Introduction
Non-small cell lung carcinoma (NSCLC) is the most
common type of lung cancer and is often fatal (1). NSCLC can be divided into different
subtypes including the common squamous cell carcinoma and
adenocarcinoma and the less common, large cell lung carcinoma.
NSCLC accounts for 70–80% of lung cancer diagnoses, and the disease
has often progressed to an advanced stage by the time it is
detected (1). Although various
treatments have been investigated, systemic chemotherapy has been
indicated to improve the quality of life and the survival of
patients with advanced NSCLC (1).
As innovative microtubule stabilizing agents,
taxanes, including paclitaxel and docetaxel, have been clinically
used for the treatment of NSCLC (2). Clinical efficacy of these agents
varies from patient to patient, with a number of individuals
exhibiting resistance to docetaxel. Resistance to chemotherapy
remains a challenge in improving the quality of life and prolonging
the survival of the patients with advanced stage NSCLC. Therefore,
it is important to explore the underlying molecular mechanisms.
MicroRNAs (miRNAs) are a class of non-coding RNAs
(18–25 nucleotides in length), that may bind to the 'seed
sequences' in the 3′-untranslated region (3′-UTR) of mRNAs and thus
trigger mRNA degradation or inhibit protein translation. In a
previous study, miRNAs were shown to have a negative impact on cell
survival, proliferation and motility by regulating the expression
of their targets (3). In addition,
miRNAs have been reported to act as oncogenes or tumor inhibitors.
It has also been indicated that disruption of miRNA functioning is
correlated with human malignant tumors (4). Furthermore, the deregulation of
certain miRNAs, including miR-7, miR-182, miR-192 and miR-17, was
associated with resistance and sensitivity to chemotherapy in NSCLC
cells (5–8).
In a previous study, miR-27b was shown to be
significantly downregulated in NSCLC tumor tissue that was
resistant to docetaxel, as compared with tumor tissues that were
sensitive to the treatment (9).
The present study aimed to investigate the expression levels of
miR-27b in samples collected from patients with NSCLC who were
resistant or sensitive to docetaxel. In addition, the online miRNA
database TargetScan was searched in order to identify the target
genes of miR-27b, and a luciferase assay was conducted in order to
confirm these predictions. Furthermore, the potential mechanisms
underlying the involvement of miR-27b in the apoptosis of A549
human lung carcinoma cells, as well as their chemoresistance, were
investigated.
Materials and methods
Tissue specimen collection
A total of 54 snap-frozen histologically confirmed
NSCLC tissue samples were collected and evaluated for miRNA and
gene expression. All of the samples were collected from the First
Affiliated Hospital of Nanjing Medical University, and this study
protocol was approved by the Institutional Review Boards at the
First Affiliated Hospital of Nanjing Medical University. None of
the patients had received any chemotherapy or radiotherapy prior to
the surgery. Following surgical intervention, patients received at
least 2–4 cycles of the docetaxel-based chemotherapy as the
first-line adjuvant therapy. Patients who experienced recurrent or
progressive disease during docetaxel-based chemotherapy or within
12 months post-completion of first line therapy were considered as
docetaxel-resistant (n=26). Patients with no recurrence or with
recurrences beyond 12 months were defined as docetaxel-sensitive
(n=28). Informed consent was obtained from each patient. Following
surgical removal, the tissue samples were stored in liquid nitrogen
for future use.
Target prediction
The online miRNA database, TargetScan (www.targetscan.org), was used to predict the target
genes of miR-27b, and the similarity of potential ̔seed sequences̓
among various species was compared and presented.
Cell lines and cell culture
A549 cells were obtained from the Shanghai Institute
of Biochemistry and Cell Biology (Shanghai, China). All cells were
incubated in RPMI 1640 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 100 U/ml penicillin, 10% fetal bovine
serum and 100 g/ml streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.) in humidified air at 37°C at an atmosphere of 5%
CO2.
Transfection
Following culturing the cells to 70% confluence,
they were transfected using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Briefly, miR-27b mimics/inhibitor and EGFR small interfering
(si)RNA (all from Shanghai GenePharma Co., Ltd., Shanghai, China)
were diluted in serum-free medium (1:50; Thermo Fisher Scientific,
Inc.), which was also used to dilute Lipofectamine 2000. Following
incubation of the cells at 37°C in 5% CO2 for 6 h, the
medium in each well was replaced by the normal serum-containing
medium and cultured for 24 h prior to the assays being
performed.
Western blot analysis of target
genes
Cells or tissue samples were suspended in 50
μl lysis buffer [1 mM ethylene diamine tetraacetic acid, pH
8.0; 5 mM dithiothreitol; 2% sodium dodecyl sulfate (SDS); and 50
mM Tris-HCl, pH 8.0] for 30 min in ice and then harvested. The
resulting lysates were centrifuged at 4,000 × g for 10 min, and the
supernatant was removed. A 10% SDS-polyacrylamide gel
electrophoresis membrane (Invitrogen; Thermo Fisher Scientific,
Inc.) was used to resolve the proteins, which were electroblotted
onto nitrocellulose membrane (Merck Millipore, Darmstadt, Germany).
Subsequently, 5% skimmed milk was used to block the blots. The
membranes were then incubated with mouse anti-EGFR antibody
(1:1,000; sc-120) and β-actin antibody (1:10,000; sc-130656; both
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at room
temperature for 2 h, and then incubated with horseradish
peroxidase-conjugated anti-goat and mouse secondary antibodies
(Sigma-Aldrich). The bands were observed with an Enhanced
Chemiluminescence kit (Invitrogen; Thermo Fisher Scientific, Inc.).
The relative density of the bands was quantified using ImageJ
software 1.41 (National Institutes of Health, Bethesda, MA, USA),
and normalized to the internal control, β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the A549 cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNA
samples (10 μg) were reverse-transcribed into cDNA using the
SuperScript III Reverse Transcriptase or miRNA-specific primers and
the TaqMan MicroRNA Reverse Transcription kit (all: Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. PCR
was conducted using 1 μl cDNA, TaqMan MicroRNA Assays,
TaqMan Universal PCR Master Mix, No AmpErase UNG and primers
(Applied Biosystems; Thermo Fisher Scientific, Inc.), on an ABI
7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The PCR cycling conditions were as follows: 95°C
for 30 sec, followed by 36 cycles at 95°C for 5 sec and 60°C for 40
sec. The primer sequences were as follows: miR-27b forward,
5′-GGGGTTCAAGTAATTCAGG-3′ and reverse, 5′-CAGTGCGTGTCGTGGA-3′; EGFR
forward, 5′-GTCAGCTAGCGCGTATGCTAAT-3′ and reverse,
5′-GCGATCGTCGTATATCTAGTCAG-3′; and β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGT ACT TGC GCT CAG GAG
G-3′ (Sangon Biotech, Co., Ltd., Shanghai, China). The PCR products
were separated by 1% agarose gel electrophoresis (Sigma-Aldich, St.
Louis, MO, USA). Each RT-qPCR assay was repeated at least three
times. The mRNA expression levels of the miRNA were normalized to
U6 snRNA, whereas those of the target genes were normalized to
β-actin. Relative expression levels were calculated using the
2−ΔΔCq method (10).
Dual luciferase reporter assay
The full length wild-type EGFR 3′-UTR was amplified
by PCR, and inserted into a psiCHECK 2 vector (Promega Corporation,
Madison, WI, USA), and the mutant type 3′-UTR of EGFR was generated
using site-directed Mutagenesis kit (Agilent Technologies, Inc.,
Santa Clara, CA, USA). The psiCHECK2-wildtype-EGFR-3′-UTR or
psiCHECK2-mutant1 or 2 EGFR-3′-UTR vectors were used to transfect
the A549 cells, in the presence or absence of 100 nM miR-27b
mimics. Following transfection, the A549 cells were cultured at
37°C at an atmosphere of 5% CO2 for 48 h. The luciferase
activity was then determined using an LD400 luminometer (Beckman
Coulter, Inc., Brea, CA USA). The activity of firefly luciferase
was standardized to the activity of Renilla.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The viability of the A549 cells was examined 48 h
following transfection. A549 cells (5×103 cells/well)
were seeded in a 96-well plate and were incubated to 70–80%
confluence. Subsequently, 10 μl of 5 mg/ml MTT
(Sigma-Aldrich, Shanghai, China) was added to all wells and
cultured at 37°C for a further 4 h. Following the removal of the
medium, 150 μl dimethyl sulfoxide (Sigma-Aldrich, Shanghai,
China) were added to dissolve the MTT formazan. The optical density
was determined at 490 nm using a spectrometer (ND-1000
Fluorospectrometer; Thermo Fisher Scientific, Inc.).
Apoptosis assay using flow cytometry
The A549 cells transfected with miR-27b
mimics/inhibitor or EGFR siRNA were stained with Annexin
V-fluorescein isothiocyanate and propidium iodide (PI) (Roche
Diagnostics GmbH Mannheim, Germany). Following collection and
washing with phosphate-buffered saline, the cells
(1×104) were cultured with Annexin V and PI at room
temperature for 20 min. Flow cytometry (LSR II; BD Biosciences, San
Jose, CA, USA) was used to analyze the A549 cells with a cell
sorter activated by fluorescence (BD Biosciences).
Statistical analysis
All data are expressed as the mean ± standard
deviation, and the difference between the groups was evaluated
using an independent t-test or one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference. Each experiment was performed three times. Statistical
analysis was performed using SPSS 19.0 (SPSS Inc., Chicago, IL,
USA).
Results
EGFR is a direct target of miR-27b
miR-27b was significantly downregulated in
docetaxel-resistant NSCLC, which may be involved in the molecular
mechanism underlying the resistance to docetaxel (9). When computational analysis was
performed in order to identify the potential target gene of
miR-27b, the results of bioinformatic analysis predicted a
regulatory association between miR-27b and EGFR (data not shown).
In addition, the putative 'seed sequences' for miR-27b in the
3′-UTR of EGFR were evolution arily conserved. To validate the
regulatory relationship between miR-27b and EGFR, the full length
wild-type EGFR 3′-UTR was sub-cloned into psiCHECK2 with a
downstream firefly luciferase gene. The two predicted binding sites
(200–207 bp and 430–436 bp) were replaced with complementary
sequences, labeled as mutant 1 and mutant 2 EGFR 3′-UTR,
respectively. Subsequently, A549 cells were transfected with
psiCHECK2-EGFR-3′-UTR or psiCHECK2-mutant 1 or mutant 2 EGFR-3′-UTR
vectors, together with miR-27b mimics or scramble controls.
Following incubation for 48 h, the luciferase activity was
examined, and the data revealed that it was significantly reduced
in A549 cells co-transfected with wild type or mutant 2 3′-UTR of
EGFR and miR-27b mimics compared with the scramble control
(P<0.01; Fig. 1), suggesting
that miR-27b may directly bind to 200–207 bp in the 3′-UTR of EGFR
mRNA in A549 cells.
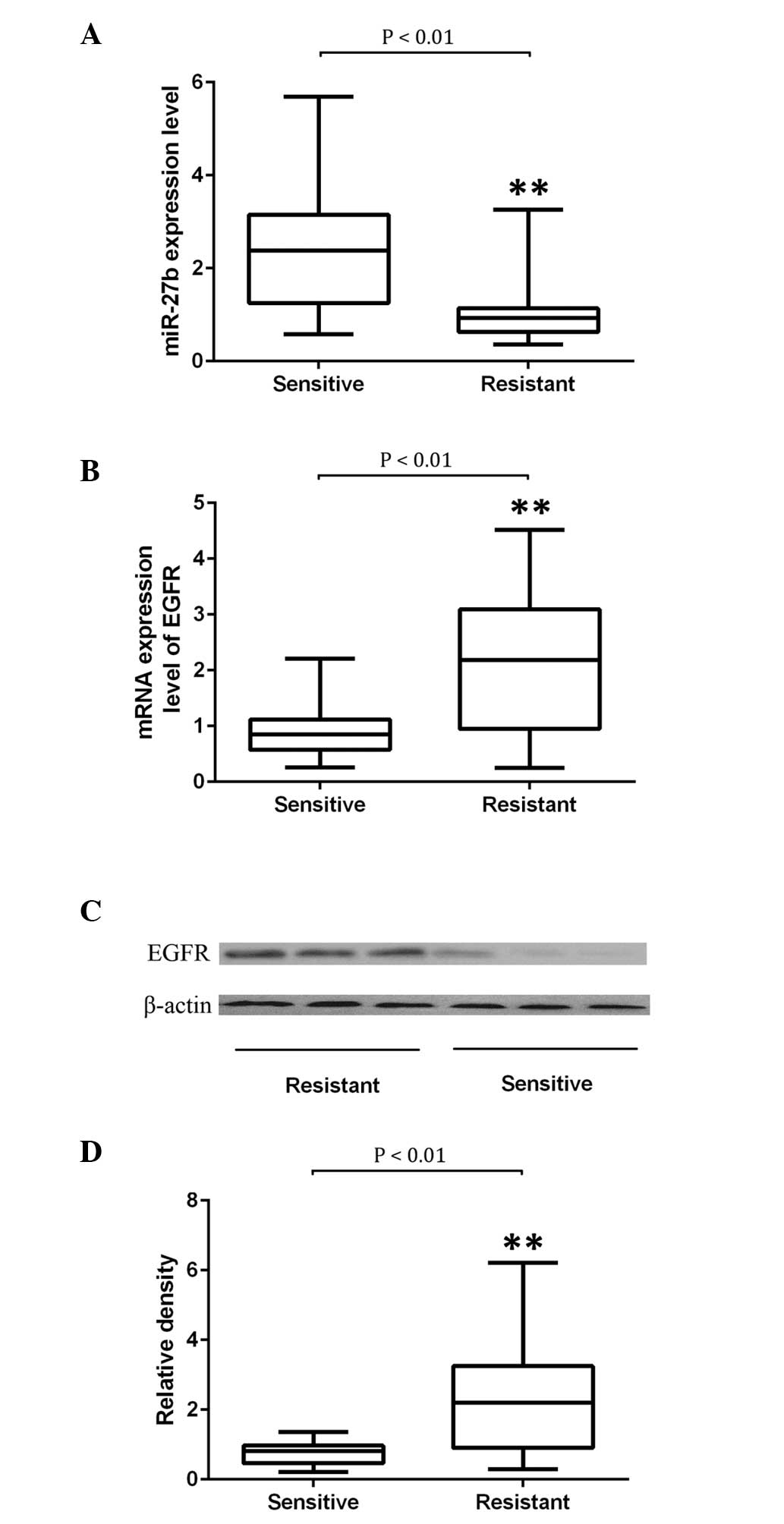
Determination of the expression of
miR-27b and EGFR in docetaxel-resistant and -sensitive NSCLC
samples
To further verify the regulatory role of miR-27b in
EGFR expression, 28 docetaxel-sensitive and 26 docetaxel-resistant
NSCLC tissue samples were collected. The expression levels of
miR-27b and EGFR were examined. As indicated by Fig. 2A, it was determined that miR-27b
was significantly downregulated in NSCLC tissues resistant to the
treatment of docetaxel compared with those sensitive to it
(P<0.01). The expression of EGFR was determined using
semiquantitative RT-PCR and western blot analysis. The mRNA and
protein expression of EGFR was significantly upregulated in NSCLC
tissues that were resistant to the treatment of docetaxel compared
with those sensitive to it (P<0.01; Fig. 2B–D).
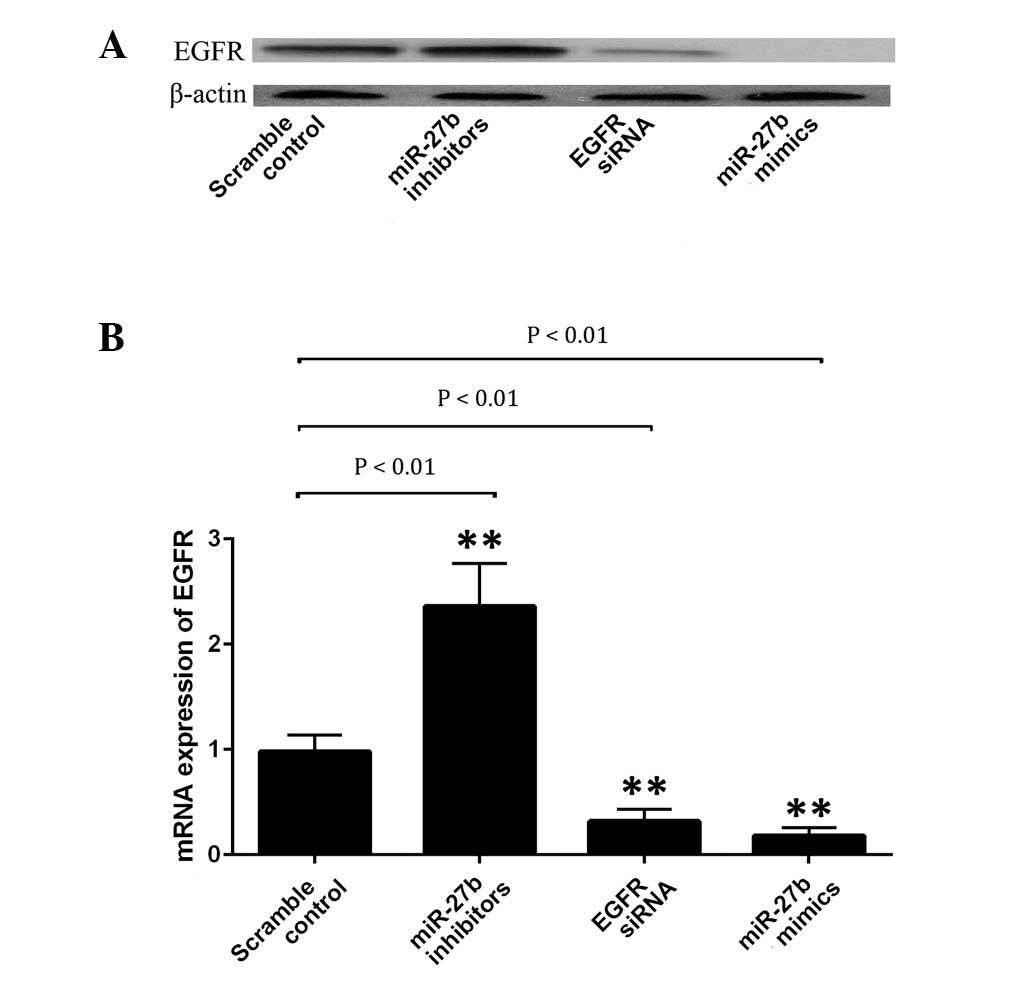
miR-27b negatively regulates EGFR
expression in A549 cells
A549 cells were transfected with scramble miRNA
control, EGFR siRNA, miR-27b mimics or its inhibitor. Subsequently,
the protein expression level of EGFR in each group was examined. As
indicated in Fig. 3A, the EGFR
protein expression level was significantly upregulated following
transfection with miR-27b inhibitors (P<0.01), whilst it was
significantly reduced subsequent to transfection with the miR-27b
mimics or EGFR siRNA (P<0.01). To explore the regulatory
association between miR-27b and EGFR, the mRNA expression level of
EGFR in A549 cells was determined following transfection with the
EGFR siRNA, miR-27b mimics or inhibitor. As indicated in Fig. 3B, upregulation of miR-27b and
introduction of EGFR siRNA significantly inhibited the protein
expression of EGFR in A549 cells (P<0.01). By contrast,
inhibition of miR-27b resulted in a significantly increased protein
expression of EGFR in the cells (P<0.01). Therefore, it was
suggested that miR-27b negatively regulates EGFR expression at the
transcriptional level in A549 cells.
miR-27b transfection suppresses A546 cell
viability by targeting EGFR
To identify the role of miR-27b in the control of
the viability of the cells, A549 cells were transfected with EGFR
siRNA, and miR-27b mimics or inhibitor. It was determined that the
transfection of miR-27b inhibitors significantly increased the
viability of the cells by inhibiting apoptosis (Fig. 4). The introduction of EGFR siRNA or
miR-27b mimics markedly decreased cell viability and induced the
apoptosis of A549 cells (Figs. 4
and 5).
Discussion
NSCLC is a major health problem worldwide with
>600,000 cases diagnosed annually in China despite improvements
in diagnosis and treatment (11).
Current treatment options for patients with NSCLC primarily include
chemotherapy, surgery, immunotherapy, radiation therapy and
targeted therapies, which are primarily dependent on the stage of
the disease (12,13). Commercially approved targeted
therapies and chemotherapeutic drugs, including docetaxel,
cisplatin, carboplatin, paclitaxel, erlotinib, gemcitabine and
pemetrexed, are used to treat advanced stage NSCLC (14–18).
Antitubulin agents are important in NSCLC
chemotherapy. Taxanes are a family of diterpenes that bind
preferentially to microtubules, leading to stabilization and arrest
of mitosis (19). As a member of
the taxane family, docetaxel is highly active and commonly used as
adjuvant therapy following resection of localized tumors in
patients with locally advanced NSCLC. However, like the majority of
chemotherapeutic agents, cellular resistance limits the clinical
success of docetaxel (20,21). It has been determined that miR-27b
was significantly downregulated in docetaxel-resistant NSCLC, and
may be responsible for the molecular mechanism underlying
resistance to docetaxel (9).
In the current study, EGFR was identified as a
potential target of miR-27b. The regulatory association between
miR-27b and EGFR was validated by a luciferase assay which
indicated that the luciferase activity was notably reduced in A549
cells co-transfected with the wild type or mutant 2 3′-UTR of EGFR
(430–436 bp was replaced with complementary sequences) and miR-27b
mimics, but had minimal effect on those cells transfected with
mutant 1 3′-UTR of EGFR (200–207 bp was replaced with complementary
sequences). Therefore, miR-27b may directly bind to 200–207 bp in
the 3′-UTR of EGFR mRNA in A549 cells. It has been previously
determined that miRNAs are involved in regulation of a wide range
of biological processes, and inhibition of their function may aid
the progression of human malignant tumors (22).
Low levels of miR-27b have been associated with
numerous types of human malignancy. A study by Wan et al
(23) demonstrated that miR-27b
levels were considerably decreased in cell lines and tissue samples
of NSCLC, and that excessive expression of miR-27b substantially
inhibited NSCLC cell invasion and proliferation. Thereby,
suggesting that miR-27b may act as a tumor inhibitor in NSCLC.
Previous studies have revealed that miR-27b acts as a tumor
suppressor in the development of various malignancies, including
prostate cancer, colon cancer and neuroblastoma (24–26).
However, other studies have also indicated that miR-27b may promote
tumorigenesis in various cancer types. A study by Jin et al
(27) demonstrated that miR-27b
was significantly upregulated in human breast cancer, and its
proliferation was inhibited by knockdown of miR-27b. Another study
by Rui et al (9)
demonstrated that miR-27b functions as an oncogene in glioma as
significant upregulation of miR-27b in glioma cells and tissues was
determined. The present study has confirmed the downregulation of
miR-27b in docetaxel-resistant NSCLC by measuring and comparing the
expression of miR-27b in 28 docetaxel sensitive and 26 docetaxel
resistant NSCLC tissue samples. EGFR was validated as a target of
miR-27b. Additionally, mRNA and protein expression of EGFR were
significantly upregulated in NSCLC tissues that were resistant to
docetaxel compared with those that were sensitive.
As a transmembrane growth factor receptor, EGFR
exhibits tyrosine kinase activity, which is important for its
involvement in controlling the survival and proliferation of cells
(28), and it has been indicated
that the expression of EGFR was negatively associated with the
apoptosis of cancer cells (29).
Various mechanisms, including gene mutation, copy number variation,
and protein overexpression are associated with the deregulation of
cellular activity of EGFR (30,31).
Alterations in the quantity or activity of EGFR are not uncommon in
lung cancer and are usually associated with numerous pathological
features, including poor differentiation, high tumor grading and an
unsatisfactory prognosis, suggesting that EGFR may be a therapeutic
target for lung cancer (31–34).
In the present study, miR-27b negatively regulated the expression
of EGFR, as shown by the observation that miR-27b transfection
mimics downregulated the expression of EGFR, whereas miR-27b
inhibitors upregulated the expression of EGFR. Furthermore, it was
demonstrated that transfection with miR-27b mimics significantly
suppressed the apoptosis and promoted the viability of A549 cells.
By contrast, the introduction of miR-27b inhibitors significantly
induced apoptosis and inhibited the proliferation of A549
cells.
In conclusion, EGFR was validated as a target of
miR-27b in NSCLC. It also mediated the apoptosis-promoting effect
of miR-27b and the transfection of miR-27 inhibitors significantly
promoted the apoptosis of lung cancer cells via targeting EGFR.
Therefore, it is possible that EGFR signaling pathway activation
underlies the observed downregulation of miR-27b in
docetaxel-resistant NSCLC cells, and administration of miR-27b in a
manner that efficiently enhances the level of miR-27b in NSCLC
tumor tissue may be a novel method to improve the sensitivity to
docetaxel in patients with NSCLC.
References
|
1
|
Jalal SI, Ademuyiwa FO and Hanna NH: The
role of maintenance chemotherapy in advanced nonsmall cell lung
cancer. Curr Opin Oncol. 21:110–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edelman MJ and Gandara DR: Promising new
agents in the treatment of non-small cell lung cancer. Cancer
Chemother Pharmacol. 37:385–393. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng Y, Yu S, Li H, Xiang H, Peng J and
Jiang S: MicroRNAs: Emerging roles in adipogenesis and obesity.
Cell Signal. 26:1888–1896. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palumbo S, Miracco C, Pirtoli L and
Comincini S: Emerging roles of microRNA in modulating cell-death
processes in malignant glioma. J Cell Physiol. 229:277–286. 2014.
View Article : Google Scholar
|
|
5
|
Liu R, Liu X, Zheng Y, Gu J, Xiong S,
Jiang P, Jiang X, Huang E, Yang Y, Ge D and Chu Y: MicroRNA-7
sensitizes non-small cell lung cancer cells to paclitaxel. Oncol
Lett. 8:2193–2200. 2014.PubMed/NCBI
|
|
6
|
Ning FL, Wang F, Li ML, Yu ZS, Hao YZ and
Chen SS: MicroRNA-182 modulates chemosensitivity of human non-small
cell lung cancer to cisplatin by targeting PDCD4. Diagn Pathol.
9:1432014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang F, Li Y, Wu H, Qi K, You J, Li X, Zu
L, Pan Z, Wang Y, Li Y, et al: MiR-192 confers cisplatin resistance
by targeting Bim in lung cancer. Zhongguo fei ai za zhi.
17:384–390. 2014.In Chinese. PubMed/NCBI
|
|
8
|
Jiang Z, Yin J, Fu W, Mo Y, Pan Y, Dai L,
Huang H, Li S and Zhao J: MiRNA 17 family regulates
cisplatin-resistant and metastasis by targeting TGFβR2 in NSCLC.
PLoS One. 9:e946392014. View Article : Google Scholar
|
|
9
|
Rui W, Bing F, Hai-Zhu S, Wei D and
Long-Bang C: Identification of microRNA profiles in
docetaxel-resistant human non-small cell lung carcinoma cells
(SPC-A1). J Cell Mol Med. 14:206–214. 2010. View Article : Google Scholar
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
11
|
Walker S: Updates in non-small cell lung
cancer. Clin J Oncol Nurs. 12:587–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gkiozos I, Charpidou A and Syrigos K:
Developments in the treatment of non-small cell lung cancer.
Anticancer Res. 27:2823–2827. 2007.PubMed/NCBI
|
|
13
|
Bernstein ED, Herbert SM and Hanna NH:
Chemotherapy and radiotherapy in the treatment of resectable
non-small-cell lung cancer. Ann Surg Oncol. 13:291–301. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pirker R: Adjuvant chemotherapy in
patients with completely resected non-small cell lung cancer.
Transl Lung Cancer Res. 3:305–310. 2014.
|
|
15
|
Bi N and Wang L: Superiority of
concomitant chemoradiation over sequential chemoradiation in
inoperable, locally advanced non-small cell lung cancer: Challenges
in the selection of appropriate chemotherapy. Semin Radiat Oncol.
25:122–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matikas A, Mistriotis D, Georgoulias V and
Kotsakis A: Current and future approaches in the management of
non-small-cell lung cancer patients with resistance to EGFR TKIs.
Clin Lung Cancer. 16:252–261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pilkington G, Boland A, Brown T, Oyee J,
Bagust A and Dickson R: A systematic review of the clinical
effectiveness of first-line chemotherapy for adult patients with
locally advanced or metastatic non-small cell lung cancer. Thorax.
Feb 6–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paul I and Jones JM: Apoptosis block as a
barrier to effective therapy in non small cell lung cancer. World J
Clin Oncol. 5:588–594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horwitz SB: Taxol (paclitaxel): Mechanisms
of action. Ann Oncol. 5(Suppl 6): S3–S6. 1994.PubMed/NCBI
|
|
20
|
Geney R, Ungureanu M, Li D and Ojima I:
Overcoming multidrug resistance in taxane chemotherapy. Clin Chem
Lab Med. 40:918–925. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McGrogan BT, Gilmartin B, Carney DN and
McCann A: Taxanes, microtubules and chemoresistant breast cancer.
Biochim Biophys Acta. 1785:96–132. 2007.PubMed/NCBI
|
|
22
|
Tay FC, Lim JK, Zhu H, Hin LC and Wang S:
Using artificial microRNA sponges to achieve microRNA
loss-of-function in cancer cells. Adv Drug Deliv Rev. 81:117–127.
2015. View Article : Google Scholar
|
|
23
|
Wan L, Zhang L, Fan K and Wang J: MiR-27b
targets LIMK1 to inhibit growth and invasion of NSCLC cells. Mol
Cell Biochem. 390:85–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye J, Wu X, Wu D, Wu P, Ni C, Zhang Z,
Chen Z, Qiu F, Xu J and Huang J: miRNA-27b targets vascular
endothelial growth factor C to inhibit tumor progression and
angiogenesis in colorectal cancer. PLoS One. 8:e606872013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishteiwy RA, Ward TM, Dykxhoorn DM and
Burnstein KL: The microRNA-23b/-27b cluster suppresses the
metastatic phenotype of castration-resistant prostate cancer cells.
PLoS One. 7:e521062012. View Article : Google Scholar
|
|
26
|
Lee JJ, Drakaki A, Iliopoulos D and Struhl
K: MiR-27b targets PPARγ to inhibit growth, tumor progression and
the inflammatory response in neuroblastoma cells. Oncogene.
31:3818–3825. 2012. View Article : Google Scholar
|
|
27
|
Jin L, Wessely O, Marcusson EG, Ivan C,
Calin GA and Alahari SK: Prooncogenic factors miR-23b and miR-27b
are regulated by Her2/Neu, EGF, and TNF-α in breast cancer. Cancer
Res. 73:2884–2896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu J, Ding JY, Lu CL, Lin ZW, Chu YW, Zhao
GY, Guo J and Ge D: Overexpression of CD88 predicts poor prognosis
in non-small-cell lung cancer. Lung Cancer. 81:259–265. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Edelman MJ: Novel taxane formulations and
microtubule-binding agents in non-small-cell lung cancer. Clin Lung
Cancer. 10:S30–S34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morales-Cano D, Calviño E, Rubio V,
Herráez A, Sancho P, Tejedor MC and Diez JC: Apoptosis induced by
paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4
human leukaemia cells is not modulated by ERK inhibition. Exp
Toxicol Pathol. 65:1101–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kavallaris M: Microtubules and resistance
to tubulin-binding agents. Nat Rev Cancer. 10:194–204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wan YF, Guo XQ, Wang ZH, Ying K and Yao
MH: Effects of paclitaxel on proliferation and apoptosis in human
acute myeloid leukemia HL-60 cells. Acta Pharmacol Sin. 25:378–384.
2004.PubMed/NCBI
|
|
34
|
Xu R, Sato N, Yanai K, Akiyoshi T, Nagai
S, Wada J, Koga K, Mibu R, Nakamura M and Katano M: Enhancement of
paclitaxel-induced apoptosis by inhibition of mitogen-activated
protein kinase pathway in colon cancer cells. Anticancer Res.
29:261–270. 2009.PubMed/NCBI
|