Introduction
Breast cancer is the most common malignancy in
women. Despite major improvements in early detection through
advanced screening and therapy, 522,000 women succumbed to this
disease worldwide in 2012 (1).
Angiogenesis is a key feature of tumor cells, which is necessary to
overcome the hypoxia that is associated with cancer outgrowth.
However, previous therapies targeting vascular development have
failed to show a clear survival benefit for patients with breast
cancer (2). Therefore, it is
hypothesized that improved understanding regarding the role of
vascular endothelial growth factors (VEGFs) and their receptors
(VEGFRs) may help to improve future therapies.
The VEGF family comprises five structurally related
factors: VEGF-A, -B, -C, -D and placenta growth factor, which act
as the primary activators of angiogenesis by binding to the
following tyrosine kinase receptors: VEGFR1, 2 and 3. The roles of
VEGFR2 and VEGFR3 as direct stimulators of angiogenesis (VEGFR2)
and lymphangiogenesis (VEGFR3) have been thoroughly characterized;
however, the function of VEGFR1 is less clear. The VEGFR1 gene
(FLT-1) encodes two proteins: Membrane-bound fibroblast growth
factor receptor 1 and a soluble form termed sVEGFR1. Both protein
forms have been reported to negatively regulate VEGFR2 via
high-affinity binding of VEGFs, which consequently become
unavailable for VEGFR2 (3).
However, it has previously been suggested that VEGFR1 may
indirectly promote tumor cell growth by activating monocytes and
macrophages, which invade the tumor and produce VEGFs and
cytokines, leading to angiogenesis and lymphangiogenesis via
activation of VEGFR2 and VEGFR3 (4,5).
Previous studies have analyzed the expression
patterns and prognostic significance of VEGFR1 in breast cancer and
cancer-associated vascular tissues. Positive associations have been
reported between high-level VEGFR1 expression and adverse tumor
features, including an increased risk for metastasis and relapse in
tumors with strongly VEGFR1-positive endothelial cells (6), as well as shortened survival
(7) and positive lymph node stage
(8) if tumor cells exhibit strong
VEGFR1 staining. Conversely, other studies have reported opposite
findings, including absence of lymph node metastases in strongly
VEGFR1-positive cancers (9), lack
of an association with overall survival (6), or generally infrequent positivity of
VEGFR1 in breast cancer cells (10). Similar discrepant findings have
also been reported in normal breast epithelial cells, with studies
describing either no expression (10,11),
or uniformly positive staining (12).
Due to these discordant findings, the present study
aimed to analyze an existing large breast cancer tissue microarray,
including >2,000 breast cancer samples, using an antibody
directed specifically against the membrane-bound form of VEGFR1.
The results detected an association between reduced membranous
expression of VEGFR1 and adverse features of breast cancer.
Materials and methods
Breast cancer tissue microarray
A breast cancer tissue microarray was used in the
present study, which has previously been described in detail
(13). Briefly, 2,197
formalin-fixed (neutral-buffered aqueous 4% solution),
paraffin-embedded tumor samples from patients with a median patient
age of 62 years (range, 26–101 years) and a median follow-up time
of 68 months (range, 1–176 months) were assembled in a tissue
microarray format (Table I). One
tissue cylinder per case, with a diameter of 0.6 mm, was obtained
from representative tumor areas of a 'donor' tissue block using a
homemade semiautomatic robotic precision instrument. Histological
grade was determined according to the Bloom-Richardson-Ellis Score
for Breast Cancer (BRE score) (14). Several molecular data used in the
present study are available from previously published studies.
These include amplification data obtained by fluorescence in
situ hybridization (FISH) for human epidermal growth factor
(HER2), MYC and cyclin D1 (CCND1), and expression data obtained by
immunohistochemistry (IHC) for estrogen receptor (ER), progesterone
receptor (PR) and Ki67 (13,15).
All tissue samples included in the present study were
double-pseudomized leftover samples from routine pathological
diagnoses, which can be used for research purposes without informed
consent according to local laws (§12 HmbKHG; Hamburg, Germany).
Manufacturing and usage of tissue microarrays for research purposes
has been approved by the local institutional review board under
protocol #WF-049/09. Control tissue was obtained from tumor
patients normal breast tissue.
 | Table IAssociation between VEGFR1 IHC results
and breast cancer phenotype, ER and PR status, HER2, MYC and CCND1
amplification, and triple negative category. |
Table I
Association between VEGFR1 IHC results
and breast cancer phenotype, ER and PR status, HER2, MYC and CCND1
amplification, and triple negative category.
| Parameters | Interpretable
(n) | VEGFR1 IHC result (%)
| P-value |
|---|
| Negative | Weak | Moderate | Strong |
|---|
| All types of
cancer | 1,630 | 3.1 | 6.3 | 10.9 | 79.7 | |
| Histology | | | | | | |
| No special type | 1,146 | 3.1 | 6.2 | 11.6 | 79.1 | |
| Lobular
carcinoma | 227 | 2.2 | 3.5 | 6.2 | 88.1 | |
| Cribriform
carcinoma | 48 | 0.0 | 6.3 | 6.3 | 87.5 | |
| Medullary
carcinoma | 38 | 13.2 | 21.1 | 15.8 | 50.0 | 0.0002b |
| Tubular
carcinoma | 40 | 0.0 | 2.5 | 7.5 | 90.0 | |
| Papillary
carcinoma | 24 | 4.2 | 4.2 | 16.7 | 75.0 | |
| Mucinous
carcinoma | 46 | 2.2 | 10.9 | 10.9 | 76.1 | |
| Other rare
typesa | 61 | 4.9 | 8.2 | 16.4 | 70.5 | |
| pT stage | | | | | | 0.0431 |
| pT1 | 540 | 1.5 | 5.2 | 10.0 | 83.3 | |
| pT2 | 784 | 3.7 | 7.1 | 12.1 | 77.0 | |
| pT3 | 99 | 7.1 | 4.0 | 8.1 | 80.8 | |
| pT4 | 198 | 3.5 | 6.6 | 9.6 | 80.3 | |
| BRE grade | | | | | | <0.0001 |
| Grade 1 | 384 | 2.9 | 3.4 | 6.5 | 87.2 | |
| Grade 2 | 627 | 1.9 | 5.6 | 9.7 | 82.8 | |
| Grade 3 | 506 | 5.1 | 9.7 | 14.2 | 70.9 | |
| Nodal stage | | | | | | 0.073 |
| pN0 | 671 | 3.4 | 7.0 | 12.1 | 77.5 | |
| pN1 | 584 | 2.2 | 5.8 | 11.0 | 81.0 | |
| pN2 | 93 | 7.5 | 11.8 | 10.8 | 69.9 | |
| ER/PR status | | | | | | <0.0001 |
| Negative | 313 | 5.1 | 11.8 | 15.7 | 67.4 | |
| Positive | 1,156 | 2.6 | 4.6 | 10.0 | 82.8 | |
| HER2 FISH | | | | | | 0.0745 |
| No
amplification | 1,051 | 3.1 | 5.8 | 10.4 | 80.7 | |
| Amplification | 227 | 0.9 | 3.5 | 10.6 | 85.0 | |
| Triple
negative | | | | | | <0.0001 |
| No | 1,021 | 2.0 | 4.0 | 9.7 | 84.3 | |
| Yes | 159 | 8.2 | 13.2 | 17.0 | 61.6 | |
| CCND1 FISH | | | | | | 0.396 |
| No
amplification | 1,129 | 2.6 | 5.1 | 10.9 | 81.4 | |
| Amplification | 281 | 2.1 | 7.8 | 10.3 | 79.7 | |
| MYC FISH | | | | | | 0.1374 |
| No
amplification | 1,146 | 2.9 | 5.8 | 10.5 | 80.9 | |
| Amplification | 66 | 6.1 | 6.1 | 18.2 | 69.7 | |
VEGFR IHC
Standard indirect immunoperoxidase procedures were
used for the detection of VEGFR1 (rabbit polyclonal antibody; cat.
no. ab2350; 1:450 dilution; Abcam, Cambridge, UK). Sections were
heated in an autoclave at 121°C for 10 min in Tris-EDTA-Citrate
buffer (pH 7.8). Primary antibody was incubated for 60 min at 37°C.
Endogenous peroxidase was blocked with Dako S2023 for 10 min at
20°C, followed by anti-rabbit peroxidase (Dako real envision
detection system K5007; Dako, Glostrup, Denmark) for 30 min at
37°C. Diaminobenzidine (Dako) was applied for 10 min at 20°C and
sections were counterstained with Mayer's hematoxylin
(Sigma-Aldrich). The VEGFR1 staining intensity and the fraction of
stained tumor cells were recorded for each tissue specimen
(Axiophot Neofluar 20×; Zeiss, Oberkochen, Germany). Staining
intensity was estimated using a 4-step scale: 0, no staining; 1+,
faint intensity; 2+, moderate intensity; 3+, strongest intensity.
The fraction of stained cells was scored according to the following
criteria: Score 0, no stained cells; score 1, ≤25% stained cells;
score 2, ≤50% stained cells; score 3, ≤75% stained cells; and score
4, >75% stained cells. A final IHC result was generated from
these scores: Negative, no staining at all; weak, intensity 1+ in
≤70% of cells, or intensity 2+ in ≤30% of cells; moderate,
intensity 1+ in >70% of cells, intensity 2+ in >30% but ≤70%
of cells, or intensity 3+ in ≤30% of cells; strong, intensity 2+ in
>70% of cells, or intensity 3+ in >30% of cells.
Statistical analysis
Pearson's χ2 test and Student's t-test
were used to study the relationship between VEGFR1 IHC results and
clinicopathological or molecular parameters. The effect of VEGFR1
on survival was assessed by Kaplan-Meier curves and log-rank tests.
A Cox proportional-hazards model was used to identify independent
factors associated with overall survival. Statistical analysis was
performed using JMP version 9.0 statistical software package (SAS
Institute Software GmbH, Böblingen, Germany). P<0.05 was
considered to indicate a statistically significant difference.
Results
Technical issues
A total of 567 of 2,197 (25.8%) tissue specimens
were non-informative for VEGFR1 IHC, due to the complete lack of
tissue or absence of unequivocal cancer cells.
Association of VEGFR1 IHC results with
breast cancer phenotype, cell proliferation, and molecular
markers
VEGFR1 immunostaining was located in the membrane,
and sometimes also the cytoplasm of luminal epithelial cells. Five
samples of normal breast epithelium included in the tissue
microarray exhibited strong VEGFR1 staining. Cancer cells typically
exhibited strong staining compared with the staining intensity
observed in the normal breast samples: Staining was strong in 1,299
(79.7%), moderate in 178 (10.9%), weak in 102 (6.3%) and negative
in 51 (3.1%), of the 1,630 interpretable cancers. Representative
images of VEGFR1 staining in normal and cancerous breast tissues
are presented in Fig. 1. VEGFR1
staining levels were comparable (70–90% with strong staining) in
the majority of different histological subtypes, apart from
medullary cancers, which exhibited a significantly lower fraction
of strongly VEGFR1-positive tumors (50%, P<0.0002), as compared
with carcinoma of no special type (NST). In addition, VEGFR1
staining was inversely associated with tumor stage (P=0.0431) and
BRE grade (P<0.0001), although the difference in numbers was
only small. Staining levels were unrelated to the presence of lymph
node metastases. Cell proliferation was previously determined
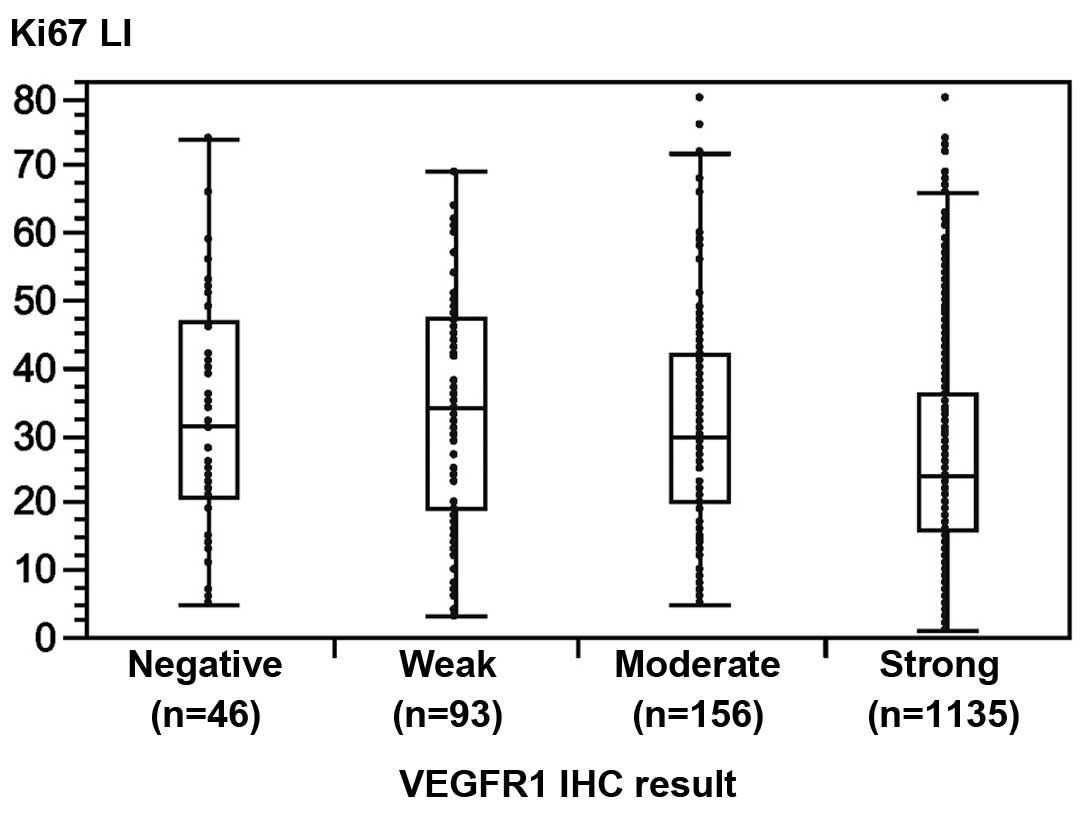
immunohistochemically using the Ki67 labeling index (LI) (13). An inverse association was detected
between VEGFR1 staining and cell proliferation: The average Ki67LI
increased from 26.5% in 1,135 cancer specimens with strong VEGFR1
staining to 33.1% in 46 tumors lacking detectable VEGFR1 staining
(P<0.0001; Fig. 2). Strong
VEGFR1 immunostaining was also associated with positive ER and PR
status and the triple negative category (all P<0.0001); however,
VEGFR staining was unrelated to amplifications in HER2, CCND1 or
MYC. The results are summarized in Table I. A multivariate analysis of
overall survival demonstrated that VEGFR1 staining had
non-significant impact on hazard ratio (Table II).
 | Table IIMultivariate analysis of overall
survival in 824 patients, including tumor stage, BRE grade, nodal
stage, ER and PR status, HER2 amplification, triple negative
category, Ki67 labeling index and VEGFR1 staining. |
Table II
Multivariate analysis of overall
survival in 824 patients, including tumor stage, BRE grade, nodal
stage, ER and PR status, HER2 amplification, triple negative
category, Ki67 labeling index and VEGFR1 staining.
| Clinicopathological
parameter | Hazard ratio | 95% Confidence
interval | P-value |
|---|
| pT stage | | | |
| pT2 vs. pT1 | 1.4 | 1.02–2.04 | 0.0357 |
| pT3 vs. pT2 | 1.0 | 0.61–1.46 | 0.8753 |
| pT4 vs. pT3 | 1.7 | 1.08–2.80 | 0.021 |
| BRE grade | | | |
| Grade 2 vs. grade
1 | 1.5 | 0.97–2.29 | 0.0707 |
| Grade 3 vs. grade
1 | 1.9 | 1.38–2.8 | <0.0001 |
| Nodal stage | | | |
| pN1 vs. pN0 | 2.7 | 2.03–3.73 | <0.0001 |
| pN2 vs. pN1 | 1.9 | 1.34–2.8 | 0.0007 |
| ER status | | | |
| Negative vs.
positive | 1.6 | 0.25–5.12 | 0.5677 |
| PR status | | | |
| Negative vs.
positive | 1.5 | 1.07–2.02 | 0.018 |
| ER/PR status | | | |
| Negative vs.
positive | 0.4 | 0.1–2.38 | 0.2485 |
| HER2 FISH | | | |
| No vs.
amplification | 0.5 | 0.36–0.78 | 0.0021 |
| Triple
negative | | | |
| Yes vs. no | 2.7 | 1.51–4.92 | 0.0009 |
| Ki67 labeling
index | | | |
| Per unit
change | 1.0 | 0.99–1.01 | 0.4404 |
| VEGFR1 | | | |
| Weak vs.
negative | 0.9 | 0.47–1.93 | 0.843 |
| Moderate vs.
weak | 0.6 | 0.33–1.08 | 0.087 |
| High vs.
moderate | 1.2 | 0.82–1.92 | 0.3337 |
Association with patient survival and
response to tamoxifen treatment
To exclude a potential influence of the histological
subtype on patient prognosis, survival analysis was limited to the
largest subset of 1,144 carcinomas of NST with interpretable VEGFR1
IHC data. Since tumors with moderate or strong staining exhibited
improved overall survival compared with tumors with negative or
weak staining (P=0.0054; Fig. 3A),
all NST specimens were grouped into subsets with low (i.e. negative
or weak) or high (i.e. moderate or strong) staining for further
survival analyses. According to these groups, tumors with high
VEGFR1 staining exhibited superior overall survival in all subsets
of NST (P=0.0015; Fig. 3B). This
association was also true for subsets of nodal-negative NST (pN0,
P=0.0256; Fig. 3C) and
nodal-positive NST (pN1-2, P=0.0018; Fig. 3D). Additional analysis in a subset
of 185 patients with breast cancer who had received tamoxifen
monotherapy revealed a significant association between high levels
of VEGFR1 and prolonged survival after treatment (P=0.0010;
Fig. 3E).
Discussion
The present study successfully analyzed >1,600
breast cancer specimens using an antibody directed against
membrane-bound VEGFR1. The results suggested that high-level
immunostaining of VEGFR1 is a common feature of normal and
cancerous breast epithelial cells, and that lost or reduced
expression of VEGFR1 is associated with tumor progression, rapid
cell proliferation and shortened survival.
All normal breast tissues (5/5) and 90% of cancer
tissues exhibited moderate to strong VEGFR1 immunostaining in the
present study, indicating that high levels of VEGFR1 represent a
physiological situation. These data thus corroborate the hypothesis
that full-length VEGFR1 physiologically regulates VEGFR2 activity
by trapping free VEGF (3,16). In addition, these results are
consistent with the findings of a previous study, which suggested
that VEGFR1 expression is indicative of better prognosis in breast
cancer (9). Based on the known
function of VEGFR2 as an activator of cell growth (17,18),
the adverse features of breast cancers with low-level VEGFR1
expression may be a consequence of unregulated VEGFR2 activation in
the absence of sufficient levels of VEGFR1.
The high rate of cancers with high-level VEGFR1
expression in the present study is consistent with the results of a
recent study reporting 100% positivity in 25 normal breast samples
and 90% positivity in 96 invasive breast cancer samples (12). Notably, in the previous study, the
same antibody was used as in the present analysis. According to the
manufacturer's data-sheet, this particular polyclonal antibody
(ab2350; Abcam) detects a 180 kD protein sequence, which is not
present in sVEGFR, and does also not detect the phosphorylated
form. Other studies using different antibodies have reported
largely variable results, including complete lack of staining in
normal breast epithelium (10),
and a broad range of positivity in cancer cells ranging from 16–91%
(7,9,10,19).
Furthermore, compared with the findings of the present study,
several of these studies also detected associations between strong
VEGFR1 staining and adverse features of breast cancer, including
early recurrence, reduced overall survival and metastasis (6–8).
Such discrepant findings are most likely attributed to the
different antibodies used in these studies. Notably, the majority
of studies described cytoplasmic staining (6–8),
which is unexpected given that full-length VEGFR1 is a
membrane-bound receptor, and that at least some membranous staining
would be expected. However, high homology exists between the
full-length membranous VEGFR1, sVEGFR1 lacking the transmembrane
and intracellular domains, and the five different intracellular
forms (iVEGFR1, possessing only one or more of the intracellular
domains) that have recently been described (20). Therefore, it appears possible that
some antibodies may cross-react with several forms of VEGFR1. This
would provide an explanation for some of the discrepant findings,
in particular since numerous studies have demonstrated that sVEGFR1
and iVEGFR1 have opposite implications for tumor biology and
patient prognosis (21,22).
In the present study, low levels of VEGFR1
expression were associated with reduced ER and PR positivity in all
cancer specimens, as well as shortened survival in the subset of
ER-positive patients receiving endocrine (tamoxifen) therapy. These
results provide additional support for the concept that VEGFR1 acts
as a negative regulator of VEGFR2 signaling, since several studies
have reported that activation of VEGFR2 is associated with high
tumor stage and grade, metastatic growth, negative ER status and
early recurrence in patients with breast cancer following tamoxifen
therapy (17,23,24).
Furthermore, the findings of the present study suggest that VEGFR1,
possibly in combination with VEGFR2, may be a suitable marker to
stratify patients for endocrine therapy.
In 2008, the Food and Drug Administration approved
the VEGF neutralizing antibody bevacizumab for the treatment of
advanced and metastatic breast cancer. However, subsequent large
clinical phase III trials (e.g. E2100, AVADO, RIBBON-1 and -2)
(25–27) have failed to detect a clear
survival benefit, resulting in withdrawal of the approval in 2011.
The reasons for the lack of a therapeutic benefit remain poorly
understood, although the pivotal role of angiogenesis in cancer
growth is undisputed (28). Since
90% of cancers in the present study, including 70–80% high grade,
advanced and metastatic tumors, exhibited high-level VEGFR1
staining, it seems obvious that the vast majority of breast cancers
are capable of regulating growth signaling via an intact
VEGF/VEGFR1 loop. It would be interesting to study the effects of
VEGF inhibitors in breast cancers with various levels of VEGFR1.
Theoretically, it may be speculated that VEGF inhibition could be
more effective in tumor cells lacking VEGFR1 expression, than in
those with physiologically high protein levels, and that VEGFR1
levels could be of potential value in selecting patients for
anti-angiogenic therapies.
A tissue microarray with a single 0.6 mm spot per
cancer was used in the present study. A limitation of the present
study is that this approach is not suitable for the detection of
possible intratumoral heterogeneity of VEGFR expression. Therefore,
it is possible that some cancers with heterogenous VEGFR expression
were overlooked in the present analysis. It has previously been
suggested that analysis of multiple spots per tumor may increase
the representativeness of microarray studies (29,30).
However, this approach bears the disadvantage that not all tissue
spots of one cancer are interpretable. Given that the likelihood of
finding a positive result increases with the number of
interpretable tissue spots, analysis of various amounts of cancer
spots per patient may introduce a statistical bias to the analysis
(31). Our previous study
demonstrated that microarrays consisting of a single spot per
cancer are superior for detecting clinically relevant associations
between molecular markers and breast cancer phenotype (32). In addition, previous tissue
microarray studies using a single spot per tumor have been able to
reproduce known associations between molecular markers and cancer
phenotype, or patient prognosis in breast cancer (13,32,33).
A limitation of the present study is that only one
(VEGFR1) of many angiogenic molecules, including VEGFR2, VEGFR3,
neuropilin (NRP)1 or NRP2, was analyzed (34). Given the complex biological
interactions of these receptors and their corresponding growth
factors it would be interesting to study their co-expression
patterns, provided that antibodies suitable for formalin-fixed
tissues become available, in order to obtain a comprehensive
picture of angiogenic factors in breast cancer.
In conclusion, the results of the present study
suggested that reduced or lost expression of full-length and
membrane-bound VEGFR1 identifies a small but clinically relevant
subset of breast cancers that are characterized by adverse tumor
features and shortened survival, which may not respond optimally to
endocrine therapy. Furthermore, the choice of antibody may have a
serious impact on the outcome of VEGFR1 expression analyses.
Acknowledgments
The authors would like to thank Mrs Christina Koop,
Mrs Janett Lütgens, Mrs Sünje Seekamp and Mrs Inge Brandt
(Institute of Pathology) for their excellent technical support.
References
|
1
|
International Agency for Research on
Cancer (IARC): GLOBOCAN 2012: Estimated cancer incidence, mortality
and prevalence worldwide in 2012. (IARC fact sheets). http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
Accessed May 27, 2016.
|
|
2
|
Rossari JR, Metzger-Filho O, Paesmans M,
Saini KS, Gennari A, de Azambuja E and Piccart-Gebhart M:
Bevacizumab and breast cancer: A meta-analysis of first-line phase
III studies and a critical reappraisal of available evidence. J
Oncol. 2012:4176732012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shibuya M: Vascular endothelial growth
factor and its receptor system: Physiological functions in
angiogenesis and pathological roles in various diseases. J Biochem.
153:13–19. 2013. View Article : Google Scholar
|
|
4
|
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu
L, Grzesik DA, Qian H, Xue XN and Pollard JW: Macrophages regulate
the angiogenic switch in a mouse model of breast cancer. Cancer
Res. 66:11238–11246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murakami M, Zheng Y, Hirashima M, Suda T,
Morita Y, Ooehara J, Ema H, Fong GH and Shibuya M: VEGFR1 tyrosine
kinase signaling promotes lymphangiogenesis as well as
angio-genesis indirectly via macrophage recruitment. Arterioscler
Thromb Vasc Biol. 28:658–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dales JP, Garcia S, Carpentier S, Andrac
L, Ramuz O, Lavaut MN, Allasia C, Bonnier P and Taranger-Charpin C:
Prediction of metastasis risk (11 year follow-up) using VEGF-R1,
VEGF-R2, Tie-2/Tek and CD105 expression in breast cancer (n=905).
Br J Cancer. 90:1216–1221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mylona E, Alexandrou P, Giannopoulou I,
Liapis G, Sofia M, Keramopoulos A and Nakopoulou L: The prognostic
value of vascular endothelial growth factors (VEGFs)-A and -B and
their receptor, VEGFR-1, in invasive breast carcinoma. Gynecol
Oncol. 104:557–563. 2007. View Article : Google Scholar
|
|
8
|
Ning Q, Liu C, Hou L, Meng M, Zhang X, Luo
M, Shao S, Zuo X and Zhao X: Vascular endothelial growth factor
receptor-1 activation promotes migration and invasion of breast
cancer cells through epithelial-mesenchymal transition. PLoS One.
8:e652172013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmidt M, Voelker HU, Kapp M, Dietl J and
Kammerer U: Expression of VEGFR-1 (Flt-1) in breast cancer is
associated with VEGF expression and with node-negative tumour
stage. Anticancer Res. 28:1719–1724. 2008.PubMed/NCBI
|
|
10
|
Wülfing P, Kersting C, Buerger H, Mattsson
B, Mesters R, Gustmann C, Hinrichs B, Tio J, Böcker W and Kiesel L:
Expression patterns of angiogenic and lymphangiogenic factors in
ductal breast carcinoma in situ. Br J Cancer. 92:1720–1728. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brown LF, Guidi AJ, Schnitt SJ, Van De
Water L, Iruela-Arispe ML, Yeo TK, Tognazzi K and Dvorak HF:
Vascular stroma formation in carcinoma in situ, invasive carcinoma,
and metastatic carcinoma of the breast. Clin Cancer Res.
5:1041–1056. 1999.PubMed/NCBI
|
|
12
|
Arias-Pulido H, Chaher N, Gong Y, Qualls
C, Vargas J and Royce M: Tumor stromal vascular endothelial growth
factor A is predictive of poor outcome in inflammatory breast
cancer. BMC Cancer. 12:2982012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruiz C, Seibt S, Al Kuraya K, Siraj AK,
Mirlacher M, Schraml P, Maurer R, Spichtin H, Torhorst J, Popovska
S, et al: Tissue microarrays for comparing molecular features with
proliferation activity in breast cancer. Int J Cancer.
118:2190–2194. 2006. View Article : Google Scholar
|
|
14
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Kuraya K, Schraml P, Torhorst J, Tapia
C, Zaharieva B, Novotny H, Spichtin H, Maurer R, Mirlacher M,
Köchli O, et al: Prognostic relevance of gene amplifications and
coamplifications in breast cancer. Cancer Res. 64:8534–8540. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hiratsuka S, Minowa O, Kuno J, Noda T and
Shibuya M: Flt-1 lacking the tyrosine kinase domain is sufficient
for normal development and angiogenesis in mice. Proc Natl Acad Sci
USA. 95:9349–9354. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Linderholm BK, Hellborg H, Johansson U,
Skoog L and Lehtiö J: Vascular endothelial growth factor receptor 2
and downstream p38 mitogen-activated protein kinase are possible
candidate markers of intrinsic resistance to adjuvant endocrine
treatment in steroid receptor positive breast cancer. Breast Cancer
Res Treat. 125:457–465. 2011. View Article : Google Scholar
|
|
18
|
Jin J, Yuan F, Shen MQ, Feng YF and He QL:
Vascular endothelial growth factor regulates primate
choroid-retinal endothelial cell proliferation and tube formation
through PI3K/Akt and MEK/ERK dependent signaling. Mol Cell Biochem.
381:267–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paradiso A, Mangia A, Chiriatti A, Tommasi
S, Zito A, Latorre A, Schittulli F and Lorusso V: Biomarkers
predictive for clinical efficacy of taxol-based chemotherapy in
advanced breast cancer. Ann Oncol. 16(Suppl 4): iv14–iv19. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mezquita B, Mezquita J, Pau M and Mezquita
C: A novel intracellular isoform of VEGFR-1 activates Src and
promotes cell invasion in MDA-MB-231 breast cancer cells. J Cell
Biochem. 110:732–742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toi M, Bando H, Ogawa T, Muta M, Hornig C
and Weich HA: Significance of vascular endothelial growth factor
(VEGF)/soluble VEGF receptor-1 relationship in breast cancer. Int J
Cancer. 98:14–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bando H, Weich HA, Brokelmann M, Horiguchi
S, Funata N, Ogawa T and Toi M: Association between intratumoral
free and total VEGF, soluble VEGFR-1, VEGFR-2 and prognosis in
breast cancer. Br J Cancer. 92:553–561. 2005.PubMed/NCBI
|
|
23
|
Ryden L, Jirström K, Bendahl PO, Fernö M,
Nordenskjöld B, Stål O, Thorstenson S, Jönsson PE and Landberg G:
Tumor-specific expression of vascular endothelial growth factor
receptor 2 but not vascular endothelial growth factor or human
epidermal growth factor receptor 2 is associated with impaired
response to adjuvant tamoxifen in premenopausal breast cancer. J
Clin Oncol. 23:4695–4704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johansson I, Aaltonen KE, Ebbesson A,
Grabau D, Wigerup C, Hedenfalk I and Rydén L: Increased gene copy
number of KIT and VEGFR2 at 4q12 in primary breast cancer is
related to an aggressive phenotype and impaired prognosis. Genes
Chromosomes. Cancer. 51:375–383. 2012.
|
|
25
|
Gray R, Bhattacharya S, Bowden C, Miller K
and Comis RL: Independent review of E2100: a phase III trial of
bevacizumab plus paclitaxel versus paclitaxel in women with
metastatic breast cancer. J Clin Oncol. 27:4966–4972. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miles DW, de Haas SL, Dirix LY, et al:
Biomarker results from the AVADO phase 3 trial of first-line
bevacizumab plus docetaxel for HER2-negative metastatic breast
cancer. Br J Cancer. 108:1052–1060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robert NJ, Diéras V, Glaspy J, et al:
RIBBON-1: randomized, double-blind, placebo-controlled, phase III
trial of chemotherapy with or without bevacizumab for first-line
treatment of human epidermal growth factor receptor 2-negative,
locally recurrent or metastatic breast cancer. J Clin Oncol.
29:1252–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Camp RL, Charette LA and Rimm DL:
Validation of tissue microarray technology in breast carcinoma. Lab
Invest. 80:1943–1949. 2000. View Article : Google Scholar
|
|
30
|
Zhang D, Salto-Tellez M, Putti TC, Do E
and Koay ES: Reliability of tissue microarrays in detecting protein
expression and gene amplification in breast cancer. Mod Pathol.
79–84. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sauter G: Representativity of TMA Studies.
Methods Mol Biol. 664:27–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Torhorst J, Bucher C, Kononen J, Haas P,
Zuber M, Köchli OR, Mross F, Dieterich H, Moch H, Mihatsch M, et
al: Tissue microarrays for rapid linking of molecular changes to
clinical endpoints. Am J Pathol. 159:2249–2256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barlund M, Forozan F, Kononen J, Bubendorf
L, Chen Y, Bittner ML, Torhorst J, Haas P, Bucher C, Sauter G, et
al: Detecting activation of ribosomal protein S6 kinase by
complementary DNA and tissue microarray analysis. J Natl Cancer
Inst. 92:1252–1259. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goel HL and Mercurio AM: VEGF targets the
tumour cell. Nat Rev Cancer. 13:871–882. 2013. View Article : Google Scholar : PubMed/NCBI
|