Introduction
MicroRNAs (miRNAs) are small non-coding regulatory
RNAs 17–25 nucleotides long, which are involved in the
post-transcriptional regulation of gene expression (1). More than 50% of miRNA genes are
located in cancer-associated genomic regions or fragile sites, thus
suggesting that miRNAs have important roles in tumor development
processes, including apoptosis, invasion, metastasis, proliferation
and drug resistance (2). Usually,
miRNAs that lead to tumorigenesis are classed as oncomiRs, whereas
miRNAs whose functional loss can contribute to the malignant
transformation of normal cells, are classed as tumor suppressors
(3). miRNA (miR)-155 is one of the
most commonly upregulated miRNAs in several types of cancer,
including non-small cell lung cancer (NSCLC) (4), breast cancer (5), pancreatic cancer (6), colon cancer (7), renal cancer (8), Hodgkin and B cell lymphoma (9), and secondary acute leukemia (10). Therefore, the dysregulated
expression of miR-155 may be an important target for the diagnosis,
prognosis and treatment of cancer (11). However, the specific mechanism of
action of miR-155 in cancer is currently only partially known.
NSCLC represents the most frequent type of lung
cancer, and has a 5-year overall survival rate of <15% (12). At present, radiation therapy is
regarded as an effective treatment strategy for NSCLC, which uses
high-energy rays or particles to destroy lung cancer cells
(13). However, radioresistance
remains a major barrier that limits the efficacy of radiotherapy
(13). Therefore, the development
of novel approaches is urgently required, in order to overcome the
radioresistance of NSCLC and improve the survival rate of patients.
A previous study reported that under hypoxic conditions, miR-155
expression was induced, leading to increased resistance of NSCLC
cells to irradiation (14).
The majority of cancer cells exhibit increased
glycolysis and dysregulated mitochondrial function, in order to
provide sufficient energy for proliferation (15); this unique feature of cancer cells
is known as the Warburg effect. Hexokinases (HKs) catalyze the
first committed step in glucose metabolism by catalyzing the
phosphorylation of glucose to glucose-6-phosphate (G6P). Therefore,
hexokinases influence the direction of glucose flux within cells,
which is tightly correlated with the processes of tumor initiation
and maintenance (15). In
addition, elevated glycolysis of cancer cells has been shown to
contribute to chemo- and radiotherapy resistance (16). It has previously been reported that
radiation induces aerobic glycolysis via reactive oxygen species
(17). Furthermore, the oncogene
Akt has been shown to promote aerobic glycolysis, which renders
cancer cells resistant to irradiation (18), thus indicating that glycolysis may
be a target for the development of anticancer therapies. The
present study investigated the role of miR-155 in the
radiosensitivity of NSCLC. The results revealed that inhibition of
miR-155 may sensitize NSCLC cells to radiation, thus suggesting
that miR-155 may be considered a therapeutic target for the
development of anticancer drugs.
Materials and methods
Cells and cell culture
The A549 and H460 NSCLC cell lines were purchased
from American Type Culture Collection (Manassas, VA, USA), and were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum, 100 U/ml penicillin and 100
µg/ml streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). All of the cells were cultured at 37°C in
a humidified incubator containing 95% air and 5% CO2,
and A549 and H460 cells were treated with 3BrPA at 50 µM for
24 h.
Antibody and reagents
The following antibodies were used in the present
study: Anti-β-actin (cat. no. 4967; Cell Signaling Technology,
Inc., Danvers, MA, USA) and anti-hexokinase 2 (HK2) (cat. no. 2106;
Cell Signaling Technology, Inc.). 3-Bromopyruvate (3BrPA) was
purchased from Sigma-Aldrich China, Inc. (Hong-Kong, China). The
activities of HK were measured using the Hexokinase Colorimetric
Assay kit (Sigma-Aldrich China, Inc.).
Ionizing radiation treatment
Approximately 5×105 cells per 6 cm-dish
were exposed to various doses of irradiation (0.2, 0.4, 0.5, 0.6,
1, 1.5, 2, 2.5, 3, 4 and 5 Gys) at room temperature using a Cs-137
irradiator (HWM D-2000; Siemens AG, Munich, Germany) at a dose rate
of 2 Gy/min. The cells were then trypsinized, re-plated in a cell
culture dish, and incubated for 16 h, prior to downstream
analysis.
Plasmid DNA and miRNA transfections
Transfection was performed using the Lipofectamine
2000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Briefly,
0.5–1×106 cells/well were plated onto a 6-well plate,
and were incubated overnight until they had reached 70–90%
confluence. The next day, plasmid DNA (4 µg; Addgene, Inc.,
Cambridge, MA, USA), pre-miR-155 (100 nM) or anti-miR-155 (100 nM)
was diluted in Opti-MEM I reduced serum medium (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the Lipofectamine 2000
transfection reagent protocol. The cells were then transfected with
a HK2 overexpression vector (Addgene, Inc., Cambridge, MA, USA),
100 nM pre-miR-155, anti-miR-155 or negative control using
Lipofectamine 2000 and Opti-MEM I reduced serum medium, according
to the manufacturer's protocol. The anti-miR negative control used
was GMR-miR microRNA inhibitors FAM-labeled single-stranded
negative control (Shanghai GenePharma Co., Ltd., Shanghai, China).
The precursor and antisense miR-155 were chemically synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). A total of 48 h
post transfection, the cells were prepared for further
analysis.
HK activity assay
The cells were seeded onto a 6-well plate at a
density of 3×105/well overnight. The cells were then
collected and HK activities were measured using the Hexokinase
Colorimetric Assay kit (Sigma-Aldrich China, Inc.), according to
the manufacturer's protocol.
Glucose consumption and lactate
production
The cells were seeded onto a 6-well plate at a
density of 3×105/well, and the culture medium was
replaced with low glucose DMEM (Invitrogen; Thermo Fisher
Scientific, Inc.) after 6 h. The concentrations of glucose and
L-lactate were measured after a further 24 h using a Glucose Test
kit (Applygen Technologies, Inc., Beijing, China) and an L-lactate
Assay kit (Eton Bioscience, Inc., San Diego, CA, USA),
respectively, according to the manufacturer's instructions.
Cell viability assay
A total of 1×104 cells/well were seeded
onto 48-well plates overnight. The medium was subsequently replaced
with fresh medium with or without 3BrPA at the indicated
concentrations, and the cells were incubated for a further 48 h.
Cell viability was measured using the MTT assay, according to the
manufactory's instructions. Briefly, an equal number of cells were
plated into 96-well plates with culture medium containing either
3BrPA or phosphate-buffered saline (PBS; Sigma-Aldrich China, Inc.)
for the untreated control. Following treatments, 0.1 mg/ml MTT was
added to each well and was incubated at 37°C for 4 h. Plates were
centrifuged at 450 × g for 5 min at room temperature, and the
medium was discarded. Dimethyl sulfoxide (0.15 ml; Sigma-Aldrich
China, Inc.) was added to each well to solubilize the crystals.
Absorbance was measured spectrophotometrically at a wavelength of
570 nm using an ELx800 universal microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
cDNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted following homogenization of
the cells using the RNeasy Mini kit (Qiagen Sciences, Inc.,
Germantown, Maryland MD). DNase digestion was performed during the
RNA extraction using the RNase free DNase set (Qiagen, Inc.,
Valencia, CA, USA). Total RNA (1 µg) was reverse transcribed
using the TaqMan microRNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and TaqMan microRNA
Assays kit (Applied Biosystems; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols. The precursor miR-155
and RNU6B (served as an internal control) primers used were as
follows: miR-155, F 5′-CTAGCCTGCAGGTATTCAAATATTTCCACAGA-3′ and R
5′-ATCCGGCCGGCCTGAAGATGGTTATGAACATA-3′; RNU6B,
TCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAATATGGAAC
(Invitrogen; Thermo Fisher Scientific, Inc.). The CFX96 Touch
Real-Time PCR thermal cycler (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) was used with the cycling conditions as follows: 95°C for
10 min, 95°C for 15 sec and 60°C for 60 sec, for 40 cycles. All
reactions were performed in triplicate. Human U6 served as an
internal control. The relative amounts of miRNA were calculated
using the comparative cycle quantification method (19).
Western blot analysis
Cells were removed from the treated or non-treated
conditions, and were immediately placed on ice. After rinsing with
phosphate-buffered saline, the cells were scraped, collected and
protein was extracted from them using lysis buffer (Thermo Fisher
Scientific, Inc.). Protein concentration was quantified using the
Bradford reagent (Sigma-Aldrich China, Inc.) according to the
manufacturer's instructions. Total protein (50 µg/each lane)
was loaded and size fractionated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and was transferred to
polyvinylidene difluoride membranes (Thermo Fisher Scientific,
Inc.). The membranes were incubated with a blocking reagent of 5%
non-fat milk in PBS-Tween 20 (PBST), washed with PBST and probed
with primary antibodies (1:1,000) at 4°C overnight. The membranes
were then incubated with horseradish peroxidase-conjugated
anti-rabbit IgG secondary antibody (1:2,000; cat. no. 7074; Cell
Signaling Technology, Inc.) for 2 h at room temperature, followed
by detection with a Super Signal Enhanced Chemiluminescence kit
(Pierce Biotechnology, Inc., Rockford, IL, USA). For sequential
blotting, the membranes were stripped with Stripping Buffer (Pierce
Biotechnology, Inc.) and re-probed with appropriate antibodies.
Statistical analysis
Statistical evaluation for data analysis was
determined by unpaired Student's t-test using the Prism software,
version 5.0 (Graphpad Software, Inc., La Jolla, CA, USA). All data
are presented as the mean ± standard error of the mean. P<0.05
was considered to indicate a statistically significant
difference.
Results
miR-155 is induced by radiation and
correlated with radiation resistance
It has previously been reported that miR-155 levels
are increased in numerous types of cancer, including NSCLC, thus
suggesting that miR-155 may act as an oncomiR (4). The present study examined the role of
miR-155 during the irradiation of cancer cells. Two NSCLC cell
lines: A549 and H460, were exposed to numerous non-toxic doses of
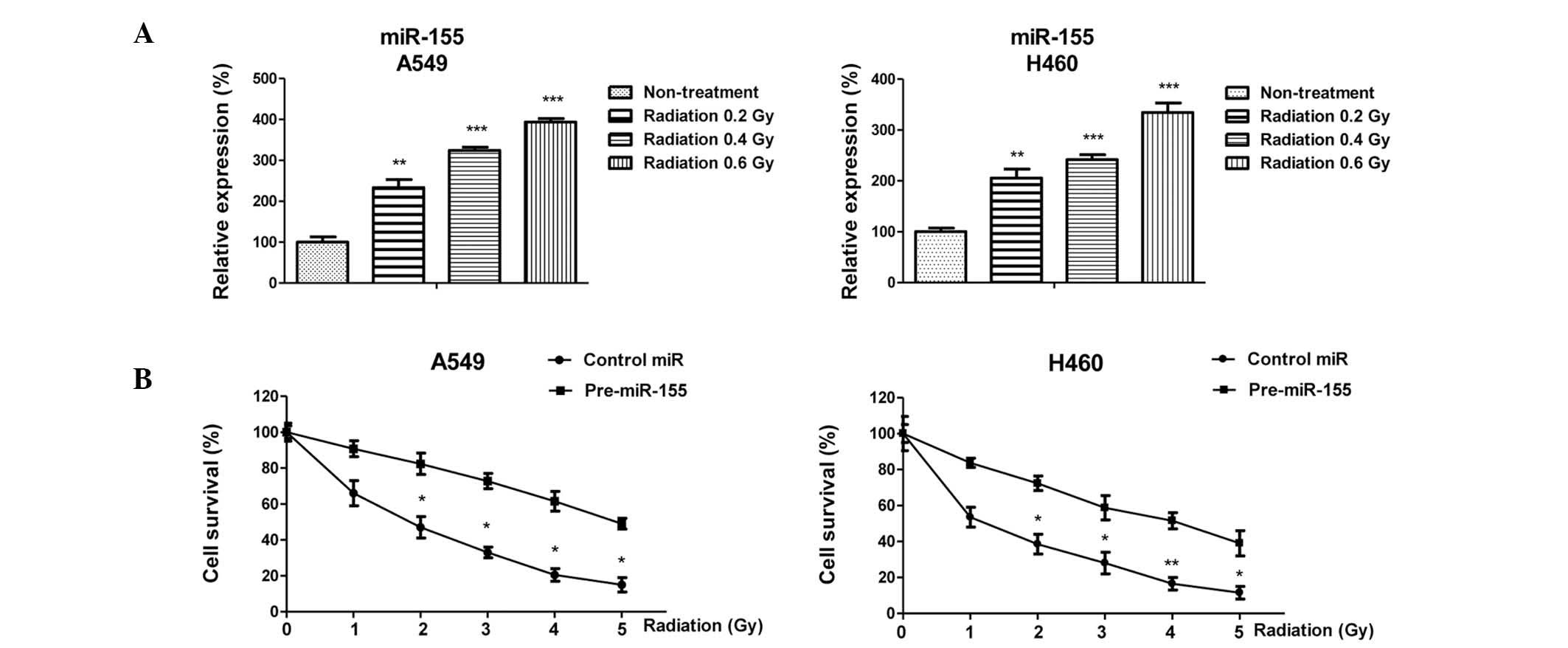
radiation. Notably, the expression levels of miR-155 were
significantly upregulated by irradiation, indicating that miR-155
may have an important role in cancer radiotherapy (Fig 1A). In addition, the present study
determined whether miR-155 was able to influence the viability of
lung cancer cells in response to radiotherapy. A549 and H460 cells
were transiently transfected with a miR-155 precursor;
overexpression of miR-155 in lung cancer cells had a
radioprotective effect on A549 and H460 cells (Fig. 1B), as compared with in cells
transfected with control miRNA. The half maximal inhibitory
concentration of control A549 or H460 cells in response to
radiotherapy was ~2 Gy, which was lower than that of the cells
overexpressing miR-155 (5 Gy). These results indicate a positive
correlation between miR-155 and radioresistance in NSCLC cells.
miR-155 promotes glycolysis of NSCLC
cells via the upregulation of HK2
The present study subsequently explored the
mechanisms underlying miR-155-mediated radioresistance. As
discussed previously, dysregulated or altered energy metabolism is
recognized as one of the hallmarks of cancer. Furthermore, it has
previously been reported that enhanced aerobic glycolysis of cancer
cells contributes to acquired radioresistance (17,18).
The present study assessed the rate of glycolysis by measuring
glucose consumption and lactate production, which are considered
the two major biochemical events of anaerobic glycolysis. As
expected, glycolysis was significantly regulated by miR-155:
Overexpression of miR-155 in A549 or H460 cells promoted glucose
consumption and lactate production, whereas inhibition of miR-155
by antisense miRNA had the opposite effect (Fig. 2A and B). These results indicate
that miR-155-mediated radioresistance may be caused by an
upregulation in glycolysis.
miR-155 upregulates the expression of
HK2
HK2 is a key enzyme of glycolysis, which catalyzes
the formation of glucose-6-phosphate from glucose (20). HK2 activity is positively
correlated with the rate of glycolysis. In the present study, HK2
was identified as an indirect target of miR-155 in NSCLC cells
(Fig. 3A and B). Overexpression of
miR-155 significantly upregulated HK2 protein expression in A549
and H460 cells. Conversely, inhibition of miR-155 downregulated HK2
expression. These results suggest a putative mechanism for the
acquired radioresistance of NSCLC cells that overexpress
miR-155.
Suppression of glycolysis by HK2
inhibitor sensitizes NSCLC cells to radiation
To investigate whether miR-155-induced glycolysis
was due to the upregulation of HK2, lung cancer cells were treated
with 3BrPA, which is a specific inhibitor of HK2. Treatment with
3BrPA significantly suppressed the HK activities of A549 and H460
cells (Fig. 4A). Furthermore, the
glucose consumption and lactate production of A549 and H460 cells
were decreased following treatment with 3BrPA (50 µM) for 24
h (Fig. 4B and C). Subsequently,
the present study examined whether inhibition of HK2 by 3BrPA was
able to sensitize lung cancer cells to radiation. A549 cells were
treated with 3BrPA (50 µM) for 24 h, and were then exposed
to various doses of radiation. As expected, A549 cells regained
sensitivity to irradiation following treatment with 3BrPA (Fig. 4D). These results suggest that
suppression of glycolysis by inhibiting HK2 may be considered a
therapeutic strategy to enhance the efficacy of radiotherapy.
Inhibition of miR-155 elevates cancer
cell sensitivity to radiation via the suppression of
glycolysis
The results of the present study revealed that
miR-155 upregulated HK2 expression, which contributed to
radioresistance. Subsequently, the radiosensitivity of lung cancer
cells was determined in response to various doses of radiation,
with or without inhibition of miR-155. As shown in Fig. 5A, inhibition of miR-155 rendered
A549 cells more sensitive to irradiation, as compared with the
cells transfected with control miRNA. These results are consistent
with the previous results that A549 cells are more sensitive to
irradiation following treatment with 3BrPA. To determine whether
inhibition of miR-155 sensitized lung cancer cells to irradiation
via the suppression of glycolysis, anti-miR-155 was transfected
into A549 cells undergoing radiation and glucose metabolism was
measured. As shown in Fig. 5B,
irradiation induced glycolysis, which is consistent with the
results of a previous study (17).
In addition, under irradiation, inhibition of miR-155 significantly
suppressed glucose metabolism (Fig.
5B), thus suggesting that miR-155-mediated radiosensitivity may
be associated with the modulation of glycolysis in lung cancer
cells.
 | Figure 5Inhibition of miR-155 sensitizes A549
cells to radiation via the suppression of glycolysis. (A) A549
cells were transfected with anti-miR-155 or control miR for 48 h,
followed by exposure to radiation at 0, 0.5, 1, 1.5, 2 or 2.5 Gy.
Subsequently, cell survival rates were determined. (B) A549 cells
were transfected with anti-miR-155 or control miR for 48 h followed
by exposure to radiation at 0 or 1 Gy. Cells were collected and
glucose consumption (left) and lactate production (right) were
measured. (C) A549 cells were transfected with control miR,
anti-miR-155, or were co-transfected with anti-miR-155 and a HK2
overexpression vector for 48 h. The cells were collected and
subjected to western blotting. β-actin was used as a loading
control. (D) A549 cells were transfected with anti-miR-155 alone,
or were co-transfected with anti-miR-155 and a HK2 overexpression
vector for 48 h, followed by exposure to radiation at 2 and 4 Gy.
Subsequently, a 3-[4,5
dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay was
performed. Data are presented as the mean ± standard error of the
mean. *P<0.05. miR, microRNA; HK2, hexokinase 2. |
Restoration of HK2 renders
miR-155-inhibited lung cancer cells resistant to irradiation
To further verify the results of the present study,
an overexpression vector containing wild type HK2 and antisense
miR-155 were co-transfected into A549 cells undergoing radiation,
control cells were transfected with a control vector (Fig. 5C). As expected, restoring the
expression of HK2 recovered the radioresistance of A549 cells in
response to radiation (Fig. 5D).
These results clearly indicate that HK2 has an important role in
lung cancer cell irradiation sensitivity, and overexpression of
miR-155 confers radioresistance at least partially via the indirect
upregulation of HK2 expression.
Discussion
The present study observed that radiation induces
the expression of miR-155, which promotes glucose metabolism via
the indirect upregulation of HK2. It has previously been reported
that miR-155 functions as an important oncomiR, which is a commonly
upregulated miRNA in solid and hematological malignancies,
including lung cancer, and is correlated with poor patient
prognosis (11). The present study
demonstrated that miR-155 is induced by radiation, and
overexpression of miR-155 in lung cancer cells contributes to
radioresistance. In addition, forced inhibition of miR-155 by
antisense transfection may render lung cancer cells sensitive to
radiation. These results suggested that miR-155 may be correlated
with radioresistance, and support the oncogenic roles of miR-155 in
NSCLC.
The present study identified an essential role for
the miR-155-HK2-glycolysis axis in the Warburg effect. Compared
with normal cells, cancer cells exhibit a metabolic switch from
oxidative metabolism to anaerobic glycolysis, even in the presence
of oxygen. This unique feature of cancer cells is known as the
Warburg effect. Activation of the glycolytic pathway is frequently
observed in radioresistant cancer cells (17,18).
Furthermore, disruption of glycolysis has been considered a
possible target for anticancer therapy. It has previously been
reported that chemo- and radioresistant cancer cells display an
elevated anaerobic glycolysis rate, as compared with parental cells
(16), thus indicating that
dysregulated glycolysis is correlated with chemo- and
radioresistance. At present, whether activation of glycolysis is
involved in miRNA regulation remains largely unknown. The present
study reported that elevated glycolysis in lung cancer cells by the
exogenous expression of miR-155 is correlated with radioresistance.
Lung cancer cells become increasingly resistant to radiation with
increased glycolysis, indicating that dysregulated glycolysis may
be a target for overcoming resistance.
Notably, miR-155 was shown to exert its role in
glycolysis via upregulation of HK2, which is a key glycolytic
enzyme responsible for catalyzing the irreversible first step of
glucose metabolism. It has previously been reported that HK2 has an
important role in controlling tumorigenesis and chemoresistance
(21). Although the indirect
regulation of HK2 by the miR-155-miR-143 cluster has been described
previously (22), the present
study investigated the novel roles of miR-155-mediated glycolysis
in radiation resistance via the upregulation of HK2. In our next
project, we will focus on the mechanisms underlying
radiation-induced miR-155 expression, and screen more
differentially regulated miRNAs involved in the radiosensitivity of
lung cancer cells. In conclusion, the results of the present study
highlight the importance of miR-155 in regulating lung cancer
radiosensitivity, and may provide potential targets for the
development of cancer therapeutic strategies.
Acknowledgments
The authors of the present study would like thank
the staff and faculty working in the Department of Respiration,
Linyi People's Hospital (Linyi, Shandong, China), including Dr Ping
Han for providing editorial assistance.
References
|
1
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng T, Wang J, Chen X and Liu L: Role of
microRNA in anticancer drug resistance. Int J Cancer. 126:2–10.
2010. View Article : Google Scholar
|
|
3
|
Ma J, Dong C and Ji C: MicroRNA and drug
resistance. Cancer Gene Ther. 17:523–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zang YS, Zhong YF, Fang Z, Li B and An J:
MiR-155 inhibits the sensitivity of lung cancer cells to cisplatin
via negative regulation of Apaf-1 expression. Cancer Gene Ther.
19:773–778. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gasparini P, Lovat F, Fassan M, Casadei L,
Cascione L, Jacob NK, Carasi S, Palmieri D, Costinean S, Shapiro
CL, et al: Protective role of miR-155 in breast cancer through
RAD51 targeting impairs homologous recombination after irradiation.
Proc Natl Acad Sci USA. 111:4536–4541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Habbe N, Koorstra JB, Mendell JT,
Offerhaus GJ, Ryu JK, Feldmann G, Mullendore ME, Goggins MG, Hong
SM and Maitra A: MicroRNA miR-155 is a biomarker of early
pancreatic neoplasia. Cancer Biol Ther. 8:340–346. 2009. View Article : Google Scholar :
|
|
7
|
Zhang GJ, Xiao HX, Tian HP, Liu ZL, Xia SS
and Zhou T: Upregulation of microRNA-155 promotes the migration and
invasion of colorectal cancer cells through the regulation of
claudin-1 expression. Int J Mol Med. 31:1375–1380. 2013.PubMed/NCBI
|
|
8
|
Li S, Chen T, Zhong Z, Wang Y, Li Y and
Zhao X: microRNA-155 silencing inhibits proliferation and migration
and induces apoptosis by upregulating BACH1 in renal cancer cells.
Mol Med Rep. 5:949–954. 2012.PubMed/NCBI
|
|
9
|
Kluiver J, Poppema S, de Jong D, Blokzijl
T, Harms G, Jacobs S, Kroesen BJ and van den Berg A: BIC and
miR-155 are highly expressed in Hodgkin, primary mediastinal and
diffuse large B cell lymphomas. J Pathol. 207:243–249. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue H, Hua LM, Guo M and Luo JM: SHIP1 is
targeted by miR-155 in acute myeloid leukemia. Oncol Rep.
32:2253–2259. 2014.PubMed/NCBI
|
|
11
|
Czyzyk-Krzeska MF and Zhang X: MiR-155 at
the heart of oncogenic pathways. Oncogene. 33:677–678. 2014.
View Article : Google Scholar :
|
|
12
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Willers H, Azzoli CG, Santivasi WL and Xia
F: Basic mechanisms of therapeutic resistance to radiation and
chemotherapy in lung cancer. Cancer J. 19:200–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Babar IA, Czochor J, Steinmetz A, Weidhaas
JB, Glazer PM and Slack FJ: Inhibition of hypoxia-induced miR-155
radiosensitizes hypoxic lung cancer cells. Cancer Biol Ther.
12:908–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Butler EB and Tan M: Targeting
cellular metabolism to improve cancer therapeutics. Cell Death Dis.
4:e5322013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong J, Rajaram N, Brizel DM, Frees AE,
Ramanujam N, Batinic-Haberle I and Dewhirst MW: Radiation induces
aerobic glycolysis through reactive oxygen species. Radiother
Oncol. 106:390–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimura T, Noma N, Sano Y, Ochiai Y,
Oikawa T, Fukumoto M and Kunugita N: AKT-mediated enhanced aerobic
glycolysis causes acquired radioresistance by human tumor cells.
Radiother Oncol. 112:302–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase-2 bound to mitochondria: Cancer's stygian link to the
“Warburg Effectˮ and a pivotal target for effective therapy. Semin
Cancer Biol. 19:17–24. 2009. View Article : Google Scholar
|
|
21
|
Suh DH, Kim MA, Kim H, Kim MK, Kim HS,
Chung HH, Kim YB and Song YS: Association of overexpression of
hexokinase II with chemoresistance in epithelial ovarian cancer.
Clin Exp Med. 14:345–353. 2014. View Article : Google Scholar
|
|
22
|
Jiang S, Zhang LF, Zhang HW, Hu S, Lu MH,
Liang S, Li B, Li Y, Li D, Wang ED and Liu MF: A novel
miR-155/miR-143 cascade controls glycolysis by regulating
hexokinase 2 in breast cancer cells. EMBO J. 31:1985–1998. 2012.
View Article : Google Scholar : PubMed/NCBI
|