Introduction
At present, superficial bladder cancer is stratified
by differentiation grade and stage into three groups of different
risk profiles (Ta G1-2 vs. T1 G1-2 vs. Tis/T1 G3). At present, the
standard therapy is fractionated transurethral resection (1). Although the rate of mortality with
this therapy is low, the recurrence rates are 50–70%, of which
10–20% cases develop into muscle-infiltration bladder cancer, and
the five-year survival rate was less than 50% (2–6). It
is necessary to have post-operative therapy and closely follow up
patients with bladder cancer following surgery. Intravesical
therapy is an effective measure used to reduce the recurrence rate
and the aggression of bladder cancer. When administered to high
risk patients with non-infiltration bladder tumors, it was
previously reported that intravesical Bacillus Calmette-Guerin
vaccine exhibited in a clear treatment effect and a reduced
recurrence rate of bladder cancer, however had no effect of
reducing tumor aggression (7,8). In
addition, bladder tumors that are resistant to this vaccine are
more likely to exhibit recurrence and develop into infiltrated
tumors (9). Thus, it was
considered important to investigate novel treatment strategies in
intravesical therapy in the current study.
When a novel p53-inducible gene was identified, a
target gene that was specifically expressed in the brain and that
inhibited in vivo neovascularization induced by basic
fibroblast growth factor (bFGF) in the rat cornea was additionally
identfied, which was named brain-specific angiogenesis inhibitor-1
(BAI-1) (10). However, it has now
been observed that BAI-1 is present not only in brain tissue,
however additionally in the colon, stomach, lung and pancreas.
Notably, Fukushima et al (11) demonstrated that the levels of BAI-1
were markedly lower in colon cancer tissue samples when compared
with normal colon tissues, and that there was a correlation between
BAI-1 levels and malignancy of the tumor. Izutsu et al
(12) additionally identified that
BAI-1 was present in renal cell carcinoma samples, and that the
BAI-1 levels were increased in normal renal tissue compared with
renal cell cancer tissue. BAI-1 encodes a seven-span transmembrane
protein, containing five thrombospondin type-1 (TSP-1) repeats that
inhibited in vivo neovascularization induced by bFGF through
interactions between its receptors and CD36 (13). In the current study, the effects of
BAI-1 plasmid transfection on T24 cells and human umbilical vein
endothelial cells (HUVECs) were investigated, with the aim to
provide experimental evidence that would aid in the development of
novel therapeutic targets for the treatment of bladder cancer.
Materials and methods
Reagents and chemicals
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was purchased from EMD Millipore (Billerica, MA,
USA). Spectrophotometer, flow cytometer, and
micro-spectrophotometer were purchased from Beckman Coulter, Inc.
(Brea, CA, USA). The fluorescence microscope was purchased from
Olympus (CX31; Olympus Corporation, Tokyo, Japan). The polyclonal
rabbit anti-BAI-1 (1:200; ab135907), polyclonal rabbit anti-β-actin
(1:200; ab8227), goat anti-rabbit secondary antibody (1:1,000;
ab97080) were obtained from Abcam (Cambridge, UK). All other
chemicals were of analytical grade and obtained from Sigma-Aldrich
(St. Louis, MO, USA).
Establishment of the p-Receiver-M61-BAI-1
plasmid
According to the design principles of establishing
an open reading frame plasmid, the NCBI website was searched for
BAI-1 mRNA (NM-001701). The mRNA length of BAI-1 was 5,535 bp, and
a BAI-1 plasmid labelled with green fluorescent protein was
established on the basis of BAI-1 primer sequences outlined by Kudo
et al (14).
0BAI-1-siR-Top,
GGACTTTAGAAGCCGTTGCTGCCCTCTCTGTCACCTGAAGCGGGGCCCTCTCCCATCCCA;
BAI-1-siR-Bot,
ATTTTTTCTCTCCTTTTCTTTTCTTCAATAAAAAGAATTAAAAACCCAAAAAAAA. BAI-1,
forward 5′-GCG GTA GGC GTG TAC GGT-3′ and reverse 5′-AGC
AGTCCCCAAGTCAGT-3′. The concentration of the plasmid was detected
using a micro-spectrophotometer, pReceiver-M61-BAI-1 plasmid
concentration was 180 ng/µl. The expression of
pReceiver-M61-BAI-1 was analyzed using agarose gel electrophoresis.
The electrophoretogram indicated that sequences of recombinant
plasmid pReceiver-M61-BAI-1 were as expected and the plasmid had
been established correctly.
Transfection of p-Receiver-M61-BAI-1 into
T24 cells and HUVECs
T24 human superficial bladder tumor cells and HUVECs
were provided by the Tianjin Institute of Department of Urinary
Surgery (Tianjin, China). The HUVECs and T24 cells were grown until
they reached the logarithmic phase. All cells were seeded into
6-well plates and cultured in a humidified incubator at 37°C under
conditions of 5% CO2. Cells were prepared for
transfection subsequent to reached 50–60% confluence. T24 cells and
HUVECs were divided into the pReceiver-M61-BAI-1 group and
p-Receiver-M61 control vector group, and each group had three
identical wells. RPMI 1640 medium containing 10% fetal calf serum
(1 ml) was added into all wells 8 h subsequent to transfection,
then the cells were cultured in a humidified incubator at 37°C
under 5% CO2 for 15 h. Each well was photographed,
subsequent to which the cells were collected after 48 h. Protein
and gene expression levels of BAI-1 were detected by western blot
analysis and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis.
RT-qPCR
Total RNA was reverse transcribed to cDNA by a
Reverse Transcription kit (Roche Diagnostics, Basel Switzerland).
RT-qPCR was performed using an Applied Biosystems 7900HT thermal
cycler, with a 20 µl PCR reaction mixture containing 10
µl of 2X LightCycler 480 SYBR Green I Master mix (Roche
Diagnostics). The following primers were used: BAI-1, forward
5′-CCGCTGTGTTTCCATTGACTA-3′ and reverse
5′-ACCACAAACACGGATGCTTCA-3′; glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), forward 5′-GAAGGTCGGAGTCAACGGAT-3′ and
reverse 5′-CTGGAAGATGGTGATGGGATT-3′. The qPCR cycling conditions
were as follows: 95°C for 10 min followed by 40 cycles of 95°C for
10 sec, 58°C for 20 sec and 72°C for 20 sec, followed by 72°C for 5
min. qPCR was used to measure the gene expression levels of BAI-1.
The results were normalized using the 2−ΔΔCq method
(15).
MTT assay to detect the effects of
BAI-1
Group A, Normal T24 cells and HUVECs cultured for
12, 48 and 72 h; group B, T24 cells and HUVECs with pReceiver-M61
cultured for 12, 48 and 72 h; group C, T24 cells and HUVECs with
pReceiver-M61-BAI-1 cultured for 12, 48 and 72 h. HUVECs and T24
cells in the logarithmic growth phase were collected and made into
a cell suspension with 10% fetal calf serum (Sigma-Aldrich, St.
Louis, MO, USA), then were plated into 96-well plates
(5×103/well) in a humidified incubator overnight at 37°C
under 5% CO2. Subsequent to 12, 48 and 72 h incubation,
10 µl MTT solution/well was added, and culture was continued
for 4 h. Subsequently, 100 µl dimethyl sulfoxide was added,
then the contents of the wells were dissolved using a vibrating
machine for 10 min. The optical density (OD) value of each well was
detected by an enzyme-linked determining instrument. Each
experiment was repeated three times. Inhibition rate = (control
group OD − experiment group OD)/(control group OD − blank group OD)
× 100%.
Flow cytometry assay to detect T24 cell
and HUVEC apoptosis subsequent to transfection
Cells in the logarithmic growth phase were plated
into 6-well plates overnight and transfected with the BAI-1
plasmid. Groups were as the same as for those used in the MTT
assay. Susbequent to culture for 12, 48 and 72 h, 1×106
cells were collected and centrifuged at 300 × g for 5 min at room
temperature, followed by being washed twice with phosphate-buffered
saline. Subsequent to fixing in cold 70% ethanol, the plates were
detected by flow cytometry.
Western blot analysis
T24 cells and HUVECs were transfected with
pReceiver-M61-BAI-1. The cell lysates were cleared by
centrifugation at 12,000 × g for 30 min at 4°C. The harvested cells
were suspended in phohsphate-buffered saline containing protease
and phosphatase inhibitors and homogenized. The homogenates were
centrifuged at 14,000 rpm for 40 min at 4°C, and the resultant
supernatant fractions were used for immunoblotting. A bicinchoninic
acid assay was used to quantify the protein. Samples containing 50
µg of protein were separated by 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis, transferred to nitrocellulose
membranes (Bio-Rad Laboratories, Hercules, CA, USA). Following
blocking with 5% (w/v) non-fat dry milk in Tris-buffered saline and
0.1% (w/v) Tween 20 (TBST), the membranes were incubated with the
following antibodies: BAI-1 and β-actin (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), at 1:200 at 4°C overnight. Following three
washes with TBST, membranes were incubated the secondary antibody
for 1 h at room temperature. Western blots were quantified with
HP-Scanjet 550c and analyzed by UN-SCAN-IT software (Silk
Scientific, Orem, UT, USA).
Statistical analysis
Statistical analysis was performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA) and all
results were presented as the mean ± standard deviation. Student's
t-test was used to compare data between the groups and the
χ2 test was used for two-sample comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
Western blotting analysis to detect the
protein expression levels of BAI-1 in T24 cells and HUVECs
pReceiver-M61-BAI-1 and pReceiver-M61 labelled with
green fluorescent protein were transfected into T24 cells and
HUVECs (Fig. 1). Plasmids were
identified to have been successfully transfected through the
detection of the protein expression levels of BAI-1 in T24 cells
and HUVECs subsequent to transfection with pReceiver-M61-BAI-1. T24
cells and HUVECs transfected with p-Receiver-M61-BAI-1 were
identified to express BAI-1 protein, however no expression of BAI-1
was observed in T24 cells and HUVECs transfected with pReceiver-M61
(Fig. 2).
qPCR assay to detect the gene expression
of BAI-1 subsequent to transfection in T24 cells and HUVECs
Plasmids were identified to have been successfully
transfected through the detection of the gene expression of BAI-1
in T24 cells and HUVECs subsequent to transfection with
pReceiver-M61-BAI-1 (Fig. 3).
Effect of BAI-1 on the proliferation of
T24 cells and HUVECs
Subsequent to transfection of BAI-1, it was
identified that BAI-1 inhibited the proliferation of HUVECs. In
addition, it was demonstrated that the longer the transfection
duration, the greater the inhibition rate was (P<0.01; Table I; Fig.
4), however, there was no significant difference prior and
subsequent to transfection in T24 cells (P=0.274; Table II; Fig. 5). Furthermore, there was no
significant difference observed between normal HUVECs and HUVECs
transfected with pReceiver-M61. These results suggest that BAI-1
significantly inhibited growth of HUVECs, however with no clear
effect on T24 cells.
 | Table IOD values at different time-points
subsequent to plasmid transfection into human umbilical vein
endothelial cells. |
Table I
OD values at different time-points
subsequent to plasmid transfection into human umbilical vein
endothelial cells.
| Time | n | Group
| Sum | F-value | P-value |
|---|
| BAI-1 | Negative plasmid | Normal |
|---|
| 12 h | 48 | 0.39±0.068 | 0.35±0.062 | 0.49±0.070 | 0.41±0.086 | | |
| 48 h | 48 | 0.16±0.016 | 0.33±0.057 | 0.46±0.071 | 0.32±0.136 | | |
| 72 h | 48 | 0.09±0.022 | 0.40±0.068 | 0.52±0.060 | 0.34±0.190 | | |
| | | | | | 12.523 | 0.000 |
| Sum | 144 | 0.22±0.138 | 0.36±0.068 | 0.49±0.069 | 0.36±0.149 | 91.934 | 0.000 |
 | Table IIOD values at different time-points
subsequent to plasmid transfection into T24 cells. |
Table II
OD values at different time-points
subsequent to plasmid transfection into T24 cells.
| Time | n | Group
| Sum | F-value | P-value |
|---|
| BAI-1 | Negative plasmid | Normal |
|---|
| 12 h | 48 | 0.83±0.163 | 0.80±0.111 | 0.79±0.121 | 0.81±0.132 | | |
| 48 h | 48 | 0.84±0.155 | 0.84±0.102 | 0.88±0.119 | 0.85±0.126 | | |
| 72 h | 48 | 0.90±0.142 | 0.85±0.163 | 0.98±0.227 | 0.91±0.185 | | |
| | | | | | 5.185 | 0.007 |
| Sum | 144 | 0.86±0.153 | 0.83±0.127 | 0.88±0.178 | 0.86±0.154 | 1.308 | 0.274 |
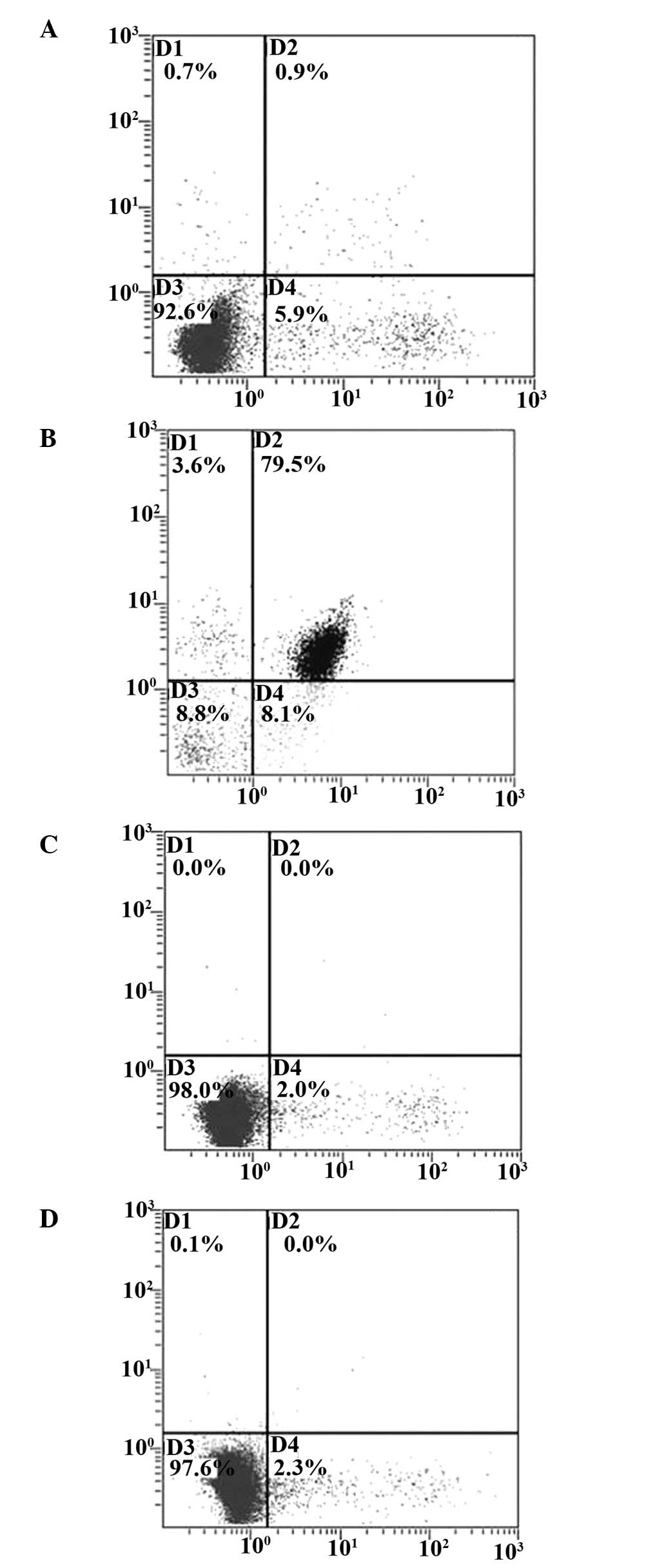
Detection of cell apoptosis subsequent to
transfection with BAI-1 in T24 cells and HUVECs
The pReceiver-M61 and pReceiver-M61-BAI-1 plasmids
were transfected into T24 cells and HUVECs, then were detected by
flow cytometry. Flow cytometry results indicated that BAI-1
resulted in an increase in HUVEC apoptosis 72 h subsequent to
transfection, however no clear effect was observed in T24 cells
(Fig. 6).
The apoptotic rate of HUVECs transfected with
pReceiver-M61-BAI-1 was 79.5% 72 h subsequent to transfection,
however the apoptotic rate of T24 cells transfected with
pReceiver-M61-BAI-1 was 0.9%. No significant difference in the
apoptotic rates of HUVECs transfected with pReceiver-M61 and T24
cells transfected with pReceiver-M61 was observed.
Discussion
BAI-1 is located in 8q24.3, is 80.99 kb and contains
30 exons and a minimum of one functional p53-binding site within an
intron. BAI-1 encodes a 1,584-amino-acid product (10) and is a member of the adhesion-G
protein-coupled receptor (GPCR) family of receptors (16). In vitro, TSP-1 has been
identified to inhibit the migration of endothelial cells and
angiogenesis mediated by CD36 (17,18).
Dawson et al (19)
identified that IgG antibodies against CD36 and
glutathione-S-trans-ferase-CD36 fusion proteins that contain the
TSP-1 binding site blocked the ability of intact TSP-1 and its
active peptides to inhibit the migration of cultured microvascular
endothelial cells. In addition, transfection of CD36-deficient
HUVECs with a CD36 expression plasmid resulted in them becoming
sensitive to TSP-1 inhibition of migration and tube formation.
Thus, TSP-1 repeats of BAI-1 had obviously effect of inhibition on
proliferation of vascular endothelial cells. Hatanaka et al
(20) examined gene expression of
BAI-1 in 48 lung adenocarcinoma specimens by qPCR and vascular
density was detected by immunohistochemistry using the anti-CD34
monoclonal antibody. They confirmed that BAI-1 gene expression was
detected in 38 out of the 48 pulmonary adenocarcinoma samples
(79.2%), and the vascular number and measurement area were
significantly reduced in the BAI-1-positive pulmonary
adenocarcinoma samples (19.3+/−4.4/µm2 and
1.7+/−0.6%) as compared with those in the BAI-1-negative carcinomas
(75.5+/−42.7/µm2 and 5.5+/−1.5%). These results
indicated that BAI-1 expression may inhibit stromal vascularization
in lung adenocarcinomas, however how angiogenesis is inhibited
remains unclear. Furthermore, Yoon et al (21) demonstrated that the extracellular
region of BAI-1 (BAI-1-ECR) could inhibit angiogenesis. Rabbits
were injected with the BAI-1-ECR gene or empty vector two or three
times at 1 week intervals beginning 1 week subsequent to
debridement and the results indicated that BAI-1-ECR gene delivery
effectively reduced experimental corneal neovascularization. In
addition, Kaur et al (22)
demonstrated that BAI-1 was proteolytically cleaved at a conserved
GPCR proteolytic cleavage site, releasing its 120 kDa extracellular
domain. This secreted fragment was termed vasculostatin as it
inhibited migration of endothelial cells in vitro and
markedly reduced in vivo angiogenesis. The site of
hydrolysis was the site of proteolytic cleavage in conservative
GPCRs (23). However, it remains
unclear which enzymes are able to recognize this site; with a
previous study indicating that BAI-1 provides a site to perform
proteolytic processing and release proteins that inhibit
angiogenesis. Previous studies identified that there was an
association between tumor growth and the concentration of vascular
inhibiting fragments; in addition, vascular inhibiting fragments
were observed to be associated with tumor prevention.
Therefore, it is suggested that BAI-1 may be
considered as a tumor suppressor gene and has been demonstrated to
exhibit low expression in cancerous tissues. In the current study,
the BAI-1 over-expression plasmid was transfected into T24 cells
and HUVECs in order to observe the alterations to T24 cells and
HUVECs. pReceiver-M61 was a eukaryotic expression vector labelled
with green fluorescence. Transfection efficiency was calculated
through detection of fluorescent intensity. The results indicated
that green fluorescent protein expression was disperse in T24 cells
and HUVECs, observed under the microscope. This indicated that
BAI-1 was located in the cytoplasm. In addition, protein and gene
expression of BAI-1 was confirmed in T24 cells and HUVECs
subsequent to transfection, observed through qPCR and western
blotting. In addition, it was identified that compared with HUVECs
transfected with p-Receiver-M61, proliferation of HUVECs
transfected with p-Receiver-M61-BAI-1 was significantly inhibited,
observed using the MTT method (P<0.05). The current study
observed 72 h subsequent to transfection, and it was identified
that with time after transfection, the concentration of
p-Receiver-M61-BAI-1 was reduced. In addition, it was identified
that BAI-1 had no direct inhibitory effect on the proliferation of
T24 cells in vitro. There was no significant difference
between T24 cells transfected with p-Receiver-M61-BAI-1 and those
transfected with p-Receiver-M61 (P>0.05). Furthermore, flow
cytometry was used to detect the apoptosis of T24 cells and HUVECs
over a 72 h time period subsequent to transfection of the
p-Receiver-M61-BAI-1 and p-Receiver-M61 plasmids. The results
indicated that BAI-1 increased the apoptosis of HUVECs, however did
not affect that of T24 cells. Thus, it is suggested that BAI-1
inhibited proliferation of vascular endothelial cells to inhibit
tumor growth, however had no direct effect on T24 cell death.
In conclusion, BAI-1 is a member of the
adhesion-GPCR family of receptors. Numerous diseases are associated
with GPCRs, which are the targets of approximately 40% of drugs.
Thus, BAI-1 is suggested to be a potential novel therapautic target
for the inhibition of tumor neovascularization.
Acknowledgments
The current study was supported by the National
Natural Science Foundation of China (grant no. 30700834) and the
Natural Science Foundation of Tianjin (grant no.
12ZCDZSY16600).
References
|
1
|
Otto T, Krege S, Noll F and Rübben H:
Therapy of superficial bladder carcinomas. Urol Int. 63:32–39.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rübben H, Lutzeyer W, Fischer N, Deutz F,
Lagrange W and Giani G: Natural history and treatment of low and
high risk superficial bladder tumors. J Urol. 139:283–285.
1988.PubMed/NCBI
|
|
3
|
Cordon-Cardo C: Molecular alterations
associated with bladder cancer initiation and progression. Scand J
Urol Nephrol Suppl. 218:154–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reuter VE: The pathology of bladder
cancer. Urology. 67(3 Suppl 1): 11–17. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sylvester RJ, van der MEIJDEN AP and Lamm
DL: Intravesical bacillus Calmette-Guerin reduces the risk of
progression in patients with superficial bladder cancer: A
meta-analysis of the published results of randomized clinical
trials. J Urol. 168:1964–1970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lamm DL, Blumenstein BA, Crissman JD,
Montie JE, Gottesman JE, Lowe BA, Sarosdy MF, Bohl RD, Grossman HB,
Beck TM, et al: Maintenance bacillus Calmette-Guerin immunotherapy
for recurrent TA, T1 and carcinoma in situ transitional cell
carcinoma of the bladder: A randomized southwest oncology group
study. J Urol. 163:1124–1129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davis JW, Sheth SI, Doviak MJ and
Schellhammer PF: Superficial bladder carcinoma treated with
bacillus Calmette-Guerin: Progression-free and disease specific
survival with minimum 10-year followup. J Urol. 167:494–500. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McGavin MK, Badour K, Hardy LA, Kubiseski
TJ, Zhang J and Siminovitch KA: The intersectin 2 adaptor links
wiskott aldrich syndrome protein (WASp)-mediated actin
polymerization to T cell antigen receptor endocytosis. J Exp Med.
194:1777–1787. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shaw RJ and Cantley LC: Ras, PI (3) K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishimori H, Shiratsuchi T, Urano T,
Kimura Y, Kiyono K, Tatsumi K, Yoshida S, Ono M, Kuwano M, Nakamura
Y and Tokino T: A novel brain-specific p53- target gene, BAI-1,
containing thrombospondin type 1 repeats inhibits experimental
angiogenesis. Oncogene. 15:2145–2150. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukushima Y, Oshika Y, Tsuchida T,
Tokunaga T, Hatanaka H, Kijima H, Yamazaki H, Ueyama Y, Tamaoki N
and Nakamura M: Brain-specific angiogenesis inhibitor 1 expression
is inversely correlated with vascularity and distant metastasis of
colorectal cancer. Int J Oncol. 13:967–970. 1998.PubMed/NCBI
|
|
12
|
Izutsu T, Konda R, Sugimura J, Iwasaki K
and Fujioka T: Brain-specific angiogenesis inhibitor 1 is a
putative factor for inhibition of neovascular formation in renal
cell carcinoma. J Urol. 185:2353–2358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shiratsuchi T, Futamura M, Oda K,
Nishimori H, Nakamura Y and Tokino T: Cloning and characterization
of BAI-associated protein 1: A PDZ domain-containing protein that
interacts with BAI1. Biochem Biophys Res Commun. 247:597–604. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kudo S, Konda R, Obara W, Kudo D, Tani K,
Nakamura Y and Fujioka T: Inhibition of tumor growth through
suppression of angiogenesis by brain-specific angiogenesis
inhibitor 1 gene transfer in murine renal cell carcinoma. Oncology
reports. 18:785–791. 2007.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Zendman AJ, Cornelissen IM, Weidle UH,
Ruiter DJ and van Muijen GN: TM7XN1, a novel human EGF-TM7-like
cDNA, detected with mRNA differential display using human melanoma
cell lines with different metastatic potential. FEBS Lett.
446:292–298. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dawson DW, Pearce SF, Zhong R, Silverstein
RL, Frazier WA and Bouck NP: CD36 mediates the in vitro inhibitory
effects of thrombospondin-1 on endothelial cells. J Cell Biol.
138:707–717. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dawson DW, Volpert OV, Pearce SF,
Schneider AJ, Silverstein RL, Henkin J and Bouck NP: Three distinct
D-amino acid substitutions confer potent antiangiogenic activity on
an inactive peptide derived from a thrombospondin-1 type 1 repeat.
Mol Pharmacol. 55:332–338. 1999.PubMed/NCBI
|
|
19
|
Dawson DW, Pearce SF, Zhong R, Silverstein
RL, Frazier WA and Bouck NP: CD36 mediates the in vitro inhibitory
effects of thrombospodin-1 on endothelial cell. J Cell Biol.
138:707–717. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hatanaka H, Oshika Y, Abe Y, Yoshida Y,
Hashimoto T, Handa A, Kijima H, Yamazaki H, Inoue H, Ueyama Y and
Nakamura M: Vascularization is decreased in pulmonary
adenocarcinoma expressing brain-specific angiogenesis inhibitor 1
(BAI1). Int J Mol Med. 5:181–183. 2000.PubMed/NCBI
|
|
21
|
Yoon KC, Ahn KY, Lee JH, Chun BJ, Park SW,
Seo MS, Park YG and Kim KK: Lipid-mediated delivery of
brain-specific angiogenesis inhibitor 1 gene reduces corneal
neovascularization in an in vivo rabbit model. Gene Ther.
12:617–624. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaur B, Brat DJ, Devi NS and Van Meir EG:
Vasculostatin, a proteolytic fragment of brain angiogenesis
inhibitor 1, is an anti-angiogenic and antitumorigenic factor.
Oncogene. 24:3632–3642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El Moustaine D, Granier S, Doumazane E,
Scholler P, Rahmeh R, Bron P, Mouillac B, Banères JL, Rondard P and
Pin JP: Distinct roles of metabotropic glutamate receptor
dimerization in agonist activation and G-protein coupling. Proc
Natl Acad Sci USA. 109:16342–16347. 2012. View Article : Google Scholar : PubMed/NCBI
|