Introduction
Colorectal cancer (CRC) is the second leading cause
of cancer-associated mortality in North America and Europe and the
fourth most common form of cancer worldwide (1). Adenocarcinoma cells, including CRC
cells, are markedly resistant to damage induced by antineoplastic
agents. Thus, these tumors are difficult to treat and often
proliferate rapidly under conditions that may adversely affect
normal cells. This proliferation is also as a result of the
inactivation of tumor suppressor genes, including, mutation of
cyclin-dependent kinase inhibitor 1 (p21Waf/Cif1) to
produce gene products that do not bind to cyclin, thus the
cyclin-dependent kinase remains active and cell division becomes
uncontrolled (2).
Numerous studies are focusing on the health benefits
of phytochemicals with regards to CRC [(3); also see references cited therein].
Increased levels of phenolic compounds have been detected in animal
intestine following oral administration of supplements, compared
with other tissues (4). Recent
evidence from epidemiological studies indicates that diets rich in
fruits and vegetables are associated with a decreased risk of
chronic diseases, including cancer (3). Anthocyanins are water-soluble
pigments in fruits and vegetables, responsible for red, blue, and
purple coloring. The daily intake of anthocyanins in human
populations has been shown to vary widely, with estimates between
10 and 215 mg/day. Previous studies indicate that anthocyanins
(ACNs) exhibit marked free radical scavenging and antioxidant
activities, which is key in the prevention of mutagenesis and
carcinogenesis [(5); also see
references cited therein]. Furthermore, two recent case control
studies have suggested that foods rich in anthocyanidins may be
chemopreventive in white men with esophageal adenocarcinoma
(6), and that consumption of
isoflavones, anthocyanidins, flavones, and flavonols is associated
with lower colorectal cancer risk (7). A previous study by Wang et al
(8) indicated that consumption of
black raspberry powder (at a level of 60 g daily), which is rich in
anthocyanins, exhibits a marked tumor suppressive activity against
colorectal cancer via modulating expression of genes associated
with the Wnt signaling pathway, proliferation, apoptosis, and
angiogenesis. However, the molecular mechanisms underlying the
antiproliferative effects of berry anthocyanins remain to be
elucidated.
Materials and methods
Reagents
All the reagents, unless otherwise specified, were
purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell cultures and treatments
The Caco-2 human colon carcinoma cell line and the
NIH/3T3 normal murine fibroblast cell line were obtained from the
American Type Culture Collection (Manassas, VA, USA). NIH/3T3 was
selected as it is a preneoplastic cell line, which demonstrates
unlimited, yet inhibitable, growth in vitro, however, the
cell line does not form colonies in semisolid media or grow as a
tumor in nude mice. In all the experiments, the undifferentiated
Caco-2 cells and NIH/3T3 cells were cultured in Dulbecco's modified
essential medium (DMEM) supplemented with 10% fetal bovine serum, 4
mM L-glutamine, streptomycin and penicillin and incubated at 37°C
in a humidified atmosphere with 95% air and 5% CO2.
Exponentially dividing Caco-2 cells are
undifferentiated, and differentiation is initiated at confluency
when the cells stop dividing. In the present study, Caco-2 cells
were investigated as undifferentiated, thus, they were cultured to
~80% confluency in 75 cm2 plastic flasks.
The anthocyanin (ACN) rich extract
(Medox®; Biolink Group AS, Sandnes, Norway) used in the
present study is a dietary supplement consisting of 17 purified
ACNs (all glycosides of cyanidin, peonidin, delphinidin, petunidin,
and malvidin) isolated from bilberries (Vaccinium myrtillus)
and blackcurrant (Ribes nigrum). The glycosides of cyanidin
and delphinidin form ~40–50% of the total anthocyanins (9). For all the experiments the ACN
extract was dissolved in dimethyl sulfoxide (DMSO) and used
immediately. The final concentration of DMSO in the culture medium
during the different treatments was <0.1%.
Cell proliferation assay
Caco-2 cell proliferation was measured using the
trypan blue dye exclusion assay (10). Cells were plated in 12-well cell
plates (initial density, 1×105 cells/well) and, after 24
h, were treated with the ACN extract (range, 50–500 µg/ml)
for 24 or 48 h. All wells were trypsinized with trypsin-EDTA and 20
µl of cell suspension was mixed with 20 µl trypan
blue isotonic solution (0.4% w/v) and loaded into a Bright-Line
hemacytometer (Hausser Scientific, Horsham, PA, USA) for live and
dead cell counting. Results are reported as the number of counted
viable cells.
Clonogenic assay
A clonogenic survival test (or colony formation
assay) was conducted on Caco-2 cells by plating 1×105
cells/well in 6-well plates. After 24 h, cells were treated for 24
or 48 h with the ACN extract (range, 25–100 µg/ml) and
control cells were treated with the vehicle alone (0.1% DMSO). At
the end of the treatment, fresh medium (DMEM) was added, and seven
days after seeding, the cell colonies that had formed were stained
with crystal violet (0.5% w/v in MeOH:H2O at ratio 1:1)
for 30 min, and counted using ImageJ software (imagej.nih.gov/ij/) (11). The colony-forming efficiency was
calculated as a percentage compared with the control cells.
Cytotoxicity assay
The cytotoxic effect of the ACN extract on the
Caco-2 cancer cell line and the NIH/3T3 normal cell line was
investigated using sulforhodamine B, a dye that binds to cellular
proteins, as described by Vichai and Kirtikara (12) with certain modifications. NIH/3T3
and Caco-2 cells were plated in 24-well plates at an initial
density of 5×104 cells/well and 1×105
cells/well, respectively. After 24 h, semi-confluent monolayers
were treated with the ACN extract for 24 and 48 h, while control
cells were exposed to the vehicle alone (0.1% DMSO). Cells were
fixed using 10% trichloroacetic acid for 1 h at 4°C and then washed
twice with water and incubated with sulforhodamine B (0.4% w/v in
1% acetic acid) for 30 min at room temperature, followed by four
washes with 1% acetic acid. The bound dye was solubilized in 1 ml
of 10 mM Tris base solution and the absorbance was measured at a
wavelength of 565 nm using a Shimadzu UV-1601 spectrophotometer
(Shimadzu, Japan). The 50% lethal concentration (LC50),
defined as the concentration required to kill 50% of the treated
cells compared with untreated control cells, and 95% confidence
limits were calculated using the Litchfield and Wilcoxon test
(13) [software: PHARM/PCS version
4 (MCS Consulting)].
Intracellular total antioxidant activity
(TAA)
Caco-2 and NIH/3T3 cells were plated in 6-well
plates at an initial density of 2×105 cells/well and
5×104 cells/well, respectively. After 3 days,
semi-confluent monolayers were treated with the ACN extract (range,
62.5–250 µg/ml) for 24 h while control cells were exposed to
the vehicle alone (0.1% DMSO). Following treatment, cells were
lysed in water. TAA in cell lysates was determined by decoloration
of the radical cation of
2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)
(ABTS•+), in terms of absorbance quenching at 740 nm.
This method determines the capacity of intracellular antioxidants
to quench the ABTS•+ radical; antioxidants inhibit the
reaction, leading to an absorbance decrease, and the extent of
inhibition is proportional to the antioxidant concentration in the
sample. Values obtained for each sample were compared with the
concentration-response curve of a standard Trolox solution, and
expressed as nmoles of Trolox Equivalents/mg of protein (TE nmol/mg
of protein) (14). Each analysis
was conducted in triplicate.
Intracellular reactive oxygen species
(ROS) measurement
Generation of ROS was measured by the
oxidation-sensitive fluorescent probe, dichloro-dihydro-fluorescein
diacetate (DCFH-DA), according to a modified method previously
described (15).
Caco-2 and NIH/3T3 cells were plated in 6-well
plates at an initial density of 2×105 cells/well and
5×104 cells/well, respectively. After 3 days,
semi-confluent monolayers were treated with the ACN extract (range,
62.5–250 µg/ml) for 24 h while control cells were exposed to
the vehicle alone (0.1% DMSO). Cells exposed to 50 µM
H2O2 for 1 h were used as a positive internal
control (data not shown). Cells were washed twice with Dulbecco's
phosphate-buffered saline (DPBS), and treated with 50 µmol/l
DCFH-DA at 37°C for 30 min in the dark. DCFH-DA was then removed,
and cells were washed with DPBS to remove excess probe. Cells were
collected using a scraper and resuspended in DPBS. Fluorescence was
measured using a spectrofluorometer (model RF5301PC; Shimadzu,
Japan) at excitation and emission wavelengths of 485 and 525 nm,
respectively. The total protein content was evaluated for each
sample using the Bradford assay (16), and results are reported as
percentage increase in fluorescence intensity/mg protein compared
with the NIH/3T3 control (untreated NIH/3T3 cells). Each analysis
was performed in triplicate.
Western blot analysis
Caco-2 cells were plated in 6-well plates at an
initial density of 2×105 cells/well. After 3 days,
semi-confluent monolayers were treated with the ACN extract (range,
62.5–250 µg/ml) for 24 h, and concentrations were selected
if they resulted in >15% cell death. Control cells were exposed
to the vehicle alone (0.1% DMSO). Cell lysates were prepared in
lysis buffer [10 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1% Triton
X-100, 5 mM disodium EDTA] containing protease inhibitors (1
µg/ml leupeptin, 1 mM benzamidine, 2 µg/ml aprotinin
and 1 mM dithiothreitol). Cells were sonicated for 30 sec, and the
cell lysates were stored at −70°C until use. Protein concentration
in lysates was determined using the Bradford assay (16). For immunoblot analyses, 40
µg of protein lysates/sample were denatured in SDS-PAGE
reducing sample buffer and subjected to SDS-PAGE on 16%
acryl-amide/bisacrylamide gels. Separated proteins (150 V for 3 h)
were transferred to a nitrocellulose membrane [Hybond-P
polyvinylidene fluoride (PVDF) membrane; GE Healthcare Life
Sciences, Chalfont, UK). The membrane was blocked with 5% (w/v in
Tris-buffered saline-Tween 20) non-fat milk overnight at 4°C and
then probed with specific primary antibodies: Rabbit monoclonal
anti-caspase-3 (cat. no. 9665; 1:1,500; Cell Signaling Technology,
Inc., Danvers, MA, USA) and rabbit monoclonal
anti-p21Waf1/Cip1 (cat. no. 2947; 1:1,000; Cell
Signaling Technology, Inc.). Subsequently, the membrane was
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (polyclonal; cat. no. 554021; 1:5,000; BD
Pharmingen, San Diego, CA, USA), and visualized with an Amersham
enhanced chemiluminescence (ECL) Plus detection system (GE
Healthcare Life Sciences). Blots were detected using High
Performance chemiluminescence film (Amersham Hyperfilm™ ECL; GE
Healthcare Life Sciences). The equivalent loading of proteins in
each well was confirmed by Ponceau S staining and β-actin control
(mouse anti-β-actin monoclonal antibody; cat. no. sc-47778; 1:700;
Santa Cruz Biotechnology, Inc. Santa Cruz, CA, USA).
Statistical analysis
All the experiments were performed in triplicate and
repeated three times. Results are expressed as the mean ± standard
deviation from three experiments and statistically analyzed by a
one-way or a two-way analysis of variance (ANOVA) test, followed by
Tukey's honest significant difference, using the statistical
software ezANOVA (www.cabiatl.com/mricro/ezanova/). P<0.05 was
considered to indicate a statistically significant difference.
Results
ACN extract influences Caco-2 cell growth
and viability
To investigate the antiproliferative effect of ACNs
on cancer cells, undifferentiated Caco-2 cells were treated with
ACN extract for 24 and 48 h at different concentrations (up to 500
µg/ml). As presented in Fig.
1, when compared with lower concentrations, the ACN extract
induced a significant dose-dependent decrease in Caco-2
proliferation, as indicated by a reduction in the viable cell
count, when cells were treated for 24 and 48 h (all P<0.05).
The antiproliferative effect of the ACN extract on
Caco-2 was investigated by clonogenic assay. This assay, also
referred to as a colony forming assay, determines the ability of a
cell to proliferate indefinitely, thereby retaining its
reproductive ability to form a large colony. It is widely used to
assess the efficacy of novel compounds in anticancer drug discovery
programs by determining the effects of cytotoxic and anticancer
agents on colony-forming ability (17). For the clonogenic assay, Caco-2
cells were treated for 24 or 48 h with the ACN extract at
increasing doses (range, 25 and 100 µg/ml) that produce only
a weak decrease in cell viability, and the formed cell colonies
were counted 7 days after seeding. The ACN extract inhibited the
capacity of Caco-2 to form colonies in a dose-dependent manner,
following 24 and 48 h exposure (all P<0.05; Fig. 2).
In order to characterize the cytotoxic effect of the
ACN extract, the viability of NIH/3T3 and Caco-2 cells exposed for
24 or 48 h to different concentrations of the ACN extract was
investigated. The findings from the present study indicate that the
cytotoxic activity of the ACN extract was weaker on normal cells
than on cancer cells, suggesting that tumor cells may be a
preferential target for its cytocidal effect (P<0.05; Table I).
 | Table ICytotoxic effect of anthocyanin
exposure for 24 or 48 h evaluated by sulforhodamine B assay. |
Table I
Cytotoxic effect of anthocyanin
exposure for 24 or 48 h evaluated by sulforhodamine B assay.
| Cell line | LC50 and
90% CL (µg/ml)
|
|---|
| 24 h | 48 h |
|---|
| NIH/3T3 | 1230 (CL
790–1506) | 477b (CL 364–724) |
| Caco-2 | 494a (CL 397–616) | 227a,b
(CL 186–242) |
Effect of ACN extract on intracellular
ROS levels and total antioxidant status
The effect of ACN extract treatment for 24 h on
intracellular TAA and ROS levels was examined in NIH/3T3 and Caco 2
cells.
TAA was determined in cell lysates according to the
capacity to quench the ABTS•+ radical. The ACN extract
increased intracellular TAA in a dose-dependent manner in NIH/3T3
and Caco-2 cells, although the effect was more evident in the
NIH/3T3 cells. Furthermore, the results suggest there is a
difference in the intracellular antioxidant status of the Caco-2
and NIH/3T3 cells. Caco-2 cells exhibit a lower TAA in basal
conditions, which is consistent with data supporting persistent
oxidative stress in cancer cells (all P<0.05; Table II) (18,19).
 | Table IIIntracellular TAA and ROS in NIH/3T3
and Caco-2 cells after 24 h exposure to ACN extract (62.5–250
µg/ml). |
Table II
Intracellular TAA and ROS in NIH/3T3
and Caco-2 cells after 24 h exposure to ACN extract (62.5–250
µg/ml).
| ACN extract
(µg/ml) | Intracellular TAA
(TE nmol/mg proteins)
| Intracellular ROS
(IF %/mg proteins)
|
|---|
| NIH/3T3 | Caco-2 | NIH/3T3 | Caco-2 |
|---|
| 0 | 424±54 | 228±3d | 100 | 135±15a,d |
| 62.5 | 542±29a | 236±3a,d | 66±2a | 235±25a,d |
| 125 | 592±55a | 268±5a,b,d | 46±13a,b | 299±20a,b,d |
| 250 | 620±39a,b | 285±7a–d | we29±15a–c | 310±25a,b,d |
ROS levels were measured by DCFH-DA assay, which
produces a fluorescence intensity that is proportional to the level
of intracellular oxidant species. ACN extract treatment induced a
significant and dose-dependent decrease of basal ROS levels in
normal NIH/3T3 fibroblasts (P<0.05; Table II). Notably, the same treatment
induced instead a dose-dependent increase of ROS levels in Caco-2
cancer cells, which already have a higher basal ROS level compared
with NIH/3T3 cells (P<0.05; Table
II).
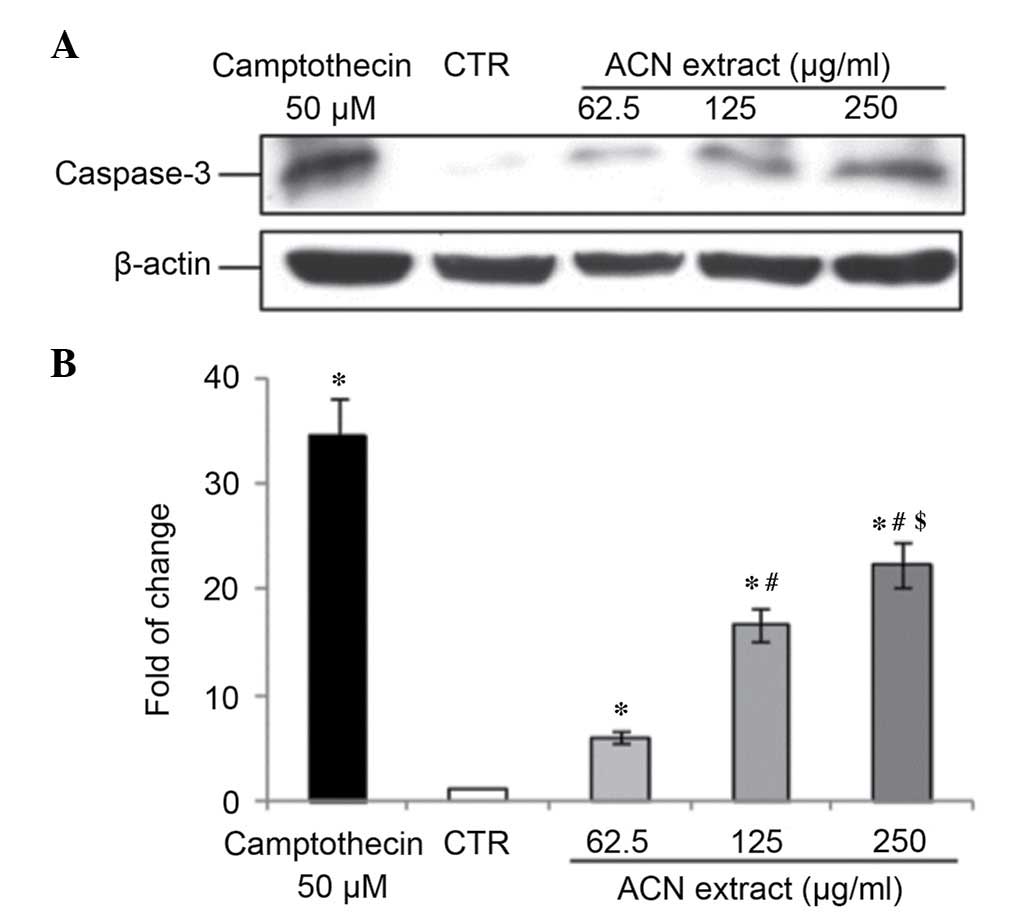
ACN extract induces apoptosis in Caco-2
cells
Caspase-3 is a cytosolic protein that exists as a
higher molecular weight inactive precursor (pro-caspase-3). It is
proteolytically cleaved into a low molecular weight (17 kDa) active
enzyme when cells undergo apoptosis (20). To determine whether caspase-3 is
involved in this process in Caco-2 cells treated with ACN, a
western blot analysis was performed to verifying caspase-3 cleavage
status. Results from the present study indicated that the ACN
extract induced caspase-3 activation in a dose-dependent manner
after 24 h of treatment (all P<0.05; Fig. 3).
ACN extract modulates cell
cycle-associated proteins in Caco-2 cells
The unlimited replication potential of cancer cells
is a result of the inactivation of tumor suppressor genes.
p21Waf1/Cip1 is a CDK2 and CDK1 inhibitory protein
(21) transcriptionally regulated
by p53, which results in G1-phase cell cycle arrest
(22). In addition,
p21Waf1/Cip1 directly inhibits proliferative cell
nuclear antigen (PCNA)-dependent DNA replication and modulates the
PCNA-dependent DNA repair system (2). Results from the present study
indicated that p21Waf1/Cip1 expression was low in
undifferentiated Caco-2 cells, in agreement with data reported by
Zhang et al (23).
Treatment with the ACN extract (at doses 62.5–250 µg/ml) for
24 h upregulated p21Waf1/Cip1 expression levels in a
dose-dependent manner (Fig.
4).
Discussion
Naturally occurring micronutrients present in the
diet are being investigated for their potential as cancer
chemopreventive agents. In particular, epidemiological studies have
shown that the consumption of fruits and vegetables, rich in
polyphenols, is associated with reduced colorectal cancer risk
(24,25). Anthocyanins, a widely distributed
class of polyphenols, have been reported for their wide variety of
biological functions and antioxidant activity, which has been
associated with the modulation of carcinogenesis. The intake of
anthocyanins, estimated to be between 10 and 215 mg/day depending
on the specific dietary profiles of the population, is markedly
higher than the 20–25 mg/day range reported for other flavonoids
including quercetin and genistein (26). This indicates the intestinal
epithelium is exposed to high concentrations of anthocyanins each
day.
In the present study, a commercially available
extract from bilberries and blackcurrants, rich in anthocyanins, is
shown to inhibit the growth of colon cancer cells. Furthermore, the
cell growth inhibition of anthocyanins in malignant cells may be
due to induction of ROS accumulation in these cells, which
interfere with basic cellular functions, including cell cycle
progression and apoptosis.
The results of the present study demonstrated that
the ACN extract markedly induced a dose-dependent decrease in
Caco-2 cell proliferation, as evidenced by a reduction in the
viable cell count and colony formation when cells were exposed to
the ACN extract for 24 and 48 h. The results from the current study
are consistent with previous studies regarding the
antiproliferative efficacy of berry anthocyanins against primary
and metastatic colorectal cancer cell lines (27–32).
The effect observed in the present study was evident at low
concentrations (25–50 µg/ml), which are easily reachable
into the colon tissue.
It is well documented that ROS are involved in
multiple signaling cascades associated with various behaviors in
cancer cells, including survival, proliferation, angiogenesis, and
metastasis. ROS are thus considered oncogenic and may be
responsible for the initiation, development, progression, invasion,
and metastasis of cancer. ROS may promote cellular proliferation
and contribute to cancer development via numerous signaling
pathways (33,34). As discussed in the 'persistent
oxidative stress in cancer' hypothesis, cancer cells generally have
an increased inherent ROS level close to the cell death-triggering
threshold and, in addition, tumor cells may also have an impaired
antioxidant system. Thus, the molecular mechanisms associated with
the antiproliferative effects of ACN were investigated, assuming a
possible modulation of intracellular redox status and ROS levels.
NIH/3T3 cells (a rapid proliferating normal cell line) and
undifferentiated Caco-2 cells were exposed for 24 h to different
concentrations of the ACN extract, and the intracellular ROS levels
and total antioxidant power were evaluated. As presented in
Table II, intracellular
antioxidant power, at a basal level, was lower in Caco-2 cancer
cells compared with normal NIH/3T3 fibroblasts, while intracellular
ROS levels being higher in Caco-2 cells is in agreement with the
above-mentioned 'persistent oxidative stress in cancer cells'
hypothesis. In the current study, the treatment with ACN extract
for 24 h ameliorated the intracellular TAA, and the effect was more
evident in normal NIH/3T3 cells compared with Caco-2 cells.
Notably, the ACN extract treatment induced ROS accumulation in
malignant cells in a dose-dependent manner, and reduced the level
of ROS in normal NIH/3T3 cells.
These results indicate that the accumulation of ROS
is an underlying mechanism involved in the cytotoxic effect of the
ACN extract on colon cancer cells. The results from the present
study further support previous studies demonstrating that numerous
plant-derived products induce an antiproliferative effect via
cellular ROS generation (34,35).
Furthermore, Noda et al (36) demonstrated that, in Caco-2 cells,
the addition of N-acetyl-cysteine (a well known antioxidant)
restored the intracellular redox balance and, correspondingly, cell
proliferative activity.
Dietary compounds have been demonstrated to affect
molecular events involved in the initiation, promotion, and
progression of cancer, thereby inhibiting carcinogenesis. Apoptosis
is one of the most important mechanisms for anti-tumor activity,
and cancer cells differ from normal cells due to the unlimited
replication potential and the absence of apoptosis. The current
study demonstrated that ACN extract induces apoptosis by activating
caspase-3 cleavage in a dose-dependent manner. These results are
supported by previous in vitro studies demonstrating that
anthocyanins exhibit pro-apoptotic effects in multiple colorectal
cancer cell types in vitro via the caspase-dependent
signaling pathways (27,29–32).
A major process in cellular transformation is the
loss of control over the mammalian cell cycle.
p21Waf1/Cip1 promotes cell cycle arrest in response to
multiple stimuli, it acts as a sensor and an effector of multiple
antiproliferative signals. p21Waf1/Cip1 has exhibited
additional and fundamental effects in other important signaling
pathways, including regulation of transcription, apoptosis, and DNA
repair. Targeting this protein kinase using natural products may be
a promising approach for development of anti-cancer therapeutic
agents (37). In the present
study, p21Waf1/Cip1 expression was low in
undifferentiated Caco-2 cells, in agreement with data reported by
Zhang et al (23).
Furthermore, in vivo loss of p21Waf1/Cip1 control
represents a major alteration in cancer cells, which is frequently
associated with carcinogenesis increase (38). Notably, the treatment with the ACN
extract (range, 62.5–250 µg/ml) for 24 h upregulated
p21Waf1/Cip1 expression in a dose-dependent manner.
Similarly, an ACN extract from chokeberries inhibited HT-29 cell
growth via a marked increase in the expression levels of the
p21Waf1/Cip1 gene (28).
In conclusion, the present study suggests exposure
to an ACN extract may promote apoptosis in colon cancer cells with
high quantities of ROS, by further increasing ROS accumulation and
modulating expression of tumor suppressor genes. Although the use
of nutritional antioxidants for cancer therapy is complex and
requires further research prior to clinical use, the results of the
present study suggest a possible chemotherapeutic role of berry ACN
extract in treatment of colon cancer and aids in elucidation of the
complex cellular mechanisms via which dietary molecules, including
ACNs, may inhibit the proliferation of cancer cells.
References
|
1
|
Boyle P and Langman JS: ABC of colorectal
cancer: Epidemiology. BMJ. 321:805–808. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waga S, Hannon GJ, Beach D and Stillman B:
The p21 inhibitor of cyclin-dependent kinases controls DNA
replication by interaction with PCNA. Nature. 369:574–578. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riboli E and Norat T: Epidemiologic
evidence of the protective effect of fruit and vegetables on cancer
risk. Am J Clin Nutr. 78(3 Suppl): 559S–569S. 2003.PubMed/NCBI
|
|
4
|
Manach C, Scalbert A, Morand C, Rémésy C
and Jiménez L: Polyphenols: Food sources and bioavailability. Am J
Clin Nutr. 79:727–747. 2004.PubMed/NCBI
|
|
5
|
Domitrovic R: The molecular basis for the
pharmacological activity of anthocyans. Curr Med Chem.
18:4454–4469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bobe G, Peterson JJ, Gridley G, Hyer M,
Dwyer JT and Brown LM: Flavonoid consumption and esophageal cancer
among black and white men in the United States. Int J Cancer.
125:1147–1154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rossi M, Negri E, Talamini R, Bosetti C,
Parpinel M, Gnagnarella P, Franceschi S, Dal Maso L, Montella M,
Giacosa A and La Vecchia C: Flavonoids and colorectal cancer in
Italy. Cancer Epidemiol Biomarkers Prev. 15:1555–1558. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang LS, Arnold M, Huang YW, Sardo C,
Seguin C, Martin E, Huang TH, Riedl K, Schwartz S, Frankel W, et
al: Modulation of genetic and epigenetic biomarkers of colorectal
cancer in humans by black raspberries: A phase I pilot study. Clin
Cancer Res. 17:598–610. 2011. View Article : Google Scholar :
|
|
9
|
Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H,
Cao L and Ling W: Anthocyanin supplementation improves serum LDL-
and HDL-cholesterol concentrations associated with the inhibition
of cholesteryl ester transfer protein in dyslipidemic subjects. Am
J Clin Nutr. 90:485–492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cimino F, Speciale A, Siracusa L, Naccari
C, Saija A, Mancari F, Raciti R, Cristani M and Trombetta D:
Cytotoxic effects induced in vitro by organic extracts from urban
air particulate matter in human leukocytes. Drug Chem Toxicol.
37:32–39. 2014. View Article : Google Scholar
|
|
11
|
Schindelin J, Arganda-Carreras I, Frise E,
Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S,
Schmid B, et al: Fiji: An open-source platform for biological-image
analysis. Nat Methods. 9:676–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vichai V and Kirtikara K: Sulforhodamine B
colorimetric assay for cytotoxicity screening. Nat Protoc.
1:1112–1116. 2006. View Article : Google Scholar
|
|
13
|
Litchfield JT and Wilcoxon FA: A
simplified method of evaluating dose-effect experiments. J
Pharmacol Exp Ther. 96:99–113. 1949.PubMed/NCBI
|
|
14
|
Cimino F, Speciale A, Anwar S, Canali R,
Ricciardi E, Virgili F, Trombetta D and Saija A: Anthocyanins
protect human endothelial cells from mild hyperoxia damage through
modulation of Nrf2 pathway. Genes Nutr. 8:391–399. 2013. View Article : Google Scholar :
|
|
15
|
Guo R, Li W, Liu B, Li S, Zhang B and Xu
Y: Resveratrol protects vascular smooth muscle cells against high
glucose-induced oxidative stress and cell proliferation in vitro.
Med Sci Monit Basic Res. 20:82–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fiebig HH, Maier A and Burger AM:
Clonogenic assay with established human tumour xenografts:
Correlation of in vitro to in vivo activity as a basis for
anticancer drug discovery. Eur J Cancer. 40:802–820. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kondo S, Toyokuni S, Iwasa Y, Tanaka T,
Onodera H, Hiai H and Imamura M: Persistent oxidative stress in
human colorectal carcinoma, but not in adenoma. Free Radic Biol
Med. 27:401–410. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perse M: Oxidative stress in the
pathogenesis of colorectal cancer: Cause or consequence? Biomed Res
Int. 2013:7257102013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shalini S, Dorstyn L, Dawar S and Kumar S:
Old, new and emerging functions of caspases. Cell Death Differ.
22:526–539. 2015. View Article : Google Scholar :
|
|
21
|
Satyanarayana A, Hilton MB and Kaldis P:
p21 inhibits Cdk1 in the absence of Cdk2 to maintain the G1/S phase
DNA damage checkpoint. Mol Biol Cell. 19:65–77. 2008. View Article : Google Scholar :
|
|
22
|
Waldman T, Kinzler KW and Vogelstein B:
p21 is necessary for the p53-mediated G1 arrest in human cancer
cells. Cancer Res. 55:5187–5190. 1995.PubMed/NCBI
|
|
23
|
Zhang X, Min KW, Wimalasena J and Baek SJ:
Cyclin D1 degradation and p21 induction contribute to growth
inhibition of colorectal cancer cells induced by
epigallocatechin-3-gallate. J Cancer Res Clin Oncol. 138:2051–2060.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang ZJ, Ohnaka K, Morita M, Toyomura K,
Kono S, Ueki T, Tanaka M, Kakeji Y, Maehara Y, Okamura T, et al:
Dietary polyphenols and colorectal cancer risk: The Fukuoka
colorectal cancer study. World J Gastroenterol. 19:2683–2690. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jedrychowski W, Maugeri U, Popiela T,
Kulig J, Sochacka-Tatara E, Pac A, Sowa A and Musial A:
Case-control study on beneficial effect of regular consumption of
apples on colorectal cancer risk in a population with relatively
low intake of fruits and vegetables. Eur J Cancer Prev. 19:42–47.
2010. View Article : Google Scholar
|
|
26
|
Speciale A, Cimino F, Saija A, Canali R
and Virgili F: Bioavailability and molecular activities of
anthocyanins as modulators of endothelial function. Genes Nutr.
9:4042014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu J, Zhang W, Jing H and Popovich DG:
Bog bilberry (Vaccinium uliginosum L.) extract reduces cultured
Hep-G2, Caco-2, and 3T3-L1 cell viability, affects cell cycle
progression, and has variable effects on membrane permeability. J
Food Sci. 75:H103–H107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malik M, Zhao C, Schoene N, Guisti MM,
Moyer MP and Magnuson BA: Anthocyanin-rich extract from Aronia
meloncarpa E induces a cell cycle block in colon cancer but not
normal colonic cells. Nutr Cancer. 46:186–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lazzè MC, Savio M, Pizzala R, Cazzalini O,
Perucca P, Scovassi AI, Stivala LA and Bianchi L: Anthocyanins
induce cell cycle perturbations and apoptosis in different human
cell lines. Carcinogenesis. 25:1427–1433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yi W, Fischer J, Krewer G and Akoh CC:
Phenolic compounds from blueberries can inhibit colon cancer cell
proliferation and induce apoptosis. J Agric Food Chem.
53:7320–7329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Renis M, Calandra L, Scifo C, Tomasello B,
Cardile V, Vanella L, Bei R, La Fauci L and Galvano F: Response of
cell cycle/stress-related protein expression and DNA damage upon
treatment of CaCo2 cells with anthocyanins. Br J Nutr. 100:27–35.
2008. View Article : Google Scholar
|
|
32
|
Cvorovic J, Tramer F, Granzotto M,
Candussio L, Decorti G and Passamonti S: Oxidative stress-based
cytotoxicity of delphinidin and cyanidin in colon cancer cells.
Arch Biochem Biophys. 501:151–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Loo G: Redox-sensitive mechanisms of
phytochemical-mediated inhibition of cancer cell proliferation
(review). J Nutr Biochem. 14:64–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sorrenti V, Vanella L, Acquaviva R,
Cardile V, Giofrè S and Di Giacomo C: Cyanidin induces apoptosis
and differentiation in prostate cancer cells. Int J Oncol.
47:1303–1310. 2015.PubMed/NCBI
|
|
36
|
Noda T, Iwakiri R, Fujimoto K and Aw TY:
Induction of mild intracellular redox imbalance inhibits
proliferation of CaCo-2 cells. FASEB J. 15:2131–2139. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Neto CC, Amoroso JW and Liberty AM:
Anticancer activities of cranberry phytochemicals: An update. Mol
Nutr Food Res. 52(Suppl 1): S18–S27. 2008.PubMed/NCBI
|
|
38
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View Article : Google Scholar : PubMed/NCBI
|