Introduction
In mammalian skeletal systems, several signaling
pathways are important for bone development, including the
transforming growth factor-β/bone morphogenetic protein, Hedgehog,
Wnt, Notch and mitogen-activated protein kinase signaling pathways.
They control osteoblast differentiation, proliferation, maturation
and cytokine secretion via a number of mechanisms. Typically, these
signaling pathways have their own canonical transduction pathways,
and each of these has a central factor, which identifies their
pathway. However, as investigations on signaling networks have
progressed, signaling crosstalk has been found to act as a critical
function to achieve tight signaling regulation and maintain a
balance between cell signaling pathways. In particular, the
orchestral regulation of Notch and Wnt signaling activity by the
pathway networks is an important method, which regulates osteoblast
differentiation and proliferation procedures, and may cause
specific osteoblast behaviors (1).
The current view is that the Notch-Wnt signaling
crosstalk is critical in controlling cell fate, and the importance
of this crosstalk between the Notch and Wnt signaling pathways has
led to suggestion that it be termed 'Wntch' (2). Typically, Notch and Wnt signaling
often have opposing roles in osteoblasts (3–5). If
one signal pathway is disrupted, the other responds to the
Notch-Wnt signaling crosstalk. Therefore, every factor that affects
Notch or Wnt signaling introduces the potential to produce marked
crosstalk disruption and cause multiple cell changes.
Among all the environmental factors that disrupt the
Notch-Wnt signaling pathways, hypoxia and hypoxia-inducible
factor-1α (HIF-1α) is of particular interest due to the frequent
presentation of hypoxic conditions in the pathologic
microenvironment of bones (6,7).
Hypoxia not only initializes the HIF-1α pathway, but also
interrupts other pathways, which are vital for bone development and
survival, including the Notch and Wnt pathways. According to
certain studies, HIF-1α induces a hypoxic condition, which can
promote Notch signaling activity by upregulating the levels of
Notch intracellular domain (NICD) via several direct and indirect
mechanisms (8,9). However, the effect of hypoxia and
HIF-1α on Notch-Wnt signaling crosstalk remains an area of
debate.
Although accumulating evidence indicates that an
increase in Wnt signaling activity is observed in hypoxic
conditions (10), other studies
have reported contradictory conclusions. In particular, the mice
osteoblast-like cell line, MC3T3-E1, showed a downregulatory trend
of Wnt signaling activity in hypoxic conditions, according to
previous studies (11,12). However, the regulation of the Wnt
signaling pathway in hypoxia and its molecular mechanisms remain to
be elucidated.
In addition, evidence has indicated that HIF-1α can
promote Notch signaling activity in hypoxic conditions (8); it is a reasonable hypothesis that
HIF-1α can modify osteoblast growth through Notch-Wnt signaling
crosstalk. However, the exact role of Notch-Wnt crosstalk in
hypoxic conditions remains to be fully elucidated.
To investigate the remaining questions, the present
study aimed to investigate how Notch-Wnt signaling crosstalk
regulates Wnt signaling and the survival of the MC3T3-E1
osteoblast-like cell line in cobalt-mimicked hypoxic conditions.
The potential role of HIF-1α in the underlying molecular mechanism
was also investigated. The current study may provide evidence to
explain the potential mechanism of how hypoxia regulates osteoblast
proliferation, and elucidate a solution to regulate the osteoblast
proliferation under pathological conditions, particularly in the
hypoxic microenvironment.
Materials and methods
Cell line and cell culture
The MC3T3-E1 cells were provided by the Cell bank of
the Chinese Academy of Sciences (Shanghai, China). All cells were
incubated with α-MEM, supplemented with 10% fetal bovine serum and
1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The culture medium was replaced every 2 days.
All cells were incubated in a cell incubator with 5% CO2
at 37°C. For all of the following assessments, the cells were
seeded in 6-well plates at 6×103 cells/cm2,
unless specifically mentioned otherwise.
DAPT and CoCl2 treatment
The γ-secretase inhibitor, DAPT (Selleck Chemicals,
Shanghai, China), was dissolved in DMSO (Sigma-Aldrich, St. Louis,
MO, USA) at a 10 mM final concentration. To inhibit Notch signaling
activity, the MC3T3-E1 cells in each treatment group were treated
with 10, 20, 30 or 40 µM DAPT, which was added into the
culture medium, according to the different experiment requirements.
To eliminate the interruption effect of DMSO, the final
concentration of DMSO in the different groups was adjusted to
ensure equal quantities by adding an additional DMSO into the
culture medium.
To establish a model of chemical hypoxia in
vitro, the cell lines were treated with 100 µM
CoCl2 (cat. no. C8661; Sigma-Aldrich), which was added
directly into the culture medium to mimic a hypoxic condition. In
the DAPT+CoCl2 treatment group, CoCl2 and
DAPT were administered simultaneously.
The medium containing DAPT and CoCl2 to
incubate the cell lines was replaced every 2 days. The treatment
was confirmed effective by performing western blotting.
Small interfering (si)RNA transfection,
and knockdown of the gene expression of HIF-1α and β-catenin
(Ctnnb1)
For the gene expression knockdown experiments, the
mRNA expression of Ctnnb1 and HIF-1α were inhibited by performing
siRNA transfection. The siRNAs were purchased from ViGene
Biosciences, Inc. (Rockville, MD, USA). The siRNA oligoconstruction
used in the experiments were as follows: siRNA for HIF-1α, sense
5′-UCAUCCAAGGAGCCUUAACTT-3′ and antisense
5′-GUUAAGGCUCCUUGGAUGATT-3′; and siRNA for ctnnb1, sense
5′-AGGACAAGCCACAGGAUUATT-3′ and antisense
5′-UAAUCCUGUGGCUUGUCCUTT-3′.
The cells were transfected using 3–5 µg siRNA
mixed with Lipofectamine 2000 (Life technologies; Grand Island, NY,
USA) in a 6-well plate at a density of 5×105/well. All
transfection procedures were performed in strict accordance with
the Lipofectamine 2000 reagent protocol. The gene knockdown
efficiency was confirmed by a reverse transcription-polymerase
chain reaction (RT-qPCR) assay, and the rates of protein production
were determined using western blotting.
Cell viability assessment using a cell
counting kit-8 (CCK-8)
The cells were seeded into a 96-well plate at a
density of 6×103/well, and each treatment group (n=5)
was treated with a different concentration of DAPT. The normal
oxygen tension (normoxia; Nx) groups were treated with 0, 10, 20,
30 and 40 µM DAPT, respectively, and the cobalt
mimicked-hypoxia (Hx) groups were treated with 0, 10, 20, 30 and 40
µM DAPT, respectively. All Hx groups received 100 µM
of CoCl2. The CCK-8 assays were performed, according to
the manufacturer's protocol. Following treatment for 24, 48 and 72
h at 37°C, the medium was replaced with 100 µl fresh α-MEM
and 10 µl CCK-8 solution (cat. no. CK04-500; Dojindo
Laboratories, Kumamoto, Japan) and incubated. The absorbance value
was assayed using an ELISA reader at 450 nm following 2 h
incubation at 37°C.
Acridine orange (AO) staining and
fluorescence microscopy
To assess cell viability under the microscope, the
cells seeded in a 24-well plate were divided into four groups
(n=4): i) Normal oxygen tension groups (Nx groups); ii) 100
µM cobalt-mimicked hypoxic conditions (Co groups); iii) 20
µM DAPT in normoxic conditions (Nx + DAPT groups); and iv)
20 µM DAPT in 100 µM cobalt-mimicked hypoxic
conditions (Co + DAPT groups). Following treatment for 48 h at
37°C, the cells were stained using AO fluorescent dye (cat. no.
A9231; Sigma-Aldrich). Images were captured using a fluorescence
microscope (Olympus Corporation, Tokyo, Japan) with 488 nm laser
emission and 515 nm laser filter at 200× magnification.
Cell apoptosis assays using flow
cytometry
The analysis of apoptosis was performed on the
following four groups: i) Control; ii) inhibitor group with 30
µM DAPT; iii) chemical hypoxia group with 100 µM
CoCl2 and iv) hypoxia and inhibitor group with 100
µM CoCl2 and 30 µM DAPT. The cells in each
group were incubated in the treatment medium for 48 h at 37°C. The
ratio of cell viability was detected using an Annexin-V/Propidium
iodide staining kit (Invitrogen; Thermo Fisher Scientific, Inc.)
and profiling was performed using flow cytometry (FACSVerse, BD
Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
To detect changes in protein levels in the MT3C3-E1
cells, the cells from the different treatment groups were harvested
and 1 ml of 1X radioimmunoprecipitation assay buffer (Cell
Signaling Technology, Inc., Beverly, MA, USA; cat. no. 9806S) was
used for protein extraction. The protein concentrations were
measured using a Bicinchoninic Acid Protein assay kit (cat. no.
23225; Pierce Biotechnology, Inc., Rockford, IL, USA). Equal
quantities of cellular proteins (30 µg) were separated using
10% SDS-PAGE. The proteins were transferred onto PVDF membranes,
and the membranes were blotted with the following primary
antibodies: NICD (1:1,000; cat. no. 2421; Cell Signaling
Technology, Inc.); activated β-catenin (1:1,000; cat. no. 05-665;
EMD Millipore, Billerica, MA, USA); HIF-1α (1:1,000; cat. no.
ab2185; Abcam, Cambridge, UK). Following overnight incubation at
4°C with the primary antibodies at a recommended dilution, the
membranes were washed with phosphate-buffered saline with Tween
(PBST) and then incubated with secondary antibodies at 20°C; for
active-β-catenin, goat anti-mouse IgG (H+L)-horseradish peroxidase
(HRP) antibody (1:10,000; cat. no BS12478; Bioworld Technology,
Inc., St. Louis, MN, USA) was used, for HIF-1α and NICD, goat
anti-rabbit IgG (H+L)-HRP (1:5,000; cat. no. AP307P; EMD Millipore)
was used. Following 90 min incubation, the membranes were washed
with PBST four times and ECL substrate (cat. no, WBKLS0500; EMD
Millipore) was used to develop protein signals, according to the
manufacturer's protocol.
The processed films were scanned, and the band
intensity was quantified as integrated optical density values using
Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA).
RT-qPCR assay
RNA was extracted from the MC3T3-E1 cells, following
the treatments described above, using TRIzol reagent (Invitrogen
Life Technologies; Thermo Fisher Scientific, Inc.). First strand
cDNA was prepared using SuperScript II reverse transcriptase
(Takara Biotechnology Co., Ltd., Dalian, China). The qPCR analysis
was performed using Power SYBR Green PCR master mix (Takara
Biotechnology Co., Ltd.). All procedures were performed according
to the manufacturer's protocol.
Each 2 µl cDNA sample was added to a well of
PCR plate with 0.25 µl forward and reverse primers, 10
µl SYBR Premix Ex Taq solution and 7.5 µl DEPC water.
The qPCR reactions were run on an Illumina-Eco qPCR monitor
(Illumina, Inc., San Diego, CA, USA) using the following cycling
parameters: 10 min at 95°C, followed by 40 cycles of 15 sec at 95°C
and 30 sec at 60–64°C. A melting curve was added to the end of the
program to confirm specific amplification. The quantification cycle
(Cq) was determined from the average of triplicate samples.
Calculations were based on the'ΔΔCq method' using the following
equation: ratio = 2−ΔΔCq. The results were standardized
using the housekeeping gene, β-actin.
The target mRNA and primer sequences were as
follows: hairy and enhancer of split 1 (Hes1), forward
5′-CTAACGCAGTGTCACCTTCC-3′ and reverse 5′-CTAGGGACTTTACGGGTAGCA-3′;
axis inhibition protein 2 (Axin2), forward
5′-ACAGCGAGTTATCCAGCGACG-3′ and reverse
5′-GTGGGTTCTCGGAAAATGAGGTAG-3′; myelocytomatosis oncogene (Myc)
forward 5′-CAGGACTGTATGTGGAGCGGT-3′ and reverse
5′-TGCTGTCGTTGAGCGGGTA-3′; vascular endothelial growth factor-A
(VEGF-A), forward 5′-AGAGAAGACACGGTGGTGGAA-3′ and reverse
5′-TGGGAAGGGAAGATGAGGAA-3′; hypoxia-inducible factor-1 α (HIF-1α),
forward 5′-TAAGGCATCAGCATACAGTGG-3′ and reverse
5′-GATTCAAAGTGGCAGACAGGT-3′; β-actin, forward
5′-TGAGAGGGAAATCGTGCGTGAC-3′ and reverse
5′-GCTCGTTGCCAATAGTGATGACC-3′.
Statistical analysis
All results were evaluated using SPSS v. 13 (SPSS,
Inc., Chicago, IL, USA). The data are presented as the mean ±
standard deviation. The statistical differences were examined using
a one-way analysis of variance Bonferroni correction for multiple
comparisons or a Student's t-test for comparison of two independent
samples. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cobalt-mimicked hypoxia and its
association with osteoblast proliferation, Wnt and Notch
signaling
As several studies have reported, hypoxia may effect
cell proliferation with multiple cell signaling pathway changes
(13–15). The present study examined the
effect of the hypoxia-mimicking agent, cobalt, on osteoblast
proliferation and the regulation of Wnt-Notch signaling pathway
coupling. The data showed that, following 48 h culture in the
cobalt medium, osteoblast proliferation decreased (Fig. 1A and B) and the apoptotic rate
increased (Fig. 1C). This showed
the negative effect of cobalt-mimicked hypoxia on osteoblast
growth.
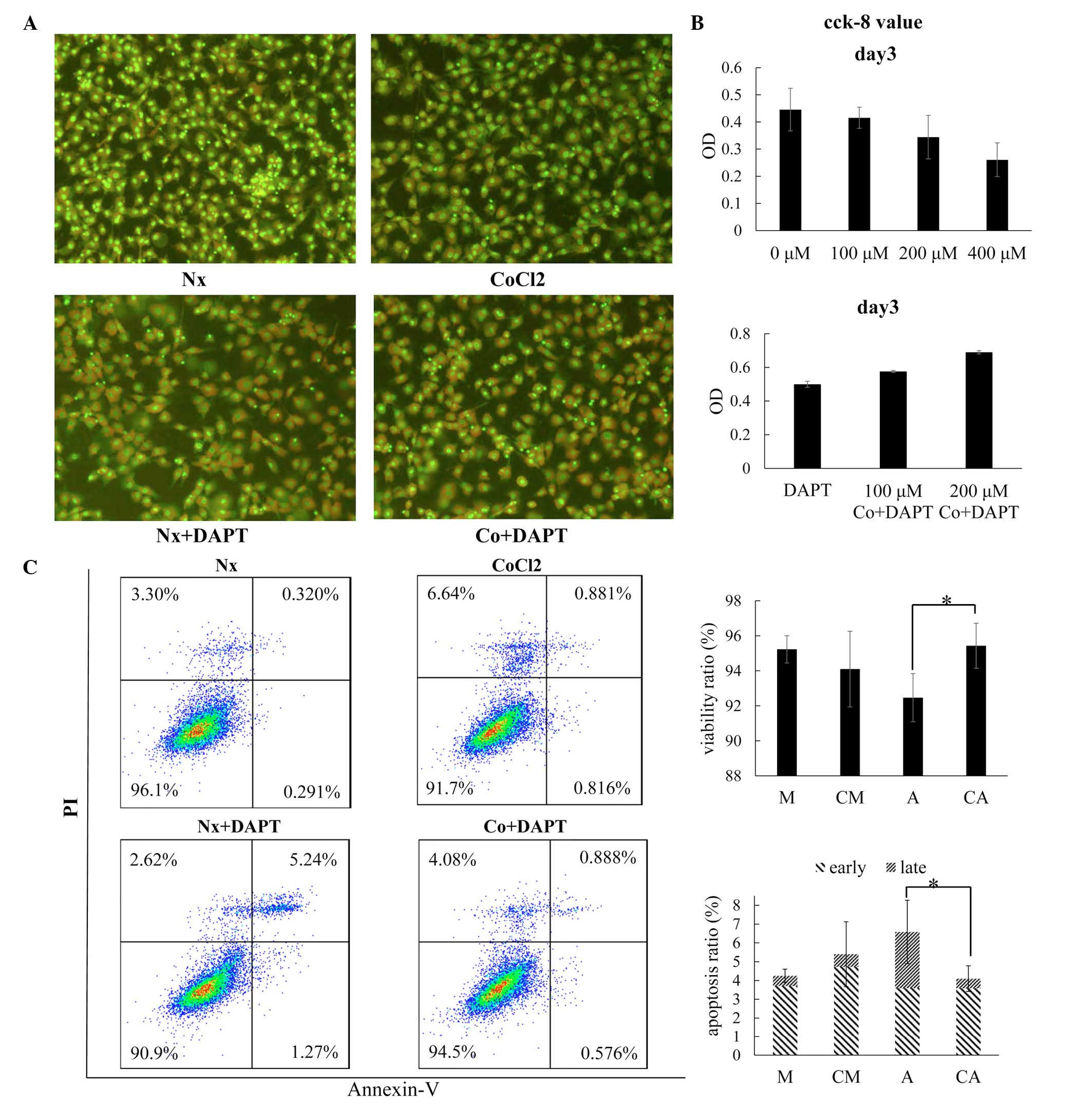 | Figure 1Inhibition of osteoblast proliferation
in cobalt-mimicked hypoxia corresponds with Wnt signaling
downregulation and Notch signaling upregulation. (A) Fluorescence
images of cells following acridine orange staining (magnification,
×200). The MC3T3E1 cells were treated in normoxic conditions (Nx),
100 µM cobalt-mimicked hypoxic conditions (Co), 20 µM
DAPT in normoxic conditions (Nx+DAPT) or 20 µM DAPT in 100
µM cobalt-mimicked hypoxic conditions (Co+DAPT). (B) CCK-8
assay for cell viability following 3 days of treatment. The cells
were treated with different concentration of cobalt (0, 100, 200
and 400 µM) for 3 days (upper figure), or with 20 µM
DAPT and different concentrations of cobalt (0, 100 and 200
µM; lower figure). (C) Detection of cell viability and
apoptotic ratio by flow cytometry under Annexin-V/PI staining
following 48 h treatment. The data are presented as the mean ±
standard deviation of three independent experiments.
*P<0.05. PI, propidium iodide; OD, optical density.
CCK-8, cell counting kit-8. |
To understand the molecular mechanism of
cobalt-induced osteoblast growth inhibition, the present study
focused predominantly on the Notch and Wnt signaling pathways, and
examined changes in their target genes under this condition. An
active component of Notch signaling, NICD (Fig. 2A and B), and the target gene of
Notch signaling, Hes1 (Fig. 2C),
were markedly increased in the cobalt-mimicked hypoxic conditions.
However, the Wnt signaling active component, β-catenin (Fig. 2A and B), was not sensitive to the
cobalt-mimicked hypoxia. The target genes, Axin2 and Myc, were also
found to decrease (Fig. 2C).
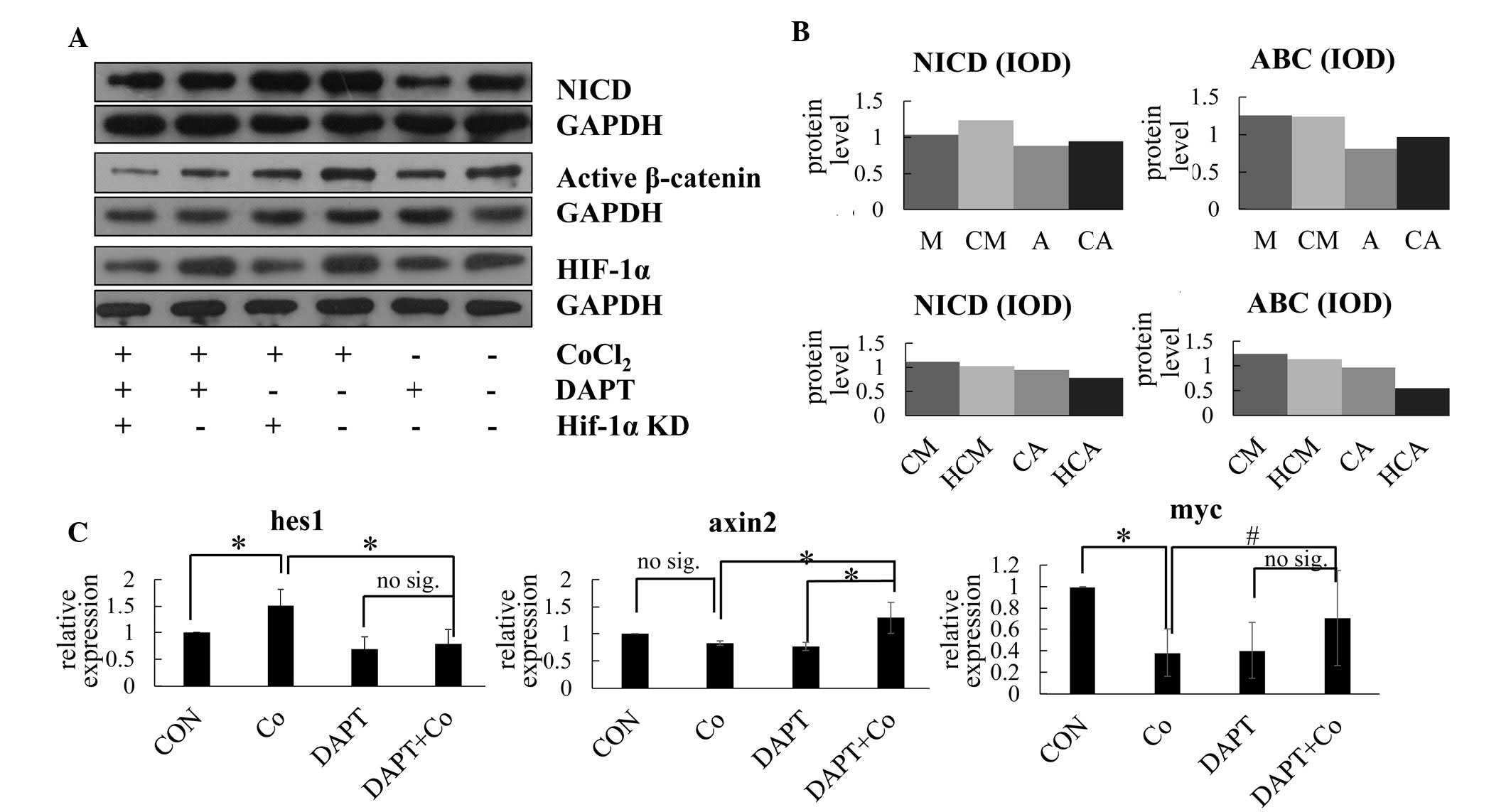 | Figure 2Notch signaling is inhibited by DAPT
in cobalt-mimicked hypoxia promoted osteoblast proliferation and
enhanced Wnt signaling pathway. (A) Western blotting for NICD, ABC
and HIF-1α under different treatment conditions. (B) Relative
protein expression levels of NICD and ABC under normoxia (M) or
hypoxia (CM), under normoxia with DAPT (A) or hypoxia with DAPT
(CA), and under hypoxia with HIF-1α knockdown (HCM) or under
hypoxia with DAPT and HIF-1α knockdown (HCA). (C) Relative
expression levels of Hes-1, Axin2 and Myc under different treatment
conditions, determined using reverse transcription-quantitative
polymerase chain reaction analysis. The data are presented as the
mean ± standard deviation of three independent experiments.
*P<0.05 for two-tailed test; #P<0.05
for one-tailed test; no sig, P>0.05. NCID, cleaved Notch1; ABC,
active-β-catenin; HIF-1α, hypoxia-inducible factor-1α; Hes1, hairy
and enhancer of split 1; Axin2, axis inhibition protein 2; Myc,
myelocytomatosis oncogene; IOD, integrated optical density; CON,
control; Co, cobalt-mimicked hypoxia. |
Cobalt-mimicked hypoxia and osteoblast
proliferation
As Notch signaling was elevated under hypoxic
conditions (Fig. 2), the present
study examined whether Notch was involved in osteoblast
proliferation under cobalt-mimicked hypoxic conditions.
To assess the function of Notch signaling under
hypoxic conditions, the Notch signal was inhibited using the
γ-secretase inhibitor, DAPT, and the effect of cobalt-mimicked
hypoxia on the osteoblasts following Notch signaling suppression
was examined. Coupled with the results from the AO staining
(Fig. 1A), CCK-8 assay (Fig. 1B) and analysis of apoptosis
(Fig. 1C), cobalt-induced hypoxia
increased osteoblast proliferation following Notch suppression, and
this occurred in a concentration-dependent manner.
To further clarify the mechanism underlying the low
concentration hypoxia rescue of cell survival by DAPT, the Notch
signaling messenger and alterations in target genes were analyzed
using western blot analysis (Fig. 2A
and B) and qPCR analysis (Fig.
2C). The data from these analyses showed no differences in the
expression level of NICD or the Hes1 gene between the normoxic and
hypoxic conditions under Notch signaling suppression. This showed
that the cobalt-mimicked hypoxia rescued cell survival in a
Notch-independent manner.
Cobalt-mimicked hypoxia promotes
osteoblast proliferation by increasing β-catenin and Wnt target
gene expression in conditions of Notch signaling suppression
To evaluate the changes in Wnt signaling induced by
cobalt hypoxia under Notch suppression, the levels of active
β-catenin (Fig. 2A and B) and Wnt
target gene (Fig. 2C) were
examined. Unlike Notch signaling, the levels of β-catenin and the
Wnt target gene increased, which indicated activation of the Wnt
signaling pathway under hypoxic conditions and Notch suppression.
The levels of active β-catenin and the Wnt target gene, Myc,
increased simultaneously, suggesting that Wnt signaling activation
enhanced osteoblast survival.
In order to evaluate the associations between Wnt
signaling activation and osteoblast survival under different
conditions, the present study used siRNA to knock down the
expression of β-catenin. The proliferation and apoptotic rates of
the β-catenin-knockdown osteoblasts and wild-type (WT) osteoblasts
under Notch-suppression and hypoxia were compared. By contrast with
the inhibition of osteoblast proliferation caused by increasing
dose of DAPT under normal oxygen condition (Fig. 3A), the proliferation rate (Fig. 3B) of the WT cells increased,
whereas proliferation (Fig. 3C)
was inhibited in the β-catenin-knockdown cells under
Notch-suppression and hypoxia
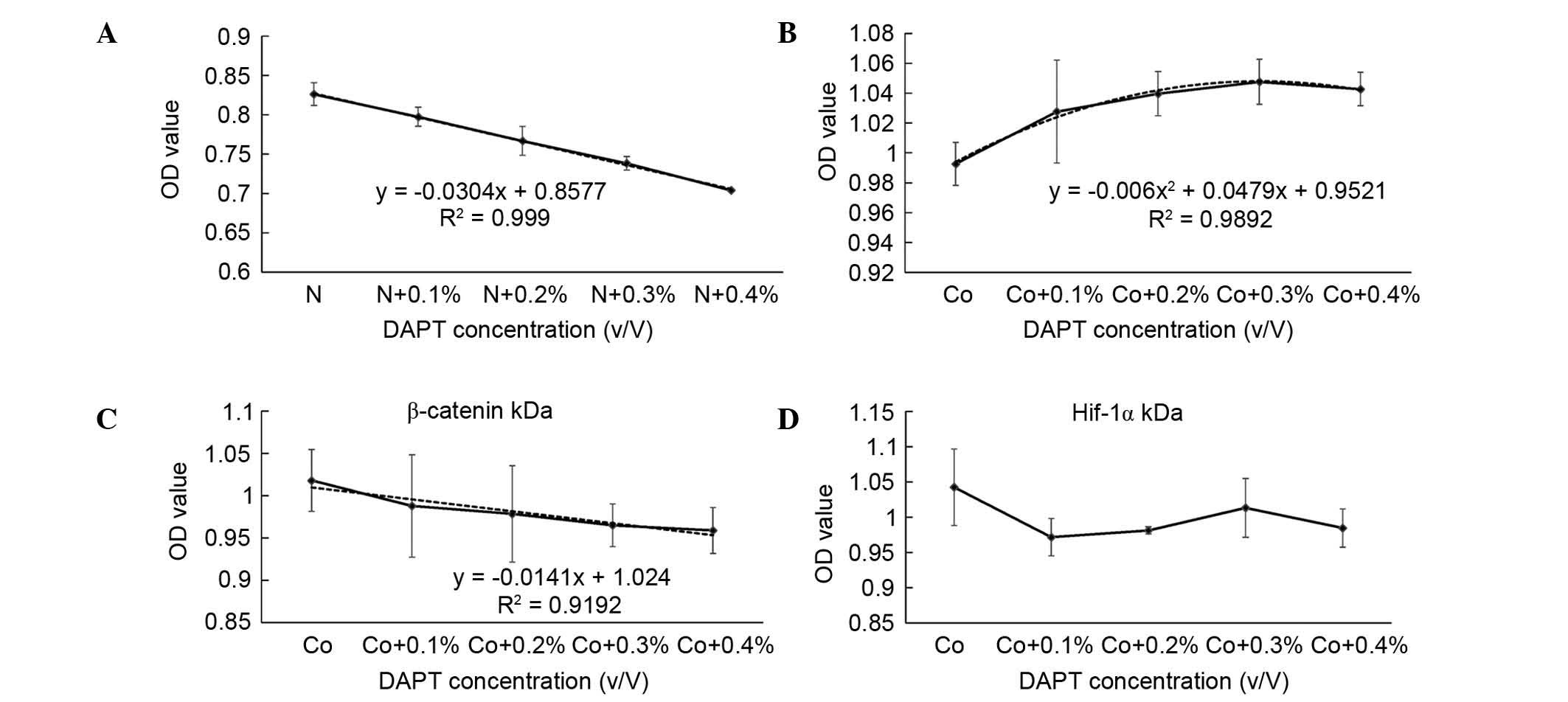 | Figure 3CCK-8 test and curve fitting. CCK-8
assay results for the different treatment groups following
treatment for 48 h and curve fitting. The cells were treated with
(A) different concentrations of DAPT (10 mM DAPT at 0, 0.1, 0.2,
0.3 and 0.4% V/V) in normoxic conditions, (B) different
concentrations of DAPT with 100 µM CoCl2, (C)
different concentrations of DAPT with 100 µM
CoCl2 following β-catenin knockdown, and (D) different
concentrations of DAPT with 100 µM CoCl2a
following HIF-1α knockdown. The data are presented as the mean ±
standard deviation. HIF-1α, hypoxia-inducible factor-1α; OD,
optical density. CCK-8, cell counting kit-8; N, normoxia; Co,
cobalt-mimicked hypoxia. |
Cobalt-mimicked hypoxia requires HIF-1α
to maintain osteoblast proliferation and the expression of Wnt
signaling target genes under Notch suppression
To understand the molecular mechanism of
cobalt-mimicked hypoxia, the HIF-1α, a critical protein induced by
hypoxia, was detected (Fig. 2A and
B), and the effects of HIF-1α on 'Wnt-Notch' signaling cross
talk and the proliferation of osteoblasts were assessed by HIF-1α
knockdown using siRNA (Fig.
4).
The results showed that, when HIF-1α was knocked
down under hypoxic conditions, the cobalt no longer maintained Wnt
signaling under Notch suppression. The level of active β-catenin
decreased more markedly when treated with DAPT (Fig. 2B) and, although no significant
differences in the cell viability ratio (Fig. 4A) were observed in the
HIF-1α-knockdown cells under the different conditions, the
expression levels of the target genes, which were increased by DAPT
(Fig. 2C), were markedly decreased
(Fig. 4B) when HIF-1α was knocked
down. This showed the critical role of HIF-1α in cobalt-induced Wnt
signaling activation and cell growth. As cobalt-mimicked hypoxia
was unable to induce Wnt activation under Notch suppression without
HIF-1α (Fig. 2B and 4B), it was confirmed that Wnt activation
was dependent on the effect of HIF-1α.
Furthermore, in comparing the WT cells and HIF-1α
knockdown cells under cobalt-mimicked hypoxic conditions, the
critical Notch signaling component, NICD, was markedly inhibited by
HIF-1α knockdown (Fig. 2A and B),
which indicated that HIF-1α activated Notch signaling under
hypoxia.
γ-secretase inhibition and osteoblast
proliferation
The CCK-8 assay was also applied to evaluate the
effect of DAPT at different concentrations on osteoblast
proliferation (Fig. 3). The CCK-8
value was marginally increased when the DAPT concentration was
elevated under cobalt-mimicked hypoxic conditions (Fig 3B); the highest OD value was observed
in the cells treated with 0.2% DAPT in the 100 µM
cobalt-mimicked hypoxic condition. This showed that the
proliferation induced by DAPT occurred in a concentration-dependent
manner.
Discussion
In several orthopedic diseases, including bone
fractures, arthritis, osteonecrosis and bone tumors, hypoxic
conditions represent a common pathologic microenvironment, which
affects local cells (7) and causes
significant biological cell alterations. Among these diseases, the
osteoblast is one of the most important cell types, which may be
affected most by the hypoxic conditions. Investigating the
regulation of cell signal transduction in osteoblasts under hypoxic
conditions may assist in developing current understanding of
different mechanisms of bone disease. Among the signaling pathways
that regulate osteoblast behavior, the Notch and Wnt signaling
pathways have been found to be important in hypoxic conditions
(3,16,17).
The associations between hypoxia and the Notch or
Wnt signaling pathways in cells have been confirmed in various
studies. Commonly, hypoxia causes activation of the HIF-1α pathway
via stabilization of the HIF-1α protein by disabling prolyl
hydroxylase domain activity (18).
Earlier studies have found that increasing the level of HIF-1α in
cells promotes the level of NICD (8), and the same effects have been
observed in bone marrow mesenchymal stem cells (19). Furthermore, multiple studies have
shown that the Wnt signaling pathway is regulated by hypoxia in
several cell types, including osteoblasts (10–12).
However, few studies have revealed the function of the Notch-Wnt
signaling interaction in osteoblasts.
In a number of cells, Wnt-Notch signaling always
results in interactions on multiple levels (1), and Notch signaling can inhibit Wnt
signaling pathway activity via several mechanisms. Typically,
canonical Notch signaling activation causes elevations in the level
of NICD and Notch target genes. Several studies have also found
that NICD can directly inhibit β-catenin binding to the T-cell
factor (TCF)/lymphoid enhancer factor (LEF), and inhibits the
transcription activity of TCF/LEF (20). Notch target genes, including Hes1
and hairy/enhancer-of-split related with YRPW motif protein 1, are
also suggested to have a repressive effect on Wnt signaling
activity (3). Certain studies have
suggested that uncleaved Notch receptor truncation inhibits Wnt
signaling, which can occur in a Notch signaling
activation-independent manner (21–23).
The above studies indicate the multi-level modification of Wnt
signaling by Notch signaling crosstalk.
Although several studies have discussed the function
of hypoxia on the proliferation and survival of different cell
types, the results from the studies are varied and complex. As
several studies have been performed in osteoblasts, hypoxia may
regulate cell proliferation via Wnt signaling (10–12).
However, the effect of hypoxia on osteoblast growth has been
debated among reports, with certain studies showing hypoxia to have
the opposite effect on cell proliferation (10), and others reporting negative
conclusions (11,12). The primary difference between these
studies was the variable level of Wnt signal activity that was
induced.
The results of the present study demonstrated that
HIF-1α induced the activation of Wnt signaling. In addition,
hypoxia induced the activation of Notch signaling, inhibiting Wnt
signaling activity and cell proliferation at the same time. When
Notch signaling was activated, the Wnt signaling activity was
relatively inhibited. The inhibition of Notch signaling activation
rescued the hypoxia-induced downregulation of Wnt signaling. This
revealed a novel effect of Wnt-Notch signaling pathway crosstalk on
osteoblast proliferation under hypoxic conditions (Fig. 5).
As shown in a previous study (24) and the present study, severe hypoxic
conditions or cobalt-mimicked hypoxia with a concentration >100
µM caused a decrease in osteoblast survival with
downregulation of the Wnt signaling pathway. Although several
mechanisms have been suggested to explain why hypoxia caused a
downregulation of Wnt, the function of Notch-Wnt crosstalk has not
been discussed. In the present study, it was shown that Notch-Wnt
crosstalk was important in osteoblasts under hypoxic conditions in
at least three ways: i) Hypoxia activated Notch and Wnt signaling
in different conditions, resulting in increased Notch-Wnt crosstalk
under hypoxic conditions in the regulation of cell biology; ii) the
activation of Notch signaling by hypoxia inhibited the Wnt
signaling activity and prevented Wnt-induced proliferation in the
osteoblasts; iii) agents that target γ-secretase regulated Notch
signaling, causing the Wnt-Notch crosstalk effect to enhance
osteoblast proliferation under hypoxic conditions.
According to the mechanism revealed in the present
study, regulation of Notch signaling may affect Notch-Wnt signal
crosstalk and rescue HIF-1α-induced Wnt signal activation, thus
promoting osteoblast proliferation. To confirm the clinical
significance of this mechanism, osteoblasts were treated with
different concentrations of DAPT under the cobalt-mimicked hypoxic
condition, which showed potential value in rescuing osteoblast
growth under hypoxic conditions.
In conclusion, the present study revealed novel
findings of an interaction between HIF-1α and Wnt-Notch signaling
pathway crosstalk. To the best of our knowledge, the present study
represents the first to demonstrate this in osteoblast-like cells.
Furthermore, the present study confirmed the critical role of
HIF-1α in this specific signaling interaction, indicating, at least
partially, a novel mechanism in the Notch-Wnt signaling
interaction. Further investigations are required to focus on the
molecular mechanism of protein interactions between the HIF-1α and
the Notch and Wnt signaling regulators, and the biological
significance of such interactions.
Acknowledgments
This study was supported by the Science and
Technology Foundation of Shenzhen, China (grant no.
JCYJ201409051532 53218) to Mr. B. Chu (Tsinghua University,
Beijing) and Dr S. J. Li (Southern Medical University,
Guangzhou).
References
|
1
|
Collu GM, Hidalgo-Sastre A and Brennan K:
Wnt-Notch signalling crosstalk in development and disease. Cell Mol
Life Sci. 71:3553–3567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayward P, Kalmar T and Arias AM:
Wnt/Notch signalling and information processing during development.
Development. 135:411–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deregowski V, Gazzerro E, Priest L,
Rydziel S and Canalis E: Notch 1 overexpression inhibits
osteoblastogenesis by suppressing Wnt/beta-catenin but not bone
morphogenetic protein signaling. J Biol Chem. 281:6203–6210. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin GL and Hankenson KD: Integration of
BMP, Wnt and notch signaling pathways in osteoblast
differentiation. J Cell Biochem. 112:3491–3501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abdallah BM, Jafari A, Zaher W, Qiu W and
Kassem M: Skeletal (stromal) stem cells: An update on intracellular
signaling pathways controlling osteoblast differentiation. Bone.
70:28–36. 2015. View Article : Google Scholar
|
|
6
|
Kusumbe AP, Ramasamy SK and Adams RH:
Coupling of angiogenesis and osteogenesis by a specific vessel
subtype in bone. Nature. 507:323–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maes C, Carmeliet G and Schipani E:
Hypoxia-driven pathways in bone development, regeneration and
disease. Nat Rev Rheumatol. 8:358–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gustafsson MV, Zheng X, Pereira T, Gradin
K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U and
Bondesson M: Hypoxia requires notch signaling to maintain the
undifferentiated cell state. Dev Cell. 9:617–628. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Villa JC, Chiu D, Brandes AH, Escorcia FE,
Villa CH, Maguire WF, Hu CJ, de Stanchina E, Simon MC, Sisodia SS,
et al: Nontranscriptional role of Hif-1α in activation of
γ-secretase and notch signaling in breast cancer. Cell Rep.
8:1077–1092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Genetos DC, Toupadakis CA, Raheja LF, Wong
A, Papanicolaou SE, Fyhrie DP, Loots GG and Yellowley CE: Hypoxia
decreases sclerostin expression and increases Wnt signaling in
osteoblasts. J Cell Biochem. 110:457–467. 2010.PubMed/NCBI
|
|
11
|
Chen D, Li Y, Zhou Z, Wu C, Xing Y, Zou X,
Tian W and Zhang C: HIF-1α inhibits Wnt signaling pathway by
activating Sost expression in osteoblasts. PLoS One. 8:e659402013.
View Article : Google Scholar
|
|
12
|
Chen D, Li Y, Zhou Z, Xing Y, Zhong Y, Zou
X, Tian W and Zhang C: Synergistic inhibition of Wnt pathway by
HIF-1α and osteoblast-specific transcription factor osterix (Osx)
in osteoblasts. PLoS One. 7:e529482012. View Article : Google Scholar
|
|
13
|
Hubbi ME and Semenza GL: Regulation of
cell proliferation by hypoxia-inducible factors. Am J Physiol Cell
Physiol. 309:C775–C782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Braunschweig L, Meyer AK, Wagenführ L and
Storch A: Oxygen regulates proliferation of neural stem cells
through Wnt/β-catenin signalling. Mol Cell Neurosci. 67:84–92.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meldrum A, Coats P, Orr D and Horgan P:
Vascular smooth muscle cell proliferation and signalling pathways –
effect of hypoxia on vascular remodelling. Atherosclerosis.
241:e762015. View Article : Google Scholar
|
|
16
|
Canalis E: Wnt signalling in osteoporosis:
Mechanisms and novel therapeutic approaches. Nat Rev Endocrinol.
9:575–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu N, Liu H, Qu F, Fan J, Mao K, Yin Y,
Liu J, Geng Z and Wang Y: Hypoxia inhibits the differentiation of
mesenchymal stem cells into osteoblasts by activation of Notch
signaling. Exp Mol Pathol. 94:33–39. 2013. View Article : Google Scholar
|
|
18
|
Chan MC, Holt-Martyn JP, Schofield CJ and
Ratcliffe PJ: Pharmacological targeting of the HIF hydroxylases – a
new field in medicine development. Mol Aspects Med. Jan
11–2016.Epub ahead of print. View Article : Google Scholar
|
|
19
|
Moriyama H, Moriyama M, Isshi H, Ishihara
S, Okura H, Ichinose A, Ozawa T, Matsuyama A and Hayakawa T: Role
of notch signaling in the maintenance of human mesenchymal stem
cells under hypoxic conditions. Stem Cells Dev. 23:2211–2224. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HA, Koo BK, Cho JH, Kim YY, Seong J,
Chang HJ, Oh YM, Stange DE, Park JG, Hwang D and Kong YY: Notch1
counteracts WNT/β-catenin signaling through chromatin modification
in colorectal cancer. J Clin Invest. 122:3248–3259. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwon C, Cheng P, King IN, Andersen P,
Shenje L, Nigam V and Srivastava D: Notch post-translationally
regulates β-catenin protein in stem and progenitor cells. Nat Cell
Biol. 13:1244–1251. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andersen P, Uosaki H, Shenje LT and Kwon
C: Non-canonical Notch signaling: Emerging role and mechanism.
Trends Cell Biol. 22:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayward P, Brennan K, Sanders P, Balayo T,
DasGupta R, Perrimon N and Martinez Arias A: Notch modulates Wnt
signalling by associating with Armadillo/β-catenin and regulating
its transcriptional activity. Development. 132:1819–1830. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Utting JC, Robins SP, Brandao-Burch A,
Orriss IR, Behar J and Arnett TR: Hypoxia inhibits the growth,
differentiation and bone-forming capacity of rat osteoblasts. Exp
Cell Res. 312:1693–1702. 2006. View Article : Google Scholar : PubMed/NCBI
|



















