Introduction
Lung cancer leads to the greatest number of
cancer-associated mortalities in China and worldwide (1–3). In
2010, nearly 600,000 newly diagnosed cases of lung cancer were
reported in China. The crude incidence rate of lung cancer was
estimated to be 46 per ten thousand individuals with a
gender-specific incidence of 62 and 30 per ten thousand individuals
for men and women, respectively (4). In China, 480,000 mortalities with a
crude mortality rate of 37 per ten thousand individuals were
estimated, which is higher by ten individuals per ten thousand
compared to world-wide statistics (5).
The etiology of lung cancer has been linked to
factors including tobacco smoking (6), genetic factors (7), diet and obesity (8,9) as
well as certain environmental factors such as air pollution
(10–14). Lung cancers are broadly classified
as small cell lung cancer and non-small cell lung cancer (NSCLC);
the latter constitutes 85% of total cases (15) and 40% of NSCLCs are adenocarcinoma
(16). Targeting of epidermal
growth factor receptor (EGFR) is currently considered to be the
most suitable way to treat NSCLC adenocarcinoma (17). Erlotinib (18), Afatinib (19) and Gefitinib (20) are the most common drugs in use that
target EGFR.
In China, the wisdom of Traditional Chinese Medicine
has been recently sourced for use in modern medicine (21), and has been applied in numerous
cancer centers in china (22).
In silico approaches are a tool for drug discovery (23), which may be utilized for lead
identification from large pools of drugs for further in
vitro analysis. Molecular dynamics simulation is a tool that
can be used to assess the stability of drug-target complexes
(24). The present study used a
computer-aided drug design (CADD) approach to identify compounds
from an in-house pool of Chinese medicinal compounds for targeting
EGFR for NSCLC treatment. The initial dataset was reduced to three
compounds and then a final lead compound using CADD.
Materials and methods
Data set preparation
The crystallographic structure of the kinase domain
of EGFR protein was retrieved from the Protein Databank repository
(www.rcsb.org/pdb; ID: 2ITY) (25), representing the crystal structure
of EGFR kinase domain with gefitinib (Iressa). After removing the
drug Iressa from the crystal structure, the Gromos force field was
used for energy minimization using Swiss-PDB viewer (http://spdbv.vital-it.ch/).
Virtual screening
The AutoDock Vina (26) platform using the Pymol interface
(27) was used for the study. A
total of 2,242 Traditional Chinese Medicinal compounds of plant
origin were docked into the active site of the kinase domain of the
EGFR protein. A uniform grid of 60 Å in each plane was created for
the run. The top compounds based on the cutoff of Gibbs free energy
(ΔG) of −9 kcal/mol were selected for further analysis.
Molecular docking
The Lamarckian algorithm of the AutoDock 4.2
(28) tool was used for automated
docking of the top compounds with the adenosine triphosphate (ATP)
binding pocket of EGFR. Based on the binding energy in kcal/mol,
the screening was limited to top three compounds. The sub-set of
the top three compounds was limited to one based on binding energy
and the Lipinski rule of five (29).
Molecular dynamics simulation
The lead compound identified was subjected to the
computational chemistry engine within GROMACS 4.6.5 (http://www.gromacs.org/) for understanding the
stability and nature of the ligand protein interaction over time.
The protocol used by Chikan et al (23) was used for this study.
Cell growth inhibition assay
H2347 cells (Hybridoma Lab., Ningbo No. 2 Hospital,
Ningbo, China), which are known to carry the wild-type EGFR gene
with a relative copy number of 4.18, were selected for the study.
The cells were grown in Gibco RPMI-1640 medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with Gibco 10%
fetal bovine serum (Thermo Fisher Scientific, Inc.) and were seeded
into a 96-well plate. Following attachment for 24 h, the cells were
incubated with erlotinib, afatinib, gefitinib, as well as the lead
compound triptolide (all purchased from Sigma-Aldrich, St. Louis,
MO, USA) at concentrations of 0.001, 0.005, 0.01, 0.05, 0.1, 0.5,
1, 5 and 10 μM for a total of 48 h. The Cell Titer-Blue cell
viability kit (Promega Corp., Madison, WI, USA) was used for a
growth inhibition assay, which was performed as five independent
experiments. Growth inhibition was calculated according to the
CellTiter-Blue-specific fluorescence at 530 nm (excitation)/590 nm
(emission) using a Fluoroskan Ascent FL plate reader (Thermo Fisher
Scientific, Inc.).
Results and Discussion
Protein preparation and virtual
screening
The crystal structure of the kinase domain of the
EGFR protein, ranging from amino acids 652–974, was observed using
the SPDB viewer to identify missing amino acids and perform energy
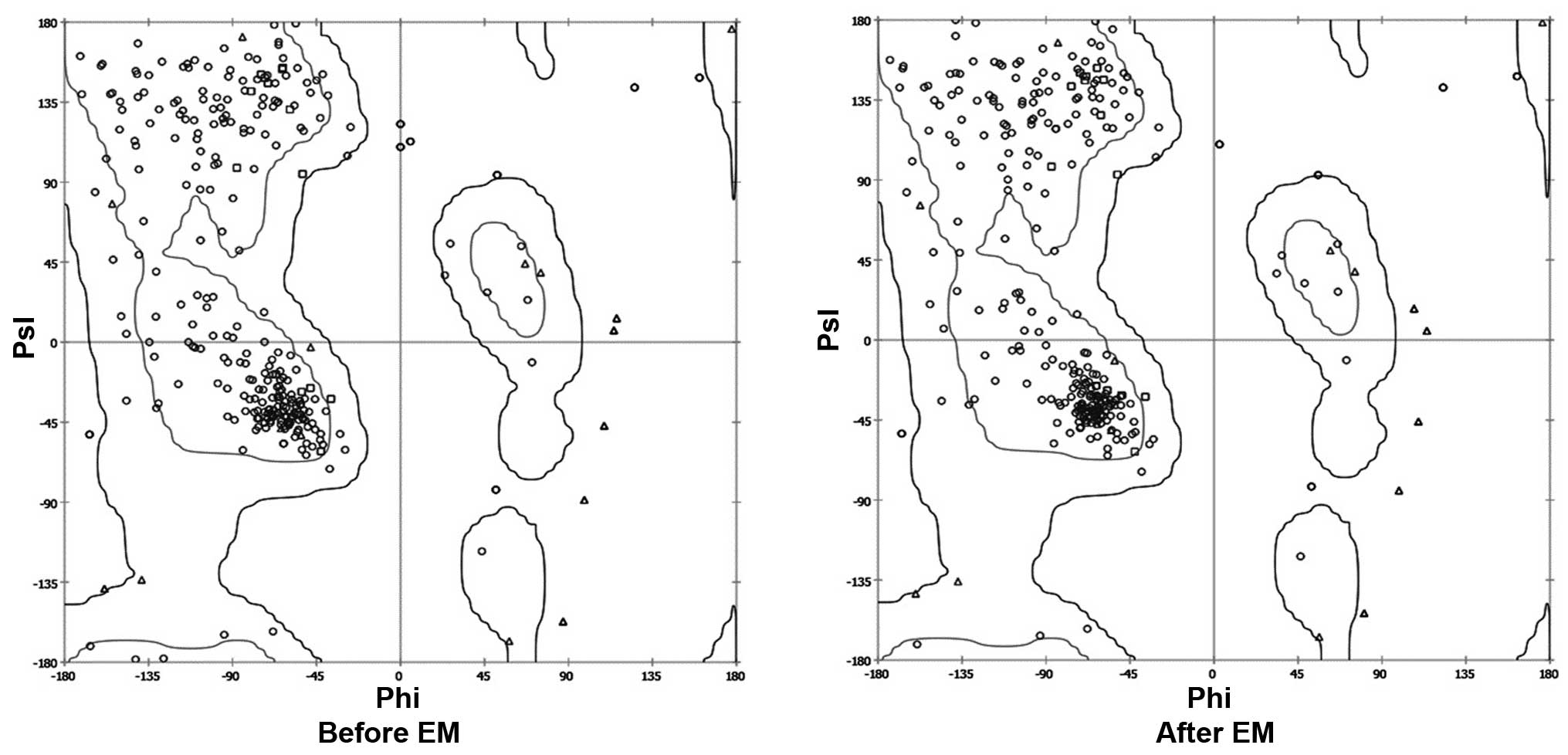
minimization. The Ramachandran plot before and after energy
minimization shows the improved acceptability of the overall
structure (Fig. 1), where points
represent residues inside and outside the acceptable region, and
the contours represent the hardsphere and overlap. The minimized
structure of the domain was then processed using the VINA plugin of
Pymol, where the grid around the ATP binding site was created. The
grid box was subjected to screening of the Traditional Chinese
Medicinal compound database (Ningbo No. 2 Hospital), which was
useful for narrowing down the number of compounds based on their
binding energy. The cutoff value of the ΔG used for initial
screening was −9 kcal/mol.
Absorption, distribution, metabolism and
excretion (ADME)/Tox prediction and molecular docking
The identified lead compounds were further screened
using the Lamarkian algorithm-based AutoDock 4.2 suit. The flexible
docking approach of 50 GA runs of maximum evaluations was performed
for the top 67 compounds. The 67 compounds were screened from the
second dataset of 337 compounds using ADME/Tox analysis, with
cutoff based on the parameters set by Lipinski. Out of these 67
compounds, three candidates showed physical interaction with the
ATP binding pocket of the kinase domain of EGFR (Fig. 2). In Table I, the top three compounds were
ranked on the basis of their binding free energy. The top ranked
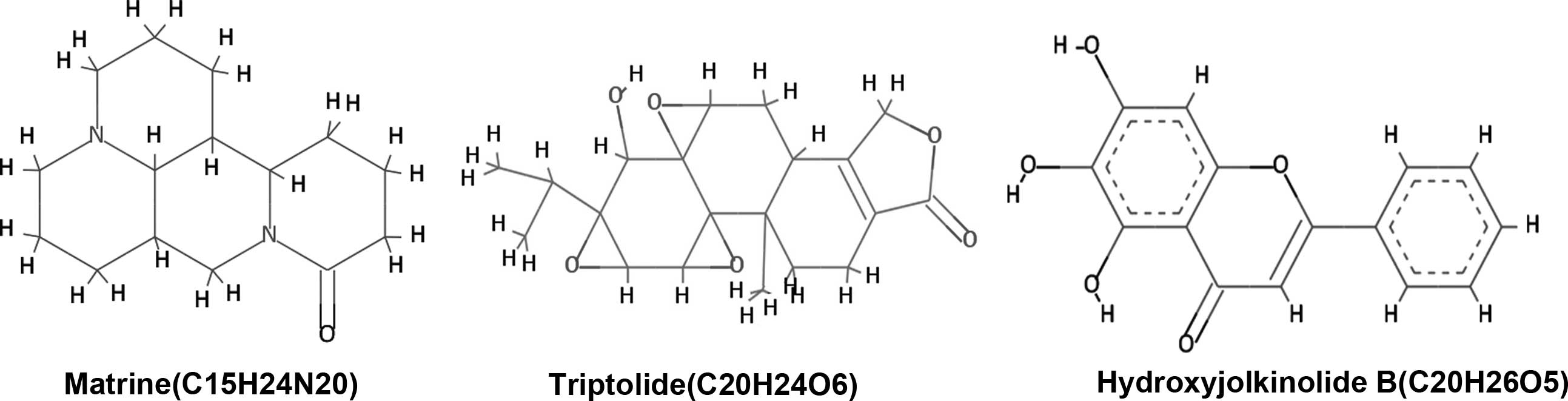
compound based on docking score was matrine, an alkaloid present in
Sophora flarescens. The compound showed a binding energy of
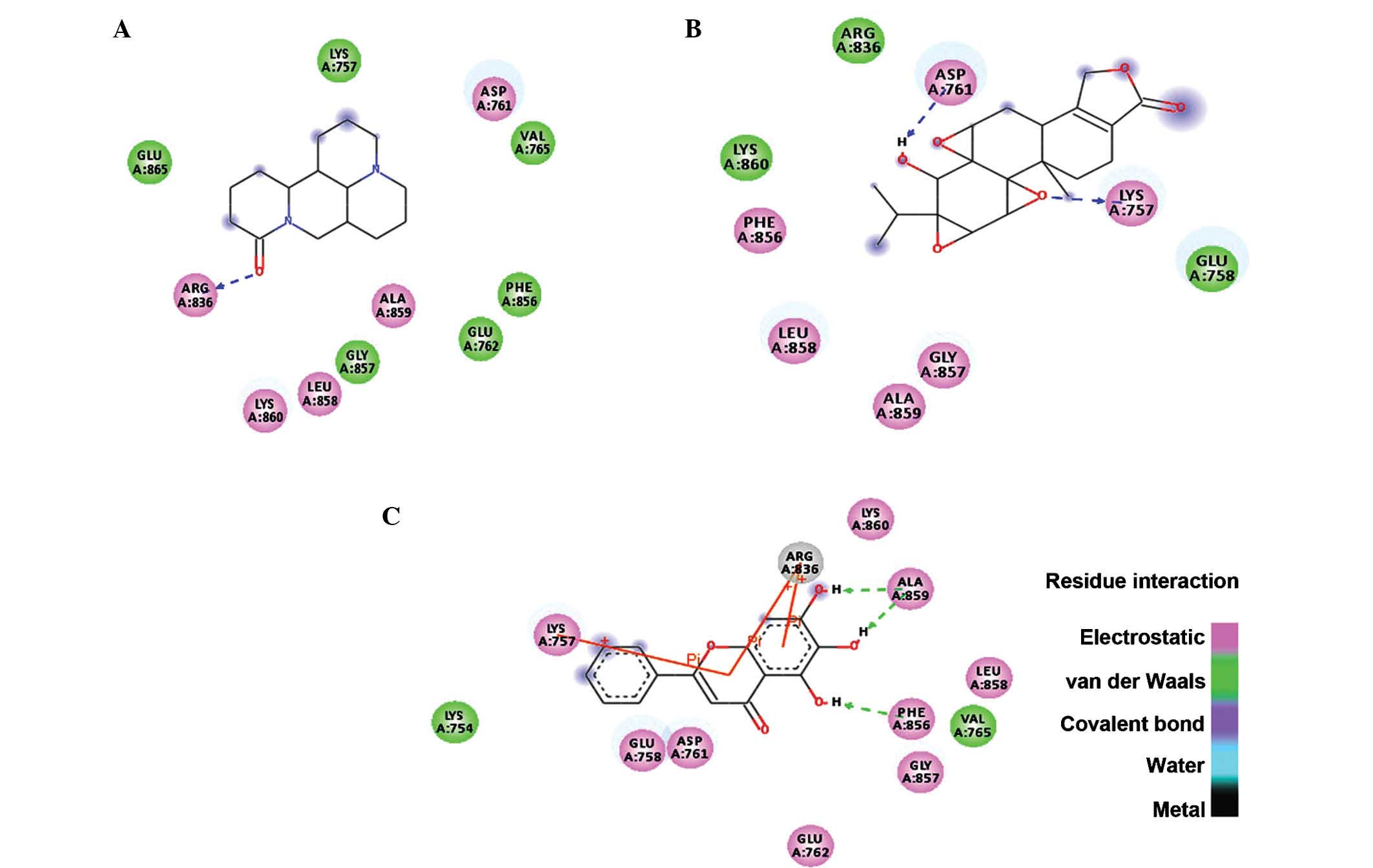
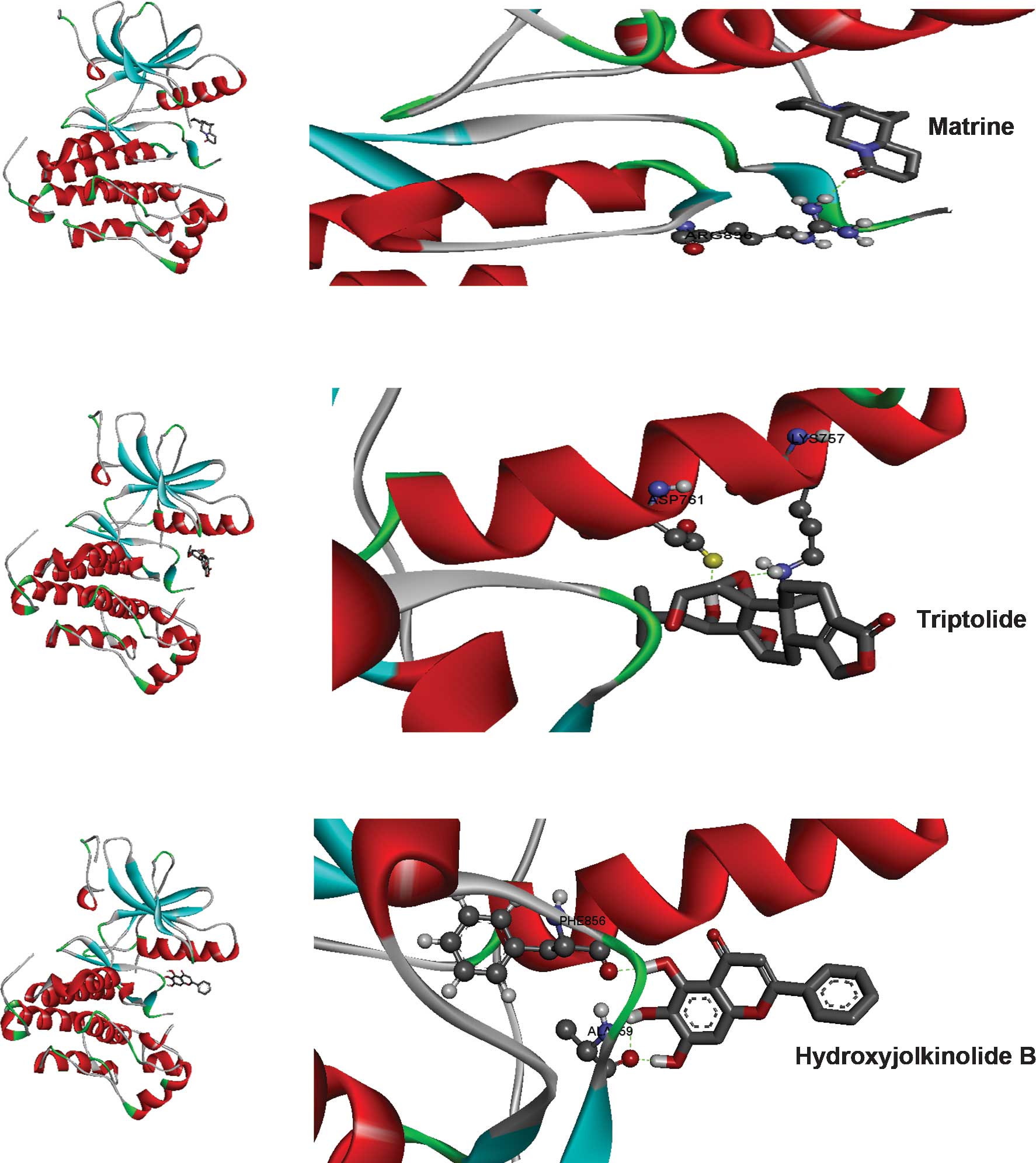
−6.19 kcal/mol and was indicated to form single hydrogen bonds with
ARG836 of the crystal structure of EGFR as shown in Fig. 3A. The oxygen at the first position
of matrine engages in a hydrogen bond with the hydrogen at the 11th
position of ARG836 over a distance of 1.91 Å. The second ranked
compound was triptolide, a diterpenoid epoxide present in
Tripterygium wilfordii, also known as thunder god vine. The
compound formed two hydrogen bonds with the ATP binding pocket of
EGFR with a binding free energy of −5.69 kcal/mol (Fig. 3B). The hydrogen bonds formed
between oxygen in the first position and hydrogen in position 40 of
triptolide with HZ2 of LYS757 and OD2 of ASP761 had a bond distance
of 2.03 and 1.71 Å, respectively. The compound ranked third
regarding interaction with the ATP binding domain of EGFR was
hydroxyjolkinolide B, found in Euphorbia fischeriana, with a
binding free energy of −3.19 kcal/mol. Two hydroxy groups engaged
in hydrogen bonds with ALA859 and one hydrogen bond was present
between another hydroxy group and PHE856 over distances of 1.93 and
1.81 Å, respectively. Furthermore, pi-pi interactions of the
aromatic core with LYS757 and ARG836 were observed (Fig. 3C). In Table II, the Lipinski rule of five
properties of the top three compounds are listed. Three-dimensional
representations of the binding interactions at the atomic level are
shown in Fig. 4.
 | Table IBinding mode of the top three
Traditional Chinese Medicinal compounds. |
Table I
Binding mode of the top three
Traditional Chinese Medicinal compounds.
| Lead | Chem ID | ΔG (kcal/mol) | Binding pocket | Physical
interaction |
|---|
| Matrine | 91466 | −6.19 | GLU865, LYS757,
ASP761, VAL765, PHE856, GLU762, ALA859, GLY857, LEU858, LYS860,
ARG863 | Single H-bond |
| Triptolide | 107985 | −5.69 | PHE856, LYS860,
ARG836, ASP761, LYS757, GLU758, GLY857, ALA859, LEU858 | Two H-bonds |
| Hydroxyjolkinolide
B | 6712607 | −3.19 | LYS754, LYS757,
ARG836, LYS860, ALA859, LEU858, VAL765, PHE856, GLY857, GLU762,
ASP761, GLU758 | Three H-bonds Three
pi-pi |
 | Table IILipinski rule of five properties of
the top three Traditional Chinese Medicinal compounds. |
Table II
Lipinski rule of five properties of
the top three Traditional Chinese Medicinal compounds.
| Lead | Hydrogen bond
donor | Hydrogen bond
acceptor | TPSA (Å) | Log P | Molecular weight
(Da) | Lipinski rule
violations |
|---|
| Matrine | 0 | 3 | 24 | 1.44 | 248.36 | 0 |
| Triptolide | 1 | 6 | 84 | 1.27 | 360.41 | 0 |
| Hydroxyjolkinolide
B | 1 | 5 | 72 | 3.07 | 332.43 | 0 |
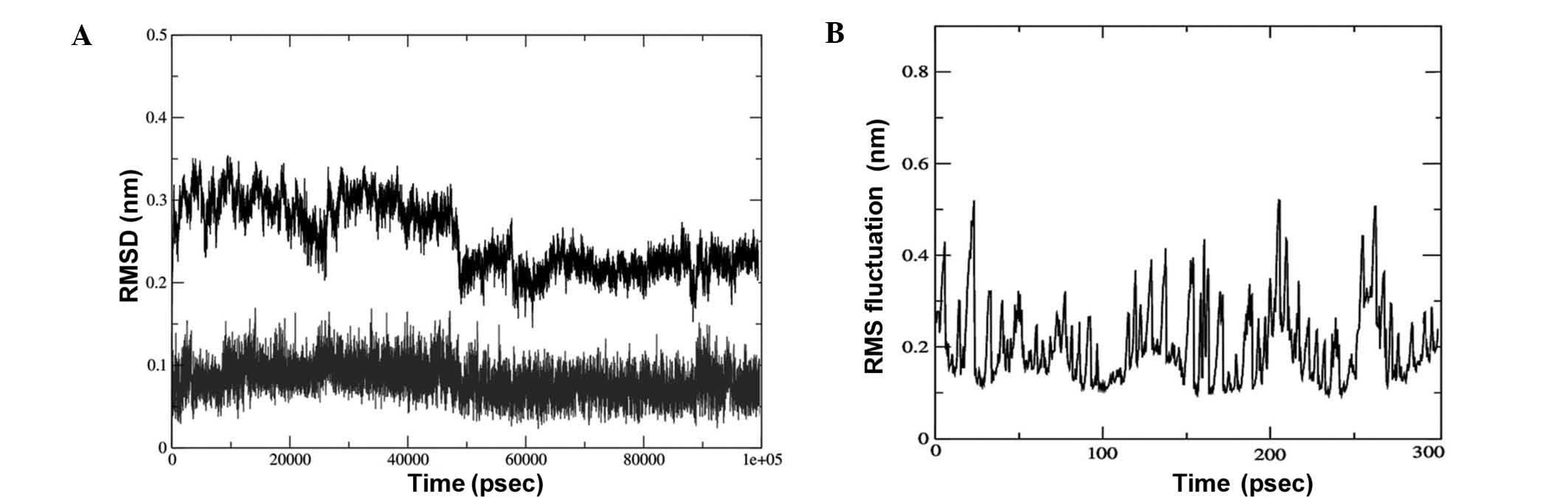
Molecular dynamics simulation
The lead compound triptolide was selected based on
the binding energy and number of hydrogen bonds. Its complex with
EGFR was subjected to root-mean-square (RMS) simulation over 100
nsec. In a neutral aqueous environment, all trajectories converged
within the first five nsec. All calculations were based on the
backbone atoms of the protein. RMS deviation (RMSD), RMS
fluctuation, radius of gyration (Rg) and hydrogen bond analysis of
the complex were performed. The stability of the complex of EGFR
with triptolide using RMSD calculations (Fig. 5A) revealed that the binding of the
drug was stable. The fluctuations observed in the plot are the
attributes of the changing position of the drug in the binding
pocket. The fluctuations at each amino acid position were also
calculated for the complex using the Grms tool,
revealing that the fluctuation of the amino acids in the binding
pocket of the complex was reduced, indicating a stable interaction
of the drug with EGFR (Fig.
5B).
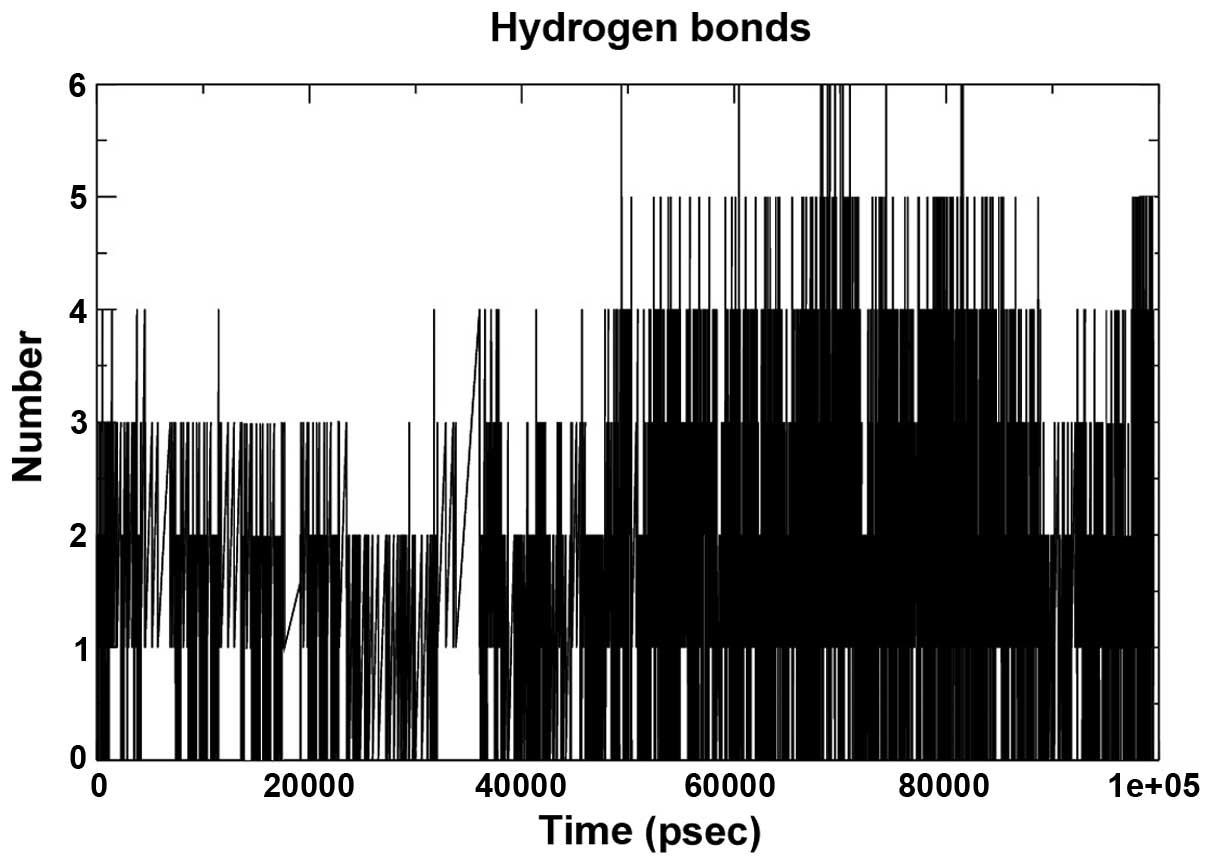
The hydrogen bond interaction between 280 potential
donors and 280 potential acceptors present in the two systems were
found to comprise 1.1 out of 15,000 possible interactions. The
pattern throughout the run is depicted in Fig. 6. The grid that was used to
calculate the hydrogen bond using the ghbond tool of gromacs was
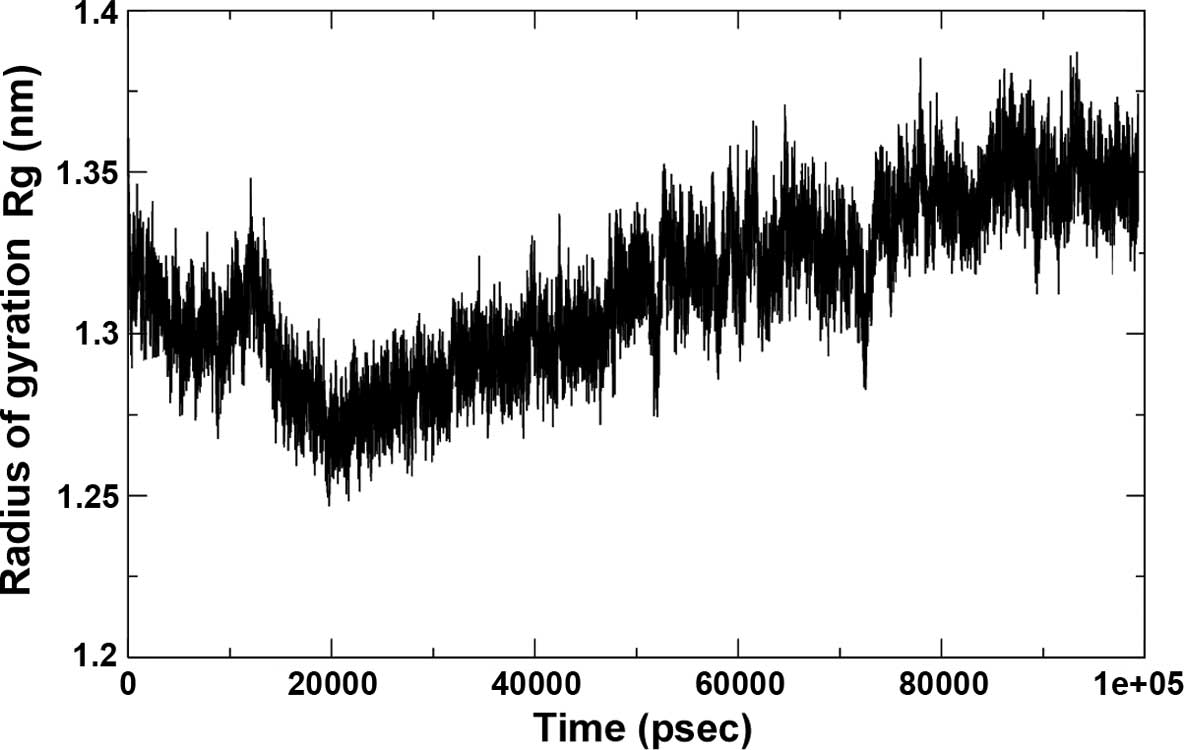
16×16×11 and the cutoff was set at 0.35 Å. The Rg of the complex,
which is indicative of its fluctuation, showed a decrease in the
area of the complex between 0 and 20,000 psec and subsequently
increased until 90,000 psec, remaining static until 100 nsec,
indicating a maximum amplitude at this time-point, with an average
Rg of ~1.32 Å (Fig. 7).
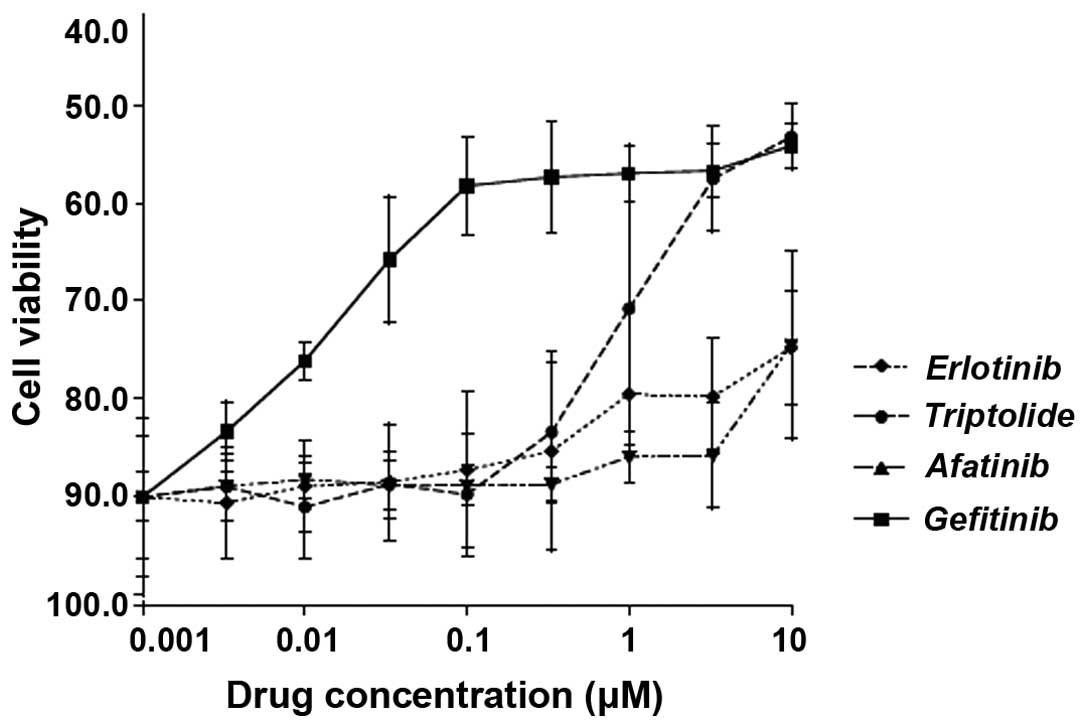
Lead compound triptolide shows potency
against lung adenocarcinoma cells
To assess the potency of triptolide in comparison
with other known anti-EGFR drugs (erlotinib, afatinib and
gefitinib) against lung adenocarcinoma cells in vitro, the
H2347 cell line was treated it with either of these drugs at
0.001–10 μM for 48 h, followed by a viability assay. As
shown in Fig. 8, the potency of
triptolide was at par with afitinib and erlotinib at concentrations
<1 μM, while being at par with gefitinib at 5 and 10
μM. The IC50 value for triptolide on H2347 cells
was 350±0.658 mg/ml (mean ± standard deviation).
Conclusions
The present study performed a computer-aided
screening approach of a database of 2,242 Chinese Traditional
Medicinal compounds with regard to their binding to EGFR for
identifying novel drugs for the treatment of lung adenocarcinoma.
This virtual screening, ADME/Tox prediction, molecular docking and
molecular dynamics simulation provided triptolide as a lead
compound. Furthermore, an in vitro study was performed to
validate the effects of triptolide on the H2347 NSCLC cell line,
which expresses wild-type EGFR. Following 48 h of treatment,
triptolide produced growth inhibition, which was comparable with
that of known EGFR-targeting drugs. Triptolide has previously been
reported to show anti-tumor effects (30–33);
the compound is reported to inhibit cell proliferation, induce cell
apoptosis, inhibit tumor metastasis and enhance the effect of other
therapeutic methods in various cancer cell lines (31). In lung cancer cell lines, the
mechanism of action of triptolide remains to be fully elucidated;
however it has been indicated that triptolide causes
hypomethylation (32) and
sensitizes lung cancer cells to cisplatin-induced apoptosis
(33). A previous study revealed
that triptolide demonstrated synergistic anti-tumor effects with
celastrol, another Chinese medicinal compound (30). Out of the two other compounds
reported in the present study, matrine has also been reported to
exert anti-tumor effects (34).
Hydroxyjolkinolide B, is a novel type of lead compound and, to the
best of our knowledge, has not undergone ample investigation,
therefore further analysis is required. The present study provided
an atomic-scale analysis of the interaction of triptolide with
EGFR, indicating the highest stability of this complex among all
compounds screened. The in silico and in vitro
investigations performed in the present study suggest the
suitability of triptolide for the treatment of NSCLC, which
warrants further in vitro and in vivo analysis.
Acknowledgments
The present study was supported by Programs
Supported by the Ningbo Science and Technology Innovation Team
Project (grant no. 2011B82016).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z: The 3th National Death Cause
Survey Report. Beijing: Chinese Academy of Medical Sciences &
Peking Union Medical College Press; 2008
|
|
4
|
Chen W, Zhang S and Zou X: Evaluation on
the incidence, mortality and tendency of lung cancer in China.
Thoracic Cancer. 1:48–53. 2010. View Article : Google Scholar
|
|
5
|
Chen W, Zheng R, Zeng H and Zhang S: The
Epidemiology of Lung Cancer in China. J Cancer Biol Res.
2:10432014.
|
|
6
|
Guindon GE and Boisclair D: Past, current
and future trend in tobacco. Washington, DC: International Bank for
Reconstruction and Development. The World Bank; pp. 13–16. 2009
|
|
7
|
Cassidy A, Myles JP, van Tongeren M, Page
RD, Liloglou T, Duffy SW and Field JK: The LLP risk model: An
individual risk prediction model for lung cancer. Br J Cancer.
98:270–276. 2008. View Article : Google Scholar
|
|
8
|
Willett WC and Trichopoulos D: Nutrition
and cancer: A summary of the evidence. Cancer Causes Control.
7:178–180. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruano-Ravina A, Figueiras A and
Barros-Dios JM: Diet and lung cancer: A new approach. Eur J Cancer
Prev. 9:395–400. 2000. View Article : Google Scholar
|
|
10
|
Rudin CM, Avila-Tang E and Samet JM: Lung
cancer in never smokers: A call to action. Clin Cancer Res.
15:5622–5625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Skillrud DM, Offord KP and Miller RD:
Higher risk of lung cancer in chronic obstructive pulmonary
disease. A prospective, matched, controlled study. Ann Intern Med.
105:503–507. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo RX, Wu B, Yi YN, Huang ZW and Lin RT:
Indoor burning coal air pollution and lung cancer-a case-control
study in Fuzhou, China. Lung Cancer. 14(Suppl 1): S113–S119. 1996.
View Article : Google Scholar
|
|
13
|
Pershagen G: Air pollution and cancer.
IARC Sci Publ. 104:240–251. 1990.PubMed/NCBI
|
|
14
|
Cohen BL: How dangerous is low level
radiation? Risk Anal. 15:645–653. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Devesa SS, Bray F, Vizcaino AP and Parkin
DM: International lung cancer trends by histologic type: Male:
Female differences diminishing and adenocarcinoma rates rising. Int
J Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morgensztern D, Ng SH, Gao F and Govindan
R: Trends in stage distribution for patients with non-small cell
lung cancer: A National Cancer Database survey. J Thorac Oncol.
5:29–33. 2010. View Article : Google Scholar
|
|
17
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang JC, Schuler MH, Yamamoto N, et al:
LUX-Lung 3: A randomized, open-label, phase III study of afatinib
versus pemetrexed and cisplatin as first-line treatment for
patients with advanced adenocarcinoma of the lung harboring
EGFR-activating mutations. J Clin Oncol. 30(Suppl): Abstract no.
LBA7500. 2012.
|
|
20
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nestler G: Traditional Chinese medicine.
Med Clin North Am. 86:63–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu W, Towers AD, Li P and Collet JP:
Traditional Chinese medicine in cancer care: Perspectives and
experiences of patients and professionals in China. Eur J Cancer
Care (Engl). 15:397–403. 2006. View Article : Google Scholar
|
|
23
|
Chikan NA, Bhavaniprasad V, Anbarasu K,
Shabir N and Patel TN: From natural products to drugs for
epimutation computer-aided drug design. Appl Biochem Biotechnol.
170:164–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chikan NA and Vipperla B: KAISO
inhibition: An atomic insight. J Biomol Struct Dyn. 33:1794–1804.
2015. View Article : Google Scholar
|
|
25
|
Yun CH, Boggon TJ, Li Y, Woo MS, Greulich
H, Meyerson M and Eck MJ: Structures of lung cancer-derived EGFR
mutants and inhibitor complexes: Mechanism of activation and
insights into differential inhibitor sensitivity. Cancer Cell.
11:217–227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Trott O and Olson AJ: AutoDock Vina:
Improving the speed and accuracy of docking with a new scoring
function, efficient optimization and multithreading. J comput chem.
31:455–461. 2010.
|
|
27
|
Seeliger D and de Groot BL: Ligand docking
and binding site analysis with PyMOL and Autodock/Vina. J Comput
Aided Mol Des. 24:417–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morris GM, Goodsell DS, Halliday RS, Huey
R, Hart WE, Belew RK and Olson AJ: Automated docking using a
Lamarckian genetic algorithm and an empirical binding free energy
function. J Comput Chem. 19:1639–1662. 1998. View Article : Google Scholar
|
|
29
|
Lipinski CA: Lead-and drug-like compounds:
The rule-of-five revolution. Drug Discov Today Technol. 1:337–341.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang QW, Cheng KJ, Mei XL, et al:
Synergistic anticancer effects of triptolide with celastrol, two
main compounds from thunder god vine. Oncotarget. 6:32790–32804.
2015.PubMed/NCBI
|
|
31
|
Meng C, Zhu H, Song H, Wang Z, Huang G, Li
D, Ma Z and Ma J, Qin Q, Sun X and Ma J: Targets and molecular
mechanisms of triptolide in cancer therapy. Chin J Cancer Res.
26:622–626. 2014.PubMed/NCBI
|
|
32
|
Li Y, Shen B, Kim J and Raz D: Triptolide
inhibits Wnt signaling due to DNA methylation alteration that is
determined by dynamic histone 3 K79 lysine methylation in NSCLC.
Cancer Res. 75(15 Suppl): Abstract no. 4777. 2015.
|
|
33
|
Wang G, Xu XS and Zhang Y: The role of
triptolide in sensitizing lung tumor cells to cisplatin-induced
apoptosis. Cancer Res. 74(19 Suppl): Abstract no. 5110. 2014.
|
|
34
|
Ma L, Wen S, Zhan Y, He Y, Liu X and Jiang
J: Anticancer effects of the Chinese medicine matrine on murine
hepatocellular carcinoma cells. Planta Med. 74:245–251. 2008.
View Article : Google Scholar : PubMed/NCBI
|