Introduction
Rectal cancer is one of the most common types of
gastrointestinal malignancy, and refers to tumors that occur
between the dentate line and the rectosigmoid junction (1). The median age of patients with rectal
cancer is ~45 years; however, the incidence in young people has
begun to increase. Rectal cancer is easily diagnosed by a digital
rectal examination and sigmoidoscopy (2–4).
However, since the cancer is located deep within the pelvic cavity,
and due to the key anatomical differences between the rectum and
colon, the management and treatment of rectal cancer is
significantly difficult, particularly in the curative setting
(5). Rectal tumors are not easily
completely removed by surgery alone, thus leading to a high
recurrence rate in patients with rectal cancer (6), which is a common cause of
cancer-associated mortality worldwide. Although great progress has
been made regarding the diagnosis and treatment of rectal cancer,
the prognosis remains poor for patients with distant metastasis of
rectal cancer (7). Clinically,
micrometastases occur in the majority of patients with rectal
cancer prior to undergoing radical operation, and are the direct
cause of metastasis and recurrence post-surgery in patients with
rectal cancer (2,5). Therefore, it is essential to further
explore the mechanisms underlying the development and progression
of rectal cancer and to clarify its biological characteristics, in
order to develop more effective therapeutic targets that may
improve the prognosis of patients with rectal cancer.
High-mobility group protein 1 (HMGB1) is a recently
discovered oncogene, with a molecular weight of ~30 kD (8). HMGB1 is a non-histone chromosomal
protein, which is widely present in the nucleus of eukaryotic
cells. It is named for its fast migration velocity in
polyacrylamide gel electrophoresis (PAGE) (9,10).
HMGB1 is regarded as a proinflammatory factor that is secreted from
the nucleus to the extracellular space. In addition, HMGB1 is
highly conserved between humans and rodents, and the amino acid
sequences are <99% homologous. Notably, the amino acid sequence
of HMGB1 is exactly the same between rats and mice. HMGB1 has two
DNA-binding motifs; A box and B box, which execute two major
functions. The HMGB1 B box exerts cytokine activity, whereas A box
has an inverse role and inhibits the activity of native HMGB1.
HMGB1 has various receptors, including receptor for advanced
glycation end products (RAGE), Toll-like receptors (TLRs), syndecan
and plasminogen, among which, RAGE and TLR4/TLR2 are the main
receptors of HMGB1.
In recent years, high expression levels of HMGB1
have been detected in breast cancer (11), colon cancer (12,13),
lung cancer (14,15), bladder cancer (16), ovarian cancer (17), and other tumors (18). HMGB1 is involved in numerous
processes during tumor formation and development, including
unlimited cell proliferation, angiogenesis, programmed cell death
evasion, and resistance to growth factor inhibitors. Previous
studies have reported that elevated expression of HMGB1 is closely
associated with a high incidence of tumor invasion and metastasis
(19–21). However, the role of HMGB1 in human
rectal cancer remains to be clarified. The present study detected
the expression levels of HMGB1 in rectal cancer specimens and
explored the mechanisms underlying the effects of HMGB1 on
colorectal cancer cells. Understanding the mechanisms underlying
rectal cancer may help to identify novel targets during the
progression and metastasis of rectal cancer.
Materials and methods
Patients
A total of 51 patients with rectal cancer and 51
normal controls were included in the present study. In the rectal
cancer group, 39 patients were male (76.5%) and 12 patients were
female (23.5%). In the control group, 32 patients were male (62.7%)
and 19 patients were female (37.3%). The median age was 60.47
years, with a range of 26–85 years. The present study was approved
by the ethics committee of Qingdao Central Medical Group (Qingdao,
China), and all patients provided written informed consent. The
subjects were well informed of the details of the study and signed
relevant contracts prior to the study. Half of each postoperative
rectal specimen was immediately fixed in 10% neutral buffered
formalin solution and embedded in paraffin. The other half of each
specimen was frozen and maintained at −80°C until further use. The
expression levels of HMGB1 were detected by immunohistochemical
analysis and western blotting. Peritumoral tissue was obtained
during he surgery.
Cell lines and agents
The SW620 and HCT116 colorectal cancer cell lines
were obtained from the American Type Culture Collection (Manassas,
VA, USA), and Colo320 colorectal cells were purchased from the
Chinese Acadamy of Tumor Cell Bank (Shanghai, China). The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) (both Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) at 37°C with 5%
CO2. The human HMGB1 short hairpin (sh)RNA and control
shRNA (cat. no. TR30013) were obtained from Origene Technologies,
Inc. (Rockville, MD, USA). The HMGB1 shRNA consisted of four unique
29mer shRNAs (cat. no. TG316576). Lipofectamine 2000 was used for
transfection of the cells (Invitrogen; Thermo Fisher Scientific,
Inc, Waltham, MA, USA).
Antibodies and small interfering
(si)RNAs
Mouse monoclonal anti-HMGB1 (cat. no. ab77302) was
obtained from Abcam (Cambridge, MA, USA). Rabbit polyclonal
anti-caspase-3 (cat. no. 3138-100) was purchased from BioVision,
Inc. (Milpitas, CA, USA). Rabbit polyclonal anti-B-cell lymphoma 2
(Bcl-2; cat. no. AP1303a-ev), mouse polyclonal
anti-Bcl-2-associated X protein (Bax; cat. no. AP1302a-ev) and
mouse monoclonal anti-β-actin (cat. no. P60709) were obtained from
Abgent Biotech Co., Ltd. (Suzhou, China), and anti-cleaved-PARP
(cat. no. ab4830; Abcam, Cambridge, MA, USA). All antibodies were
used at a dilution of 1:1,000. The siRNAs were designed and
synthesized by GenePharma Corp. (Shanghai, China). The
HMGB1-specific siRNA sequences were as follows: HMGB1 siRNA1, sense
5′-GGAGAUCCUAAGAAGCCGATT-3′, antisense 5′-UCGGCUUCUUAGGAUCUCCTT-3′;
HMGB1 siRNA2, sense 5-CUGCGAAGCUGAAGGAAAATT-3′, antisense
5′-UUUUCCUUCAGCUUCGCAGTT-3′; and HMGB1 siRNA3, sense
5′-GGGAGGAGCAUAAGAAGATT-3′, antisense 5′-UUCUUCUUAUGCUCCUCCCTT-3′.
Negative control (N.C.) siRNA was purchased from GenePharma Corp.
(cat. no. A01004), which was purified by HPLC and the unpaired
single chains were truly removed.
Transfection
The SW620 cells (1×106 cells/well) were
plated into a 48-well plate. After 8 h, 200 ng of three pairs of
HMGB1-specific siRNA and 2 µl Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The mixture was added into the medium of human colorectal cancer
cells and cultured for 48 h.
MTT assay
A
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay was used to determine the proliferation of SW620 and
Colo320 cells. The SW620 and Colo320 colorectal cancer cells were
plated into 48-well plates at a density of 2×104
cells/well. After 6 h, the cells were transfected with HMGB1 shRNA
or N.C. shRNA using Lipofectamine 2000, and the cells were cultured
for 24, 48, 72 and 96 h. Subsequently, 10 µl 5 mg/ml MTT
(Sigma-Aldrich, St. Louis, MO, USA) was added to the cells for 4 h
at 37°C. The medium was gently aspirated and 100 µl dimethyl
sulfoxide was added and the cells were incubated for 15 min.
Absorbance was measured at a wavelength of 490 nm using a
microplate reader. The cells transfected with N.C. shRNA were used
as negative controls and the experiment was repeated three
times.
Immunohistochemical analysis
Immunohistochemical staining was performed to detect
the expression and distribution of HMGB1 in rectal cancer tissues.
Briefly, the sections were dewaxed in xylene for 5–10 min and were
exposed to a graded alcohol solution at concentrations of 100, 95,
90, 80 and 70%, respectively, for 3–5 min for rehydration.
Following this, the sections were placed in distilled water for 3
min. Finally, the sections were sealed with neutral gum and
incubated overnight with a primary anti-HMGB1 antibody at room
temperature. The specimens were then washed three times with
phosphate-buffered saline for 5 min, and were incubated with goat
anti-mouse secondary antibody for 1 h at room temperature. The
stained slides were visualized and images were captured using a
Nikon Eclipse Ti microscope (Nikon Corporation, Tokyo, Japan). The
positivity of immunostaining was identified in eight different
fields at 400× magnification.
Western blotting
For total protein, the samples and cells were washed
in phosphate-buffered saline and were lysed in
radioimmunoprecipitation assay buffer [50 mmol/l of Tris, 1% NP-40,
150 mmol/l of NaCl, 1 mmol of EDTA, 1% sodium dodecyl sulfate (SDS)
and 0.25% SDC]. When assessing cytoplasmic or nuclear proteins, a
nuclear protein extraction kit (Beyotime Institute of
Biotechnology, Beijing, China), according to the manufacturer's
protocol. The concentration of whole proteins in the lysates was
determined using the Bradford assay (Beyotime Institute of
Biotechnology). Protein samples (10 µg) were separated by
10% SDS-PAGE and were then transferred electrophoretically onto a
nitrocellulose membrane. Subsequently, the membrane was blocked
with 5% bovine serum albumin (Beyotime Institute of Biotechnology)
in Tris-buffered saline containing Tween (50 mmol/l Tris-HCl, 150
mmol/l NaCl and 0.1% Tween). The membranes were incubated with
primary antibodies overnight at 4°C. The membranes were then
incubated with horseradish peroxidase-conjugated goat anti-mouse
(cat. no. sc-2031; 1:1,000) or goat anti-rabbit immunoglobulin G
(cat. no. sc-2004; 1:1,000; each from Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) secondary antibodies for 1 h at room
temperature. The resulting bands were detected using an enhanced
chemiluminescence western blotting detection system (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
β-actin was used as an internal reference. The gray value of the
protein band was analyzed using ImageJ version 1.38 (NIH, Bethesda,
MD, USA).
Statistical analysis
Statistical analyses were performed using SPSS 20.0
statistical software (IBM SPSS, Armonk, NY, USA). Clinical
indicators were compared using χ2 test. The data are
presented as the mean ± standard deviation. P<0.01 was
considered to indicate a statistically significant difference.
Results
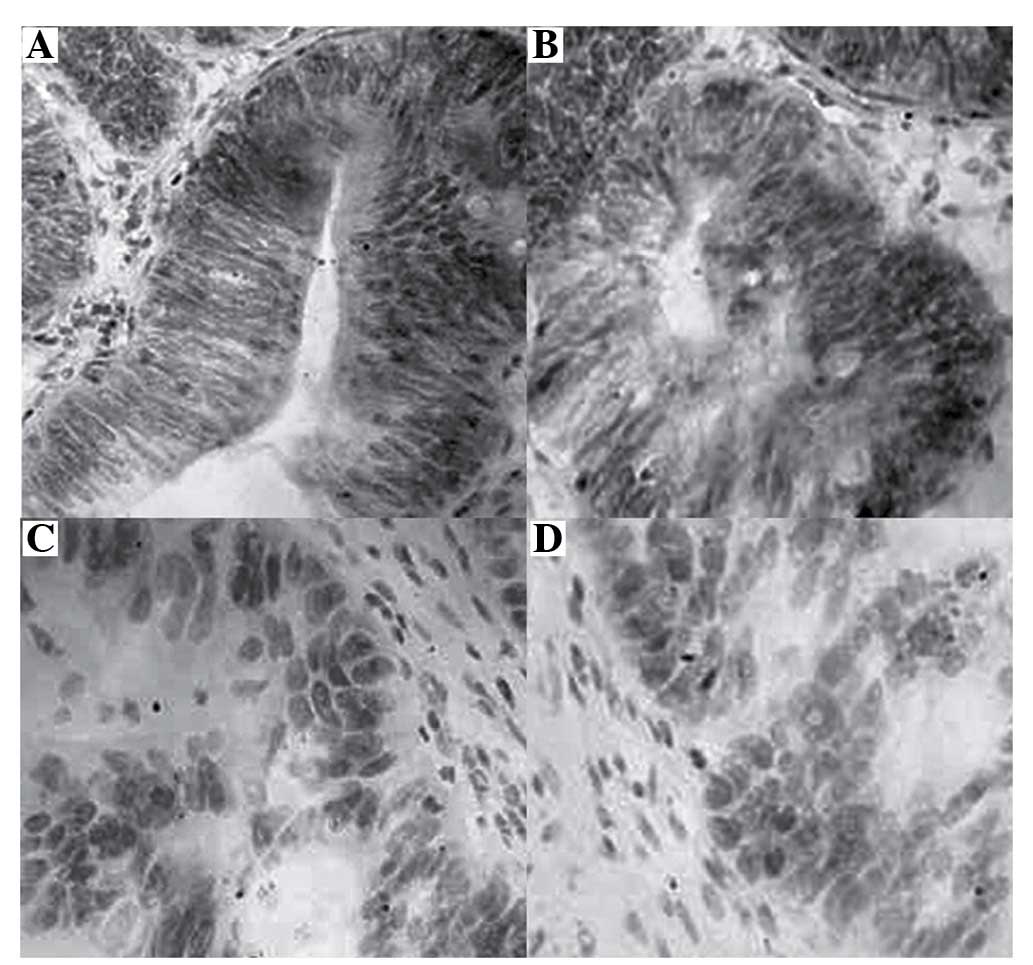
HMGB1 is highly expressed in samples from
patients with rectal cancer, as detected by immunohistochemical
analysis
A total of 51 patients and 51 normal controls were
studied in the present study; the median age was 60.47 years, with
a range of 26–85 years. HMGB1 expression was detected by
immunohistochemical analysis, and images of the specimens were
captured (original magnification, ×400; Fig. 1). HMGB1 protein was predominantly
located in the cytoplasm and nucleus of rectal tumor cells, with
control tissues exhibiting no positive staining for HMGB1. Positive
HMGB1 expression appeared as brown cytoplasmic and nuclear
staining. The whole panel of rectal cancer and normal tissues was
tested. As presented in Table I,
the positive rate of HMGB1 expression was 96.08% (49/51) in rectal
cancer tissues, which was significantly higher than in normal
tissues (3.92%; 2/51).
 | Table IHMGB1 expression in rectal cancer and
normal tissues. |
Table I
HMGB1 expression in rectal cancer and
normal tissues.
| Group | Normal tissues | Rectal cancer
tissues |
|---|
| HMGB1 | 2 (3.92%) | 49 (96.08%) |
| P-value | <0.001 | |
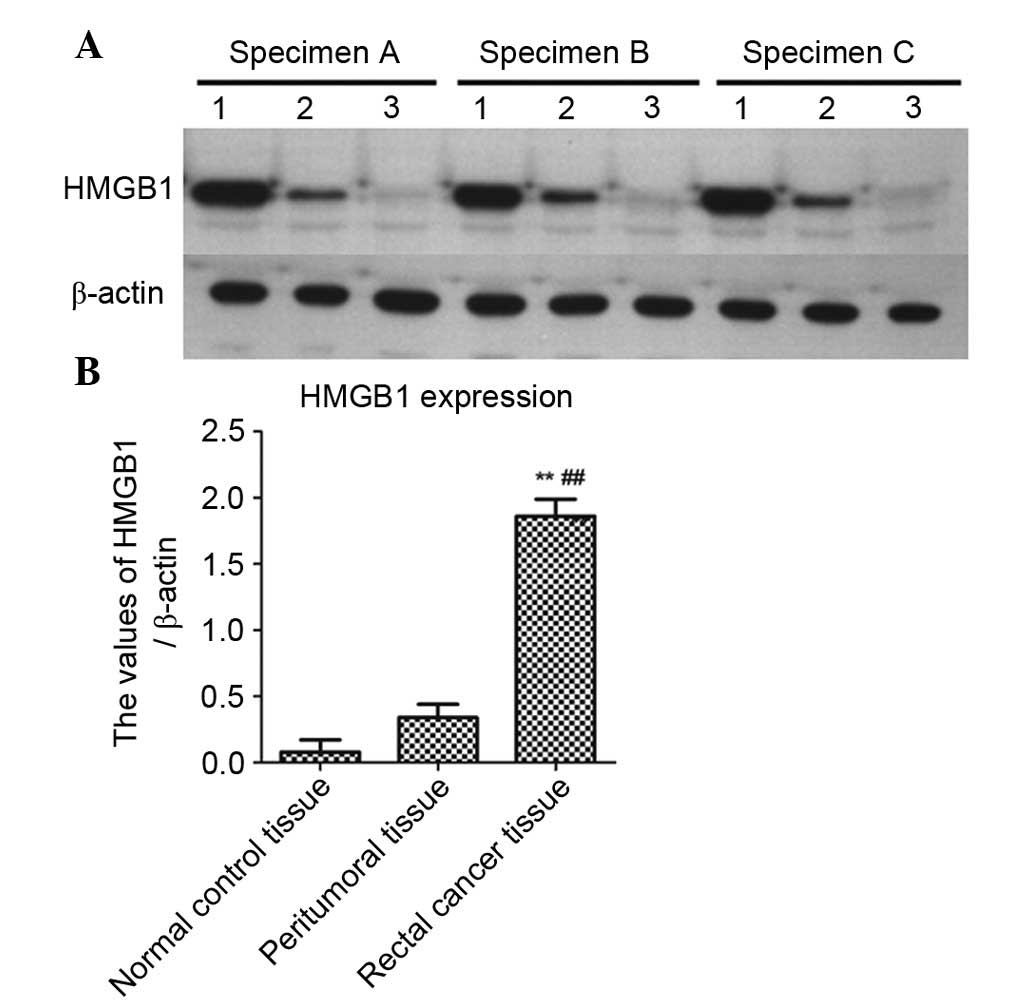
Western blot analysis of HMGB1 expression
in rectal cancer tissues
In order to further detect the expression levels of
HMGB1 in rectal cancer, rectal cancer tissues, peritumoral tissues
and normal control tissues (three specimens each) underwent western
blotting. As shown in Fig. 2A, the
expression levels of HMGB1 were markedly increased in rectal cancer
tissues compared with in peritumoral or normal control tissues,
which was consistent with the immunohistochemical results. β-actin
was used as an internal reference. As shown in Fig. 2B, the data were normalized to
β-actin and expressed as mean ± standard deviation. The expression
levels of HMGB1 were significantly higher in rectal cancer tissues
compared with in peritumoral tissues (P<0.01) or normal control
tissues (P<0.01).
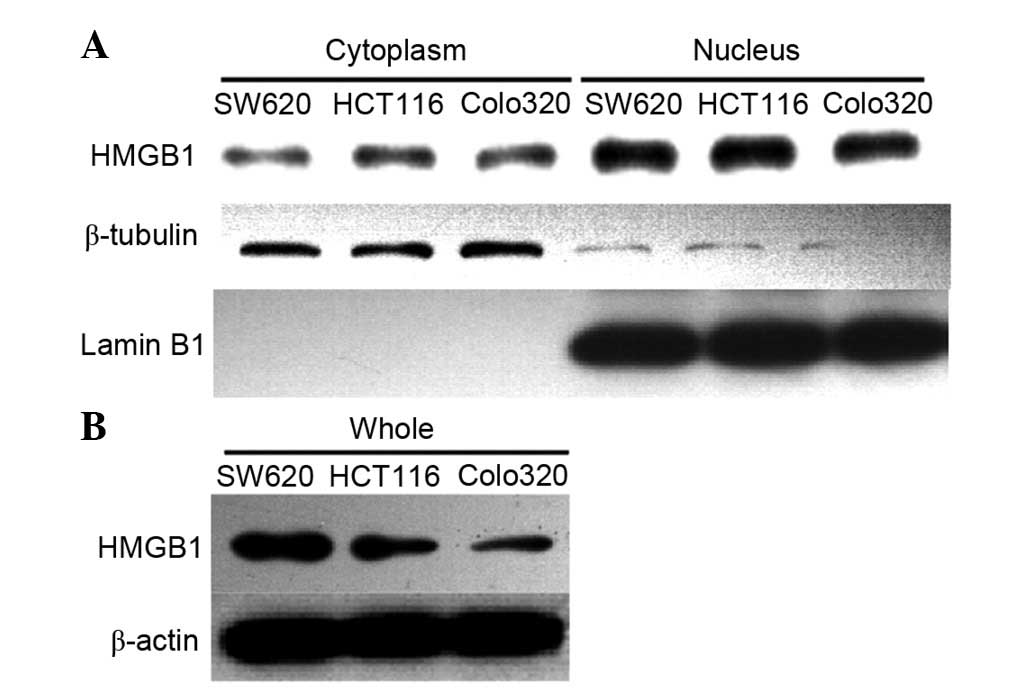
HMGB1 is located in the cytoplasm and
nucleus of colorectal cancer cells
Three colorectal cancer cell lines were selected as
a cell model. Western blotting was preformed to detect the
expression of HMGB1 in Colo320, SW620 and HCT116 cells (Fig. 3). In order to detect the location
of HMGB1, cytoplasmic and nuclear proteins were extracted,
according to the kit protocol. β-tubulin was used as a cytoplasmic
marker and Lamin B1 was used as a nuclear marker. The results
demonstrated that HMGB1 was mainly located in the nucleus, but was
also partly distributed in the cytoplasm of colorectal cancer
cells. These findings are consistent with the results of the
immunohistochemical analysis.
HMGB1 expression is successf ully
silenced by HMGB1-specific shRNA
In order to further confirm the role of HMGB1 in
cancer cells, RNA interference technology was used to knockdown the
expression of HMGB1 in SW620 cells. Three siRNA pairs specific to
HMGB1 were generated to interfere with endogenous HMGB1 expression
in SW620 cells; however, the knockdown efficiency was not
significantly altered compared with that of the N.C. siRNA
(Fig. 4A). Therefore,
HMGB1-specific shRNA was used to knockdown the endogenous
expression of HMGB1 in SW620 cells. As shown in Fig. 4B, transfection with HMGB1-shRNA for
48 h effectively downregulated the endogenous expression of HMGB1,
as determined by western blotting.
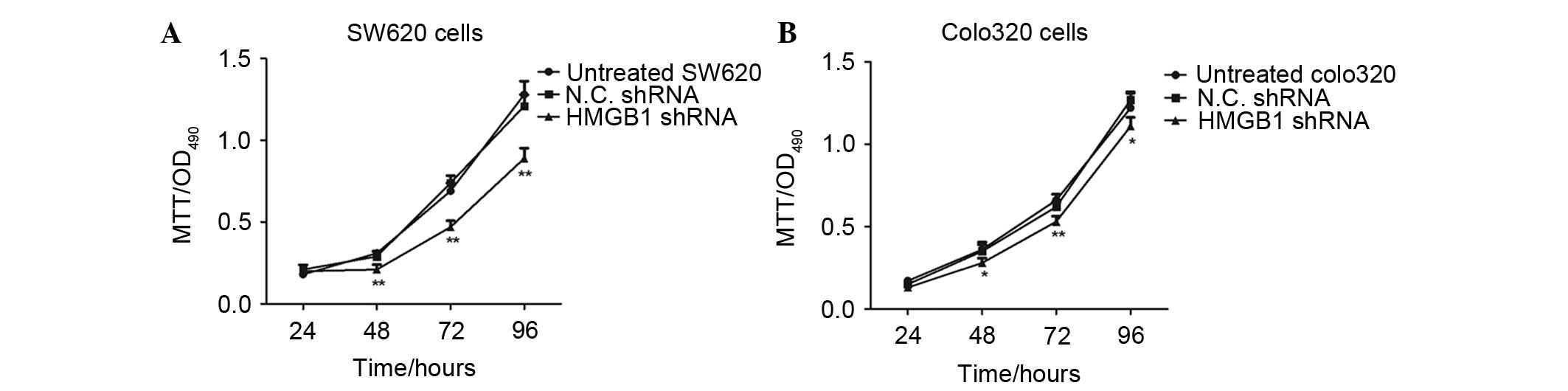
Knockdown of endogenous HMGB1 expression
inhibits the proliferation of colorectal cancer cells
The present study aimed to determine whether
knockdown of endogenous HMGB1 expression would affect the
proliferation of colorectal cancer cells. Therefore, two cell lines
were used as a cell model and an MTT assay was conducted to detect
the proliferation of cancer cells transfected with HMGB1 shRNA. As
shown in Fig. 5, the HMGB1
shRNA-transfected SW620 cells had much lower proliferative ability
compared with the untreated or N.C. shRNA-transfected cancer cells.
In addition, the survival rate of HMGB1 shRNA-transfected Colo320
cells was determined by MTT assay. Knockdown of endogenous HMGB1
expression inhibited the proliferation of Colo320 cells, compared
with those transfected with N.C. shRNA; however, the results
demonstrated that SW620 cells were more appropriate for
transfection with shRNA.
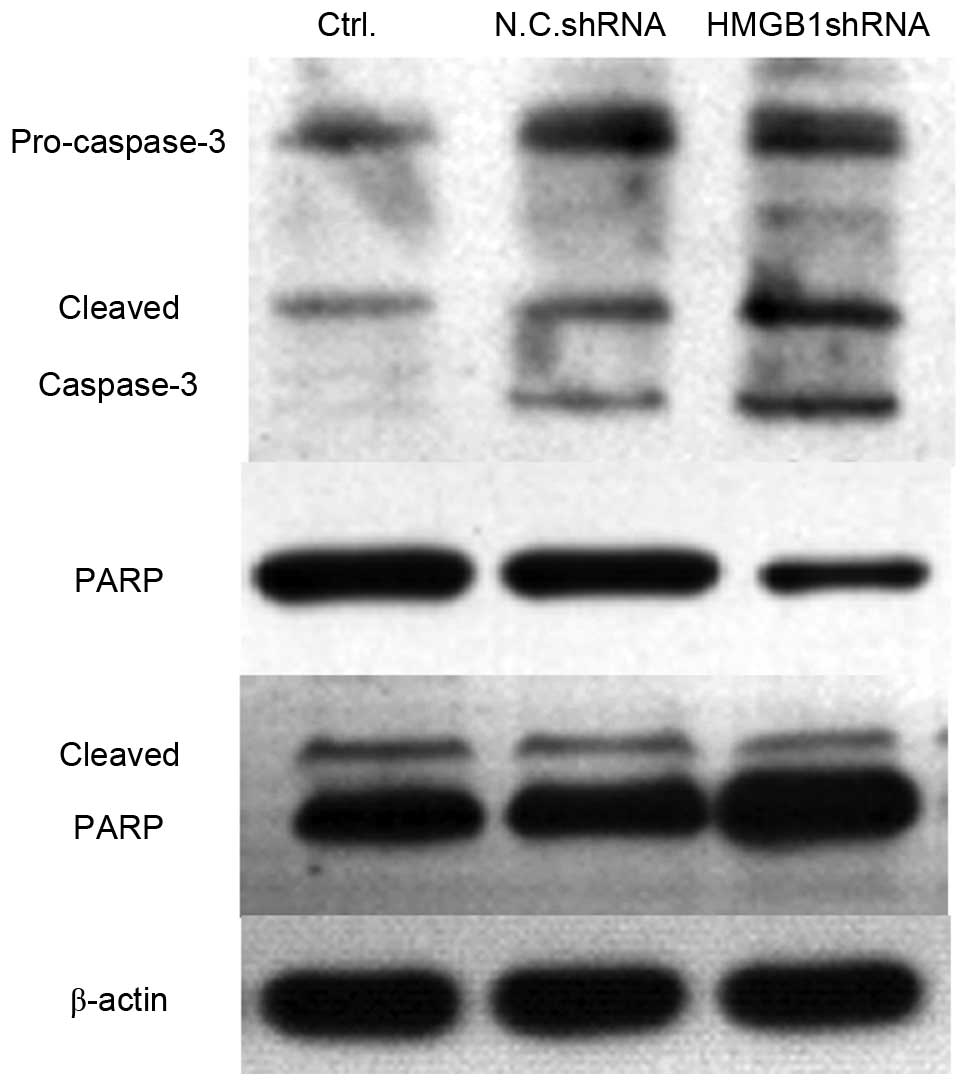
Knockdown of endogenous HMGB1 expression
activates caspase-3 and its substrate poly (ADP-ribose) polymerase
(PARP)
The present study indicated that knockdown of HMGB1
expression significantly inhibited the proliferation of SW620 and
Colo320 cells. Subsequently, the present study aimed to determine
whether cell apoptosis was induced in HMGB1 shRNA-transfected
cells. Caspase-3 is the executor of cell apoptosis, and the
activation of caspase-3 and its substrate PARP were detected using
western blotting. As shown in Fig.
6, the expression levels of cleaved capspase-3 were markedly
enhanced in HMGB1 shRNA-transfected SW620 cells, compared with in
the untreated or N.C. shRNA-transfected cells. Furthermore, the
expression levels of PARP were decreased, whereas the levels of
cleaved PARP were upregu-lated in HMGB1 shRNA-transfected SW620
cells. These data suggest that transfection with HMGB1 shRNA may
significantly increase cell apoptosis in colorectal cancer
cells.
Knockdown of HMGB1 contributes to the
activation of cell apoptosis-associated proteins
The present study also detected the expression
levels of the cell apoptosis-associated proteins Bcl-2 and Bax. Bax
is an apoptosis inducer, whereas Bcl-2 is an apoptosis inhibitor.
The Bax/Bcl-2 ratio reflects the situation of cell apoptosis. In
the present study, the expression levels of Bax and Bcl-2 were
detected by western blotting, and the results demonstrated that
knockdown of HMGB1 resulted in downregulation of Bcl-2 and
upregulation of Bax; therefore, the Bax/Bcl-2 ratio was increased
in the HMGB1 shRNA-transfected colorectal cancer cells (Fig. 7). These results indicate that
knockdown of endogenous HMGB1 expression may activate the intrinsic
mitochondrial apoptotic pathway.
Discussion
HMGB1 is a recently discovered oncogene that is
associated with tumor progression and metastasis. It has previously
been reported that HMGB1, through interactions with RAGE and TLRs,
induces inflammation and promotes cancer progression (22). In addition, it has been
demonstrated that HMGB1 may induce autophagy or apoptosis in cancer
cells, depending on its redox status (23). Flohr et al (10) analyzed the expression patterns of
HMGB1 in 13 breast cancer samples, and northern blot analyses of
the 1.4 kb and 2.4 kb transcripts of HMGB1 revealed a strong
intertumoral variation by a factor of 8.5 and 14.5, respectively,
indicating that HMGB1 may be a marker of considerable clinical
interest. The role of HMGB1 has been discussed in several types of
cancer, including breast cancer (24), colorectal cancer (9,25),
gastric cancer (26–28), non-small cell lung cancer (29), pancreatic cancer (30), head and neck cancer (31), and gallbladder cancer (32). However, the role of HMGB1 in rectal
cancer remains to be elucidated. The present study collected rectal
cancer specimens, and used colorectal cancer cell lines as cell
models to explore the role of HMGB1 in the progression of rectal
cancer. The aim of the present study was to identify novel targets
in rectal cancer therapy.
In the present study, immunohistochemistry and
western blotting were used to analyze the expression levels of
HMGB1 in pathological specimens from patients with clinically
identified rectal cancer. In addition, the distribution of HMGB1 in
tumor cells was detected by western blot analysis. The results
demonstrated that HMGB1 was highly expressed in specimens from
patients with rectal cancer. Notably, HMGB1 was distributed and
located not only in the nucleus, but also in the cytoplasm of
colorectal cancer cells. These results indicated that HMGB1 may
have an important role in the progression and development of rectal
cancer. Consistent with these findings, Song et al (18) demonstrated that HMGB1 was
overexpressed in ~85% of gastric cancers, and downregulation of
HMGB1 increased the proportion of cells in
G0/G1 phase and sensitized gastric cancer
cells to apoptosis via the caspase-3 pathway.
Livesey et al (33) hypothesized that direct molecular
interactions between HMGB1 and TP53 in colorectal cancer regulated
the balance between apoptosis and autophagy through regulation of
the cytosolic localization of the reciprocal binding partner.
Increased cytosolic HMGB1 was shown to enhance autophagy, whereas
increased cytosolic TP53 enhanced apoptosis in colon cancer cells.
In addition, Zhang et al (21) reported that knockdown of HMGB1
inhibited growth and invasion of gastric cancer cells via the
nuclear factor-κB pathway in vitro and in vivo.
In conclusion, the present study demonstrated that
knockdown of endogenous HMGB1 expression in SW620 and Colo320
colorectal cancer cells was able to significantly inhibit cell
proliferation. In addition, apoptosis was induced in HMGB1
shRNA-transfected colorectal cancer cells. However, the mechanism
underlying the effects of HMGB1 on the regulation of cell apoptosis
requires further study, and the downstream targets or receptors of
HMGB1 remain to be clearly explored.
References
|
1
|
Yaffee P, Osipov A, Tan C, Tuli R and
Hendifar A: Review of systemic therapies for locally advanced and
metastatic rectal cancer. J Gastrointest Oncol. 6:185–200.
2015.PubMed/NCBI
|
|
2
|
Harji DP, Griffiths B, Velikova G, Sagar
PM and Brown J: Systematic review of health-related quality of life
issues in locally recurrent rectal cancer. J Surg Oncol.
111:431–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poulsen LØ, Qvortrup C, Pfeiffer P, Yilmaz
M, Falkmer U and Sorbye H: Review on adjuvant chemotherapy for
rectal cancer - why do treatment guidelines differ so much? Acta
Oncol. 54:437–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arezzo A, Passera R, Salvai A, Arolfo S,
Allaix ME, Schwarzer G and Morino M: Laparoscopy for rectal cancer
is oncologically adequate: A systematic review and meta-analysis of
the literature. Surg Endosc. 29:334–348. 2015. View Article : Google Scholar
|
|
5
|
Nussbaum N and Altomare I: The neoadjuvant
treatment of rectal cancer: A review. Curr Oncol Rep. 17:4342015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghouti L, Pereira P, Filleron T, Humeau M,
Guimbaud R, Selves J and Carrere N: Pelvic exenterations for
specific extraluminal recurrences in the era of total mesorectal
excision: Is there still a chance for cure? A single-center review
of patients with extraluminal pelvic recurrence for rectal cancer
from March 2004 to November 2010. Am J Surg. 209:352–362. 2015.
View Article : Google Scholar
|
|
7
|
Guren MG, Undseth C, Rekstad BL,
Brændengen M, Dueland S, Spindler KL, Glynne-Jones R and Tveit KM:
Reirradiation of locally recurrent rectal cancer: A systematic
review. Radiother Oncol. 113:151–157. 2014. View Article : Google Scholar
|
|
8
|
Lotze MT and DeMarco RA: Dealing with
death: HMGB1 as a novel target for cancer therapy. Curr Opin
Investig Drugs. 4:1405–1409. 2003.
|
|
9
|
Süren D, Yildirim M, Demirpençe Ö, Kaya V,
Alikanoğlu AS, Bülbüller N, Yıldız M and Sezer C: The role of high
mobility group box 1 (HMGB1) in colorectal cancer. Med Sci Monit.
20:530–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Flohr AM, Rogalla P, Meiboom M, Borrmann
L, Krohn M, Thode-Halle B and Bullerdiek J: Variation of HMGB1
expression in breast cancer. Anticancer Res. 21:3881–3885.
2001.
|
|
11
|
Jiao Y, Wang HC and Fan SJ: Growth
suppression and radiosensitivity increase by HMGB1 in breast
cancer. Acta Pharmacol Sin. 28:1957–1967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohmori H, Luo Y and Kuniyasu H:
Non-histone nuclear factor HMGB1 as a therapeutic target in
colorectal cancer. Expert Opin Ther Targets. 15:183–193. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moriwaka Y, Luo Y, Ohmori H, Fujii K,
Tatsumoto N, Sasahira T and Kuniyasu H: HMGB1 attenuates
anti-metastatic defense of the lymph nodes in colorectal cancer.
Pathobiology. 77:17–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang C, Fei G, Liu Z, Li Q, Xu Z and Ren
T: HMGB1 was a pivotal synergistic effecor for CpG oligonucleotide
to enhance the progression of human lung cancer cells. Cancer Biol
Ther. 13:727–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Zhang P, Wu Z, Chen J and Zhou Q:
Screening of highly-expressed-HMGB1-gene human lung cancer cell
lines. Zhongguo Fei Ai Za Zhi. 12:965–968. 2009.In Chinese.
|
|
16
|
Yang GL, Zhang LH, Bo JJ, Huo XJ, Chen HG,
Cao M, Liu DM and Huang YR: Increased expression of HMGB1 is
associated with poor prognosis in human bladder cancer. J Surg
Oncol. 106:57–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen J, Liu X, Zhang J and Zhao Y:
Targeting HMGB1 inhibits ovarian cancer growth and metastasis by
lentivirus-mediated RNA interference. J Cell Physiol.
227:3629–3638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song B, Song WG, Li ZJ, Xu ZF, Wang XW,
Wang CX and Liu J: Effect of HMGB1 silencing on cell proliferation,
invasion and apoptosis of MGC-803 gastric cancer cells. Cell
Biochem Funct. 30:11–17. 2012. View
Article : Google Scholar
|
|
19
|
Zhang J, Liu C and Hou R: Knockdown of
HMGB1 improves apoptosis and suppresses proliferation and invasion
of glioma cells. Chin J Cancer Res. 26:658–668. 2014.
|
|
20
|
Gong W, Wang ZY, Chen GX, Liu YQ, Gu XY
and Liu WW: Invasion potential of H22 hepatocarcinoma cells is
increased by HMGB1-induced tumor NF-κB signaling via initiation of
HSP70. Oncol Rep. 30:1249–1256. 2013.PubMed/NCBI
|
|
21
|
Zhang J, Kou YB, Zhu JS, Chen WX and Li S:
Knockdown of HMGB1 inhibits growth and invasion of gastric cancer
cells through the NF-κB pathway in vitro and in vivo. Int J Oncol.
44:1268–1276. 2014.PubMed/NCBI
|
|
22
|
Sims GP, Rowe DC, Rietdijk ST, Herbst R
and Coyle AJ: HMGB1 and RAGE in inflammation and cancer. Annu Rev
Immunol. 28:367–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang D, Loze MT, Zeh HJ and Kang R: The
redox protein HMGB1 regulates cell death and survival in cancer
treatment. Autophagy. 6:1181–1183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang BP, Wang DS, Xing JW, Yang SH, Chu Q
and Yu SY: MiR-200c inhibits metastasis of breast cancer cells by
targeting HMGB1. J Huazhong Univ Sci Technolog Med Sci. 34:201–206.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu W, Zhang Z, Zhang Y, Chen X, Guo S,
Lei Y, Xu Y, Ji C, Bi Z and Wang K: HMGB1-mediated autophagy
modulates sensitivity of colorectal cancer cells to oxaliplatin via
MEK/ERK signaling pathway. Cancer Biol Ther. 16:511–517. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Zhang R, Lu WW, Zhu JS, Xia LQ,
Lu YM and Chen NW: Clinical significance of hmgb1 expression in
human gastric cancer. Int J Immunopathol Pharmacol. 27:543–551.
2014.
|
|
27
|
Li ZJ, Song B, Liu J, Han JJ, Wang CX, Zhu
YX and Xu ZF: Inhibitory effect of silencing of HMGB1 gene
expression on the invasive and metastatic abilities of MGC-803
gastric cancer cells. Zhonghua Zhong Liu Za Zhi. 35:244–248.
2013.In Chinese. PubMed/NCBI
|
|
28
|
Zhang J, Zhu JS, Zhou Z, Chen WX and Chen
NW: Inhibitory effects of ethyl pyruvate administration on human
gastric cancer growth via regulation of the HMGB1-RAGE and Akt
pathways in vitro and in vivo. Oncol Rep. 27:1511–1519.
2012.PubMed/NCBI
|
|
29
|
Zhang X, Wang H and Wang J: Expression of
HMGB1 and NF-κB p65 and its significance in non-small cell lung
cancer. Contemp Oncol (Pozn). 17:350–355. 2013.
|
|
30
|
Wittwer C, Boeck S, Heinemann V, Haas M,
Stieber P, Nagel D and Holdenrieder S: Circulating nucleosomes and
immunogenic cell death markers HMGB1, sRAGE and DNAse in patients
with advanced pancreatic cancer undergoing chemotherapy. Int J
Cancer. 133:2619–2630. 2013.PubMed/NCBI
|
|
31
|
Wild CA, Brandau S, Lotfi R, Mattheis S,
Gu X, Lang S and Bergmann C: HMGB1 is overexpressed in tumor cells
and promotes activity of regulatory T cells in patients with head
and neck cancer. Oral Oncol. 48:409–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li ML, Wang XF, Tan ZJ, Dong P, Gu J, Lu
JH, Wu XS, Zhang L, Ding QC, Wu WG, et al: Ethyl pyruvate
administration suppresses growth and invasion of gallbladder cancer
cells via downregulation of HMGB1-RAGE axis. Int J Immunopathol
Pharmacol. 25:955–965. 2012.
|
|
33
|
Livesey KM, Kang R, Zeh HJ III, Lotze MT
and Tang D: Direct molecular interactions between HMGB1 and TP53 in
colorectal cancer. Autophagy. 8:846–848. 2012. View Article : Google Scholar : PubMed/NCBI
|