Introduction
Bronchopulmonary dysplasia (BDP) is a disease
affecting neonatals, particularly premature neonatals in which the
structural development of the alveoli is blunted as a consequence
of inflammation and oxygen toxicity (1). The disease is complex and is
characterized by disturbed alveologenesis (2). The pathogenesis depends on the
interaction of a susceptible host with a multitude of environmental
risk factors, including growth factors, cytokines, other substances
that may act as ligands, receptors, signaling molecules and
transcription factors, and the protein products of cell activity,
including enzymes involved in matrix reconstruction, retinoids and
elastin (3,4).
Hyperoxia-induced lung injury is a model of lung
disease similar to BPD, with rarification and simplification of
alveoli, thickened septa and vascular damage (5,6).
Cell therapy is a potential therapeutic approach for lung
disorders, including BDP. Previous advances have shown that
mesenchymal stromal cells (MSCs) can protect the lung in the repair
of injured lung tissues in several animal models, including
endotoxin, bleomycin, monocrotaline and hypoxia-induced lung injury
(7–9). Studies have also shown that the
intravenous or intratracheal administration of MSCs can protect
against hyperoxic lung injury through reducing inflammation and
improving alveolar structure (10,11).
However, MSC engraftment in this disease is low, and therapeutic
benefit is likely to be triggered by a paracrine-mediated
mechanism, immunomodulation (12)
or other mechanisms, which remain to be elucidated.
The vascular endothelial growth factor (VEGF)
pathway and nuclear factor-κB (NF-κB), a transcription factor
traditionally associated with inflammation, are essential for
alveolarization in the neonatal lung (13). The overexpression of transforming
growth factor β (TGF-β), a stimulus for myofibroblastic
differentiation, in neonatal mouse lungs results in structural
changes of BPD, including abnormal alveolar structure and vascular
development (14). Erythropoietin
(EPO) is a glycoprotein hormone produced primarily by the adult
kidney, which regulates the production of red blood cells, exerting
its hematopoietic effects by stimulating the proliferation of
committed erythroid progenitor cells (15). In the last decade, it has emerged
that EPO is an important cytoprotective cytokine against various
stress-inducing events in several organs (16,17).
The beneficial effects of EPO in pulmonary diseases have also been
reported (18). Ozer et al
(19) reported that treatment with
recombinant human erythropoietin (rhEPO) during hyperoxia exposure
is associated with improved alveolar structure, enhanced
vascularity and decreased fibrosis, therefore, treatment of preterm
infants with EPO may reduce the risk of developing BPD. Shang et
al (20) reported that ERO
attenuates pulmonary inflammation and suppresses the levels of
tumor necrosis factor-α (TNF-α) and interleukin-1 β (IL-1β)
overproduced during acute endotoxemia, which is partially mediated
by the inhibition of NF-κB (20).
Although treatment with rhEPO is associated with improved alveolar
structure, enhanced vascularity and decreased fibrosis, these
effects require cautious interpretation due to the limited number
of animals included, and further investigation is required.
Previous studies have investigated the potential
therapeutic effect of stem cells in hyperoxic lung injury in
neonatal rats, and of MSCs, which are able to prevent arrested
alveolar and vascular growth, partly via paracrine activity
(11,21). These may offer novel therapeutic
strategies for treatment of lung disease; however, their potential
roles in neonatal lung injury have not been identified. In the
present study, the different effects of MSCs, EPO alone or MSCs +
EPO in the treatment of BPD were investigated as promising
therapeutic targets for the treatment of BPD.
Materials and methods
Animals
Neonatal C57BL/6 mice (age, 24 h; weight, 12 g) were
used in all experiments in the present study. The C57BL/6-green
fluorescent protein (GFP) mice were purchased from the Animal
Resource Center of the Fourth Military Medical University (Xi'an,
China) and maintained in a temperature controlled environment
(22–24°C) under a 12-h light/dark cycle with access to food and
water ad libitum. The mice were randomized into various
groups and weighed, and blood samples were collected. All animal
procedures were approved by the animal ethics committee of Shandong
University (Jinan, China) and were performed in accordance with the
Guide for the Care and Use of Laboratory Animals, published by the
US National Institutes of Health (NIH Publication no. 85–23,
revised 1996).
Cell isolation and culture
BMSCs were isolated from the bone marrow from the
tibia and femurs of all four limbs of 6–8-week-old C57BL/6-GFP
transgenic mice using a previously reported approach (22). Briefly, fresh BMSCs were isolated
by flushing Dulbecco's modified Eagle's medium (DMEM; American Type
Culture Collection, Manassas, MD, USA) containing 1%
penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO, USA) through
the medullary cavity of the mouse femurs. The cells were isolated
using a Ficoll density gradient centrifugation method (1.077;
Sigma-Aldrich) at 800 x g for 20 min at room temperature. The
isolated BMSCs were cultured (1×106 cells per 100-mm
cell culture dish (Corning Incorporated, Corning, NY, USA) and
expanded in low glucose culture containing culture medium (GE
Healthcare Life Sciences, Logan, UT, USA) containing 10% fetal calf
serum (Becton Dickinson; BD Biosciences, San Diego, CA, USA) at
37°C in 5% CO2. After 48 h, the nonadherent cells were
removed, fresh medium was added, and the culture was maintained for
7 days. The cells were then washed three times with Tris-buffered
saline, which was followed by a 1-h incubation in the dark with
fluorescein isothiocyanate, (FITC)-conjugated goat anti-rabbit IgG
(H+L) secondary antibody (cat. no. ZF-0311; dilution, 1:200,
ZSGB-Bio Co., Beijing, China) in phosphate-buffered saline (PBS).
4,6-Diamino-2-phenyl indole (DAPI) was used for nuclear
counterstaining. Images of the cells were captured using a camera
system connected to a fluorescence microscope (ECLIPSE 90i and
NIS-Elements AR system, Nikon Corporation, Tokyo, Japan). The cells
had characteristic immunoreactivities for cell markers CD44, CD117,
CD34 and CD106 (Santa Cruz Biotechnology, Inc., CA, USA) on the
basis of immunocytochemistry. Briefly, 1×104 cells grown
on a poly-l-lysine-coated 24-well plate were washed three times
with PBS and fixed in 4% paraformaldehyde for 30 min at room
temperature, following which the cells were permeabilized with 0.1%
Triton X-100/PBS for 20 min and blocked with 4% goat serum for 1 h.
The cells were then incubated with rat monoclonal CD44 (1:100; cat.
no. sc-18849; Santa Cruz Biotechnology, Inc.), CD106 (1:100; cat.
no. ab24853; Abcam,Cambridge, MA, USA) and CD117 (1:100; cat. no.
ab24870; Abcam) antibodies, and a goat polyclonal CD34 (1:100; cat.
no. sc-7045, Santa Cruz Biotechnology, Inc.) antibody for 1 h at
room temperature in the dark (23).
BMSCs were suspended with trypsin and
5×105 cells, washed twice with PBS containing 0.5%
bovine serum albumin (Sigma-Aldrich), were incubated with 1:200
dilutions of fluorochrome-conjugated specific or isotype control
antibodies for 30 min at 4°C. The BMSCs were subsequently
resuspended in PBS and incubated with specific or isotype control
antibodies: Phycoerythrin (PE)/Cy5-conjugated anti-CD44 (cat. no.
ab25579), FITC-conjugated anti-CD106 (cat. no. ab24853),
PE-conjugated anti-CD34 (cat. no. ab23830), and FITC-conjugated
anti-CD117 (cat. no. ab24870; all Abcam) resuspended in PBS and
incubated with mouse anti-human monoclonal antibodies for 30 min at
4°C. After being washed, the cells were resuspended in PBS for
fluorescence-activated cell sorting (FACS) analysis.
Preparation of hyperoxia-induced lung
injury and experimental groups
The neonatal C57BL/6 mice were randomly divided into
the following four groups (n=10 in each group): Control, high
oxygen, BMSCs alone and BMSCs/rHuEPO. Hyperoxia exposure of the
neonatal mice was prepared, as previously reported (24). Briefly, the BPD group of neonatal
C57BL/6 mice (age, 24 h) were placed in chambers in which the
oxygen concentration was maintained at FiO2=0.60. The
exposure to hyperoxia was continuous, and the animals were exposed
to the hypoxic environment for 14 days, with a brief interruption
for animal care (<10 min/day). Subsequently, 1×106
BMSCs were administered intravenously and 5,000 U/kg rHuEPO was
administered by intraperitoneal injection, 1 h prior to and 7 days
following hyperoxia exposure of the neonatal mice.
Lung histology and morphometric
analysis
The lung tissues were prepared for histological
examination, as previously described (24). Briefly, the mice were sacrificed by
intra-peritoneal injections of pentobarbital (100 mg/kg) at 14 days
old. Following sacrifice, the lungs were fixed with 4%
paraformaldehyde solution overnight, and the left lower lobe was
embedded in paraffin. Tissue sections were produced using a
microtome set at 4–5 µm (Leica RM226; Leica Microsystems
GmbH, Heidelberg Germany). Alveolarization was assessed by
performing radial alveolar counts (RACs), according to standard
methods, as previously described (25). Briefly, from the center of the
respiratory bronchiole, a perpendicular line was drawn to the edge
of the acinus, as defined by a connective tissue septum or the
pleura, and the number of septa intersected by this line were
counted. A total of five counts were performed for each animal, and
the average count from the five high-power fields was randomly
selected. These experiments were performed by two examiners blinded
to treatment assignment.
Immunohistochemical staining
On day 14, the mice were sacrificed by intracoronary
perfusion of 10% KCl solution, and the lungs were removed and fixed
in 4% paraformaldehyde/PBS for 24 h, followed by storage in 70%
ethanol. The left lower lobe was embedded in paraffin, sectioned
into 4–5 µm-thick sections, deparaffinized in xylene, and
rehydrated by serial immersions in 100, 95, 85 and 75% ethanol, and
100% water. Following blocking with 4% normal goat serum in PBS,
the slides were incubated with primary antibodies overnight at 4°C,
and biotinylated HRP-conjugated rabbit IgG (H+L) polyclonal
secondary antibody (1:200; cat. no. ZB-2306; ZSGB-Bio Co.) for 20
min at room temperature. The following primary antibodies were
used: Rabbit polyclonal MMP-9 (1:200; cat. no. ab3889; Abcam) and
VEGF (1:200; cat. no. ab46154; Abcam), which was followed by a 1-h
incubation in the dark with tetrametrylrhodarnine
isothiocyante-conjugated goat anti-rabbit IgG (H+L) secondary
antibody (cat. no. ZF-0316; dilution, 1:200; ZSGB-Bio Co., Ltd). A
total of 10 serial cortex and hippocampal sections (50 µm
interval for each section) from each animal (n=10 for each group)
were used to quantify each parameter. The staining was analyzed
using the image-analyzing system, Image Pro Plus 6 (Media
Cybernetics, Rockville, MD, USA), as previously described (26).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Comparisons of parameters between two groups were made
using an unpaired Student's t-test. Comparisons of
parameters among three groups were made using one-way analysis of
variance, followed by Scheffe's multiple comparison test.
Statistical analysis performed using the SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
FACS
The surface markers of BMSCs were determined using
FACS and showed that the BMSCs were positive for CD44 (93.14%) and
CD106 (95.37%), but negative for CD34 (1.69%) and CD117 (2.58%;
Fig. 1).
Body weights
A total of 10 animals were represented in each
treatment group and at each time point. The body weights of the
animals were lower in the BPD group (positive control) when the
mice were 7- (5.89±0.26 g) and 14- (7.95±0.22 g) days-old, compared
with the mice exposed to room air (8.34±0.25 and 11.05±0.28,
respectively, P<0.05). The body weights in the BMSC alone and
BMSC/rHuEPO treatment groups were higher at 7 days old (6.84±0.20
and 7.42±0.23 g, respectively) and 14 days old (9.27±0.21 and
10.38±0.26 g, respectively), compared with the hyperoxia group
(P<0.05). Weight preservation was most marked in the
BMSCs/rHuEPO-treated mice (P<0.05; Fig. 2).
Lung histology and morphometric
analyses
To investigate whether treatment with BMSCs and
BMSCs/rHuEPO have beneficial effects on hyperoxia exposure-induced
impairment in lung structure, morphometric analysis was performed
using RACs. Compared with the room air control, RACs were lower in
the 14-day-old C57BL/6 mice exposed to hyperoxia, but higher in the
mice treated with BMSCs alone and with BMSCs/rHuEPO during
hyperoxia on postnatal days 7 and 14, compared with those of the
BPD group. In addition, improved weight preservation was shown in
the BMSCs/rHuEPO-treated mice, compared with the mice treated with
BMSCs alone (Fig. 3).
Protein expression levels of MMP-9 and
VEGF in lung tissues
The results of the immunohistochemical staining
analysis revealed that exposure of the neonatal mice to hyperoxia
for 14 days resulted in significant increases in the protein
expression levels of MMP-9 in the lungs, and a decrease in VEGF.
The alterations in these proteins were significantly improved
following treatment with BMSCs alone and with BMSCs/rHuEPO on day
14. Of note, the improvement in these protein expression levels
were more marked in the BMSCs/rHuEPO group, compared with the BMSCs
only group (Fig. 4).
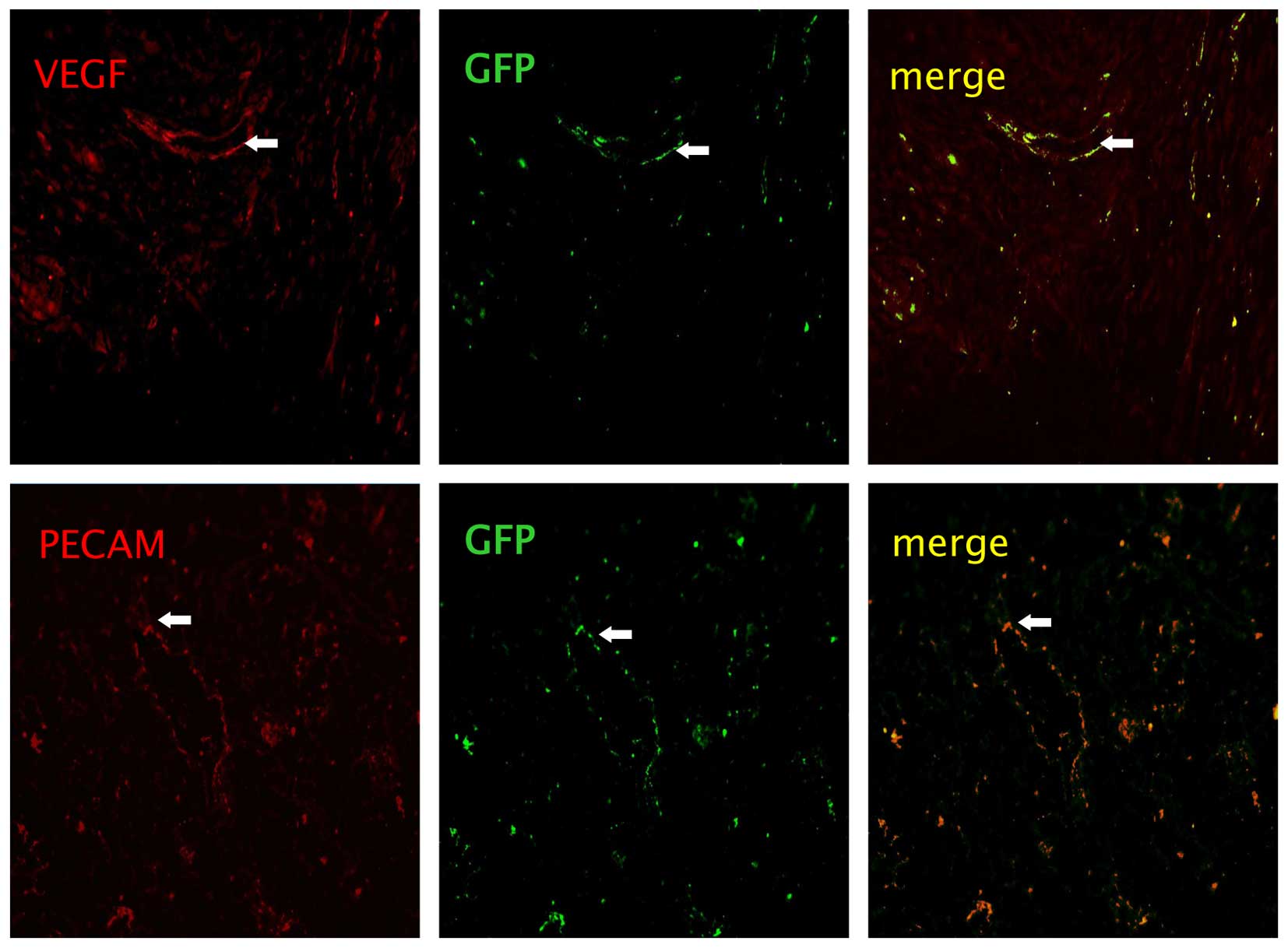
Identification of the injected BMSCs
On day 14 following the injection of BMSCs from the
C57BL/6-GFP mice,cytochemical staining of vessel markers, PECAM
(red) and VEGF (red), in the lung tissues were observed. Images
were captured using a camera system connected to a fluorescence
microscope (Fig. 5). However, the
differentiation did not differ between the BMSCs alone and
BMSCs/rHuEPO groups.
Discussion
There has been substantial interest in the potential
therapeutic effect of stem cells as a novel approach to a diverse
range of lung diseases, including hyperoxic injury in neonatal rats
(21,27). The intravenous or intra-alveolar
administration of BMSCs attenuates the severity of lung damage in
adult rats following bleomycin- and endotoxin-induced lung injury
(5,8). Potential mechanisms for BMSC
therapy-induced improvements in lung structure include engraftment,
anti-inflammatory or immunomodulatory functions, and anti-apoptotic
effects. Previous studies have indicated that BMSCs can prevent
arrested alveolar and vascular growth, in part, through paracrine
activity in an experimental model of neonatal lung injury in rats
(11,28). These reports may offer novel
therapeutic strategies for lung diseases, which currently lack
efficient treatments. However, several issues require further
investigation (29) and the
potential role of BMSCs in the setting of neonatal lung injury
remain to be fully elucidated (30). BMSCs can migrate to, or be involved
in the development of lung tissue (31–34),
and several studies have demonstrated that stem/progenitor cells
have the potential for use as cellular therapies to contribute to
lung repair mechanisms following acute lung injury (34,35).
The present study demonstrated that the intravenous injection of
BMSCs significantly improved body weight and airway structure
following lung injury in hyperoxia-exposed neonatal mice, Of note,
the injection of BMSCs in combination with rHuEPO showed more
marked improvement, compared with injection of the BMSCs alone.
MMPs are a family of proteolytic enzymes that
degrade various components of the ECM, and members of MMPs family
include MMP-1, MMP-2, MMP-8 and MMP-9 (36). MMP-9 has been shown to be a key
proteinase, promoting the destruction of matrix and basement
membranes (37). Studies have
shown that the concentrations of MMP-9 are elevated in the tracheal
aspirates of neonates with BPD (38,39).
The findings of the present study may support the suggestion that
MMP-9 and TGF-β are important in neonatal lung injury and BPD.
Treatment with rhEPO during hyperoxia exposure is associated with
improved alveolar structure, enhanced vascularity and decreased
fibrosis in BPD (19). In
addition, EPO can attenuate pulmonary inflammation and suppress the
overproduction of TNF-α and IL-1β induced by acute endotoxemia
(20). In the present study, the
results showed that the protein expression levels of MMP-9 were
significantly lower following treatment with BMSCs alone and in
combination with rHuEPO following exposure of the neonatal mice to
hyperoxia for 14 days. However, the expression of MMP-9 was lower
in the BMSCs/rHuEPO group, compared with the BMSCs only group.
These results showed that treatment with BMSCs or BMSCs/rHuEPO
reduced lung injury, and that EPO promoted the effect of the BMSCs
through paracrine or anti-inflammatory mechanisms.
VEGF is recognized as being essential in the
regulation of pulmonary vascular growth and development,
stimulating angiogenesis and endothelial survival (40). In BDP animal models and in the
lungs of premature infants who succumbed to BPD-associated
mortality, the expression levels of VEGF are decreased (41–43).
Inhibiting VEGF receptor 2 (VEGFR2) causes rarefaction of pulmonary
vessels and impairs alveolar formation (41) in neonatal rats, whereas the
enhancement of VEGF signaling rescues the alveolar disruption
induced by hyperemia (44). By
contrast, inhibiting postnatal angiogenesis impairs alveolarization
(27), and decreased pulmonary
capillary density is observed in animal models and patients who
have succumbed to BPD-associated mortality (45). At present, although the association
between pulmonary angiogenesis and lung development is clear, the
transcription mediation of pulmonary angiogenesis remains to be
elucidated. As reported, NF-κB directly regulates the expression of
the proangiogenic molecule, VEGFR2, in the developing pulmonary
vasculature (13). The findings of
the present study showed that the protein expression levels of VEGF
were significantly improved 14 days following treatment with BMSCs
alone or with BMSCs/rHuEPO during exposure of the neonatal mice to
hyperoxia. These results supported the hypothesis that VEGF is
involved in neonatal lung injury. Of note, the present study found
that EPO further promoted this effect.
In conclusion, supplemental oxygen is required for
the survival of premature infants, however, this may lead to the
accumulation of reactive oxygen species, impairment of
alveolarization and dysmorphic pulmonary vasculature. The present
study demonstrated that intravenous injection of BMSCs
significantly improved the damaged airway structure, and rescued
the levels of MMP-9 and VEGF in hyperoxia-exposed neonatal mouse
lungs. It was found that treatment with BMSCs in combination with
intraperitoneal injection of rHuEPO further enhanced these
improvements, compared with BMSCs alone.
Taken together, the data obtained in the present
study suggested that BMSCs from C57BL/6-GFP mice provided a novel
approach for the treatment of BPD in an in vivo model of
lung injury. The results also indicated that the combination of
BMSCs and EPO had more marked effects, compared with BMSCs alone 14
days following injection. These findings suggested the potential to
rescue BPD damage by the injection of BMSCs alone or BMSCs/EPO in
mice, providing valuable information for future clinical
trials.
Acknowledgments
This study was supported by a grant from the Seed
Foundation of the Second Hospital of Shandong University (grant no.
S2013010001).
References
|
1
|
Madurga A, Miziková I, Ruiz-Camp J and
Morty RE: Recent advances in late lung development and the
pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell
Mol Physiol. 305:L893–L905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pietrzyk JJ, Kwinta P, Wollen EJ,
Bik-Multanowski M, Madetko-Talowska A, Günther CC, Jagła M, Tomasik
T and Saugstad OD: Gene expression profiling in preterm infants:
New aspects of bronchopulmonary dysplasia development. PLoS One.
8:e785852013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Warburton D, Bellusci S, De Langhe S, Del
Moral PM, Fleury V, Mailleux A, Tefft D, Unbekandt M, Wang K and
Shi W: Molecular mechanisms of early lung specification and
branching morphogenesis. Pediatr Res. 57:R26–R37. 2005. View Article : Google Scholar
|
|
4
|
Thébaud B: Angiogenesis in lung
development, injury and repair: Implications for chronic lung
disease of prematurity. Neonatology. 91:291–297. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rojas M, Xu J, Woods CR, Mora AL, Spears
W, Roman J and Brigham KL: Bone marrow-derived mesenchymal stem
cells in repair of the injured lung. Am J Respir Cell Mol Biol.
33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yam J, Frank L and Roberts RJ: Oxygen
toxicity: Comparison of lung biochemical responses in neonatal and
adult rats. Pediatr Res. 12:115–119. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mei SH, McCarter SD, Deng Y, Parker CH,
Liles WC and Stewart DJ: Prevention of LPS-induced acute lung
injury in mice by mesenchymal stem cells overexpressing
angiopoietin 1. PLoS Med. 4:e2692007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ortiz LA, Gambelli F, McBride C, Gaupp D,
Baddoo M, Kaminski N and Phinney DG: Mesenchymal stem cell
engraftment in lung is enhanced in response to bleomycin exposure
and ameliorates its fibrotic effects. Proc Natl Acad Sci USA.
100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu J, Woods CR, Mora AL, Joodi R, Brigham
KL, Iyer S and Rojas M: Prevention of endotoxin-induced systemic
response by bone marrow-derived mesenchymal stem cells in mice. Am
J Physiol Lung Cell Mol Physiol. 293:L131–L141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aslam M, Baveja R, Liang OD,
Fernandez-Gonzalez A, Lee C, Mitsialis SA and Kourembanas S: Bone
marrow stromal cells attenuate lung injury in a murine model of
neonatal chronic lung disease. Am J Respir Crit Care Med.
180:1122–1130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Haaften T, Byrne R, Bonnet S,
Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J,
Haromy A, Eaton F, et al: Airway delivery of mesenchymal stem cells
prevents arrested alveolar growth in neonatal lung injury in rats.
Am J Respir Crit Care Med. 180:1131–1142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JW, Fang X, Krasnodembskaya A, Howard
JP and Matthay MA: Concise review: Mesenchymal stem cells for acute
lung injury: Role of paracrine soluble factors. Stem Cells.
29:913–919. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iosef C, Alastalo TP, Hou Y, Chen C, Adams
ES, Lyu SC, Cornfield DN and Alvira CM: Inhibiting NF-κB in the
developing lung disrupts angiogenesis and alveolarization. Am J
Physiol Lung Cell Mol Physiol. 302:L1023–L1036. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vicencio AG, Lee CG, Cho SJ, Eickelberg O,
Chuu Y, Haddad GG and Elias JA: Conditional overexpression of
bioactive transforming growth factor-beta1 in neonatal mouse lung:
A new model for bronchopulmonary dysplasia? Am J Respir Cell Mol
Biol. 31:650–656. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jelkmann W: Erythropoietin: Structure,
control of production, and function. Physiol Rev. 72:449–489.
1992.PubMed/NCBI
|
|
16
|
Lipsic E, Schoemaker RG, van der Meer P,
Voors AA, van Veldhuisen DJ and van Gilst WH: Protective effects of
erythropoietin in cardiac ischemia: From bench to bedside. J Am
Coll Cardiol. 48:2161–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
King VR, Averill SA, Hewazy D, Priestley
JV, Torup L and Michael-Titus AT: Erythropoietin and carbamylated
erythropoietin are neuroprotective following spinal cord
hemisection in the rat. Eur J Neurosci. 26:90–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shang Y, Li X, Prasad PV, Xu S, Yao S, Liu
D, Yuan S and Feng D: Erythropoietin attenuates lung injury in
lipopolysaccharide treated rats. J Surg Res. 155:104–110. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ozer EA, Kumral A, Ozer E, Yilmaz O, Duman
N, Ozkal S, Koroglu T and Ozkan H: Effects of erythropoietin on
hyperoxic lung injury in neonatal rats. Pediatr Res. 58:38–41.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shang Y, Jiang YX, Xu SP, Wu Y, Wu ZY,
Yuan SY and Yao SL: Reduction of pulmonary inflammatory response by
erythropoietin in a rat model of endotoxaemia. Chin Med J (Engl).
122:834–838. 2009.
|
|
21
|
Zhang H, Fang J, Su H, Yang M, Lai W, Mai
Y and Wu Y: Bone marrow mesenchymal stem cells attenuate lung
inflammation of hyperoxic newborn rats. Pediatr Transplant.
16:589–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okabe M, Ikawa M, Kominami K, Nakanishi T
and Nishimune Y: 'Green mice' as a source of ubiquitous green
cells. FEBS Lett. 407:313–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han X, Zhao L, Lu G, Ge J, Zhao Y, Zu S,
Yuan M, Liu Y, Kong F, Xiao Z and Zhao S: Improving outcomes of
acute kidney injury using mouse renal progenitor cells alone or in
combination with erythropoietin or suramin. Stem Cell Res Ther.
4:742013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balasubramaniam V, Mervis CF, Maxey AM,
Markham NE and Abman SH: Hyperoxia reduces bone marrow,
circulating, and lung endothelial progenitor cells in the
developing lung: Implications for the pathogenesis of
bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol.
292:L1073–L1084. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kunig AM, Balasubramaniam V, Markham NE,
Seedorf G, Gien J and Abman SH: Recombinant human VEGF treatment
transiently increases lung edema but enhances lung structure after
neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol.
291:L1068–L1078. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang H, Xie Z, Wei L, Yang H, Yang S, Zhu
Z, Wang P, Zhao C and Bi J: Human umbilical cord mesenchymal stem
cell-derived neuron-like cells rescue memory deficits and reduce
amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem
Cell Res Ther. 4:762013. View
Article : Google Scholar
|
|
27
|
Zhang H, Fang J, Wu Y, Mai Y, Lai W and Su
H: Mesenchymal stem cells protect against neonatal rat hyperoxic
lung injury. Expert Opin Biol Ther. 13:817–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tropea KA, Leder E, Aslam M, Lau AN,
Raiser DM, Lee JH, Balasubramaniam V, Fredenburgh LE, Alex
Mitsialis S, Kourembanas S and Kim CF: Bronchioalveolar stem cells
increase after mesenchymal stromal cell treatment in a mouse model
of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol.
302:L829–L837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abman SH and Matthay MA: Mesenchymal stem
cells for the prevention of bronchopulmonary dysplasia: Delivering
the secretome. Am J Respir Crit Care Med. 180:1039–1041. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hennrick KT, Keeton AG, Nanua S, Kijek TG,
Goldsmith AM, Sajjam US, Bentley JK, Lama VN, Moore BB, Schumacher
RE, et al: Lung cells from neonates show a mesenchymal stem cell
phenotype. Am J Respir Crit Care Med. 175:1158–1164. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Krause DS, Theise ND, Collector MI,
Henegariu O, Hwang S, Gardner R, Neutzel S and Sharkis SJ:
Multi-organ, multi-lineage engraftment by a single bone
marrow-derived stem cell. Cell. 105:369–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wen ST, Chen W, Chen HL, Lai CW, Yen CC,
Lee KH, Wu SC and Chen CM: Amniotic fluid stem cells from EGFP
transgenic mice attenuate hyperoxia-induced acute lung injury. PLoS
One. 8:e753832013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gore AV, Bible LE, Livingston DH, Mohr AM
and Sifri ZC: Mesenchymal stem cells enhance lung recovery after
injury, shock, and chronic stress. Surgery. 159:1430–1435. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mitsialis SA and Kourembanas S: Stem
cell-based therapies for the newborn lung and brain: Possibilities
and challenges. Semin Perinatol. 40:138–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luan Y, Zhang X, Kong F, Cheng GH, Qi TG
and Zhang ZH: Mesenchymal stem cell prevention of vascular
remodeling in high flow-induced pulmonary hypertension through a
paracrine mechanism. Int Immunopharmacol. 14:432–437. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Buckley S and Warburton D: Dynamics of
metalloproteinase-2 and -9, TGF-beta, and uPA activities during
normoxic vs. hyperoxic alveolarization. Am J Physiol Lung Cell Mol
Physiol. 283:L747–L754. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ekekezie II, Thibeault DW, Simon SD,
Norberg M, Merrill JD, Ballard RA, Ballard PL and Truog WE: Low
levels of tissue inhibitors of metalloproteinases with a high
matrix metallopro-teinase-9/tissue inhibitor of metalloproteinase-1
ratio are present in tracheal aspirate fluids of infants who
develop chronic lung disease. Pediatrics. 113:1709–1714. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harijith A, Choo-Wing R, Cataltepe S,
Yasumatsu R, Aghai ZH, Janér J, Andersson S, Homer RJ and Bhandari
V: A role for matrix metalloproteinase 9 in IFNγ-mediated injury in
developing lungs: Relevance to bronchopulmonary dysplasia. Am J
Respir Cell Mol Biol. 44:621–630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abman SH: Impaired vascular endothelial
growth factor signaling in the pathogenesis of neonatal pulmonary
vascular disease. Adv Exp Med Biol. 661:323–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
De Paepe ME, Mao Q, Powell J, Rubin SE,
DeKoninck P, Appel N, Dixon M and Gundogan F: Growth of pulmonary
microvasculature in ventilated preterm infants. Am J Respir Crit
Care Med. 173:204–211. 2006. View Article : Google Scholar
|
|
42
|
Maniscalco WM, Watkins RH, Pryhuber GS,
Bhatt A, Shea C and Huyck H: Angiogenic factors and alveolar
vasculature: Development and alterations by injury in very
premature baboons. Am J Physiol Lung Cell Mol Physiol.
282:L811–L823. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hosford GE and Olson DM: Effects of
hyperoxia on VEGF, its receptors and HIF-2 in the newborn rat lung.
Am J Physiol Lung Cell Mol Physiol. 285:L161–L168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Acarregui MJ, Penisten ST, Goss KL,
Ramirez K and Snyder JM: Vascular endothelial growth factor gene
expression in human fetal lung in vitro. Am J Respir Cell Mol Biol.
20:14–23. 1999. View Article : Google Scholar
|
|
45
|
Le Cras TD, Markham NE, Tuder RM, Voelkel
NF and Abman SH: Treatment of newborn rats with a VEGF receptor
inhibitor causes pulmonary hypertension and abnormal lung
structure. Am J Physiol Lung Cell Mol Physiol. 283:L555–L562. 2002.
View Article : Google Scholar : PubMed/NCBI
|