao Z,
Zhou L, Zheng S, Zhang J, Chen Y, Han Y, et al: Inhibition of
glycogen synthase kinase 3beta ameliorates D-GalN/LPS-induced liver
injury by reducing endoplasmic reticulum stress-triggered
apoptosis. PloS One. 7:e452022012. View Article : Google Scholar
|
|
90
|
Qiu ZL, Zhang JP and Guo XC: Endoplasmic
reticulum stress and vascular endothelial cell apoptosis. Acta
Academiae Medicinae Sinicae. 36:102–107. 2014.In Chinese.
|
|
91
|
Fujita A, Sasaki H, Doi A, Okamoto K,
Matsuno S, Furuta H, Nishi M, Nakao T, Tsuno T, Taniguchi H and
Nanjo K: Ferulic acid prevents pathological and functional
abnormalities of the kidney in Otsuka Long-Evans Tokushima Fatty
diabetic rats. Diabetes Res Clin Pract. 79:11–17. 2008. View Article : Google Scholar
|
|
92
|
Thyagaraju BM and Muralidhara: Ferulic
acid supplements abrogate oxidative impairments in liver and testis
in the streptozotocin-diabetic rat. Zoolog Sci. 25:854–860. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xu X, Xiao H, Zhao J and Zhao T:
Cardioprotective effect of sodium ferulate in diabetic rats. Int J
Med Sci. 9:291–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ju Y, Untereiner A, Wu L and Yang G:
H2S-induced S-sulfhydration of pyruvate carboxylase
contributes to gluconeogenesis in liver cells. Biochim Biophys
Acta. 1850:2293–2303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xue R, Hao DD, Sun JP, Li WW, Zhao MM, Li
XH, Chen Y, Zhu JH, Ding YJ, Liu J and Zhu YC: Hydrogen sulfide
treatment promotes glucose uptake by increasing insulin receptor
sensitivity and ameliorates kidney lesions in type 2 diabetes.
Antioxid Redox Signal. 19:5–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hu N, Dong M and Ren J: Role of
mitochondrial injury and apoptosis. Am J Physiol Regul Integr Comp
Physiol. 306:R761–R771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
He C, Zhu H, Li H, Zou MH and Xie Z:
Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances
cardiac autophagy and protects against cardiomyocyte apoptosis in
diabetes. Diabetes. 62:1270–1281. 2013. View Article : Google Scholar :
|