Introduction
Diabetes mellitus, known as simply diabetes, is a
group of metabolic diseases in which there are high blood sugar
levels over a prolonged period. Symptoms of high blood sugar
include frequent urination, increased thirst, and increased hunger.
Additionally, the increased blood glucose concentration in patients
with diabetes frequently results in concomitant illness including
microvascular complications (nephropathy, neuropathy and
retinopathy) and macrovascular complications (coronary artery
disease, stroke and peripheral arterial disease) (1–3).
Furthermore, high blood sugar levels are able to damage blood
vessels for prolonged periods. Serious long-term complications of
diabetes include heart disease, stroke, kidney failure, foot ulcers
and eye damage (4–6). Patients with diabetes experience
delayed wound healing, resulting in diabetes being a leading cause
of non-traumatic lower extremity amputation, which is often
preceded by a non-healing ulcer. It has been estimated that around
15–27% patients with diabetes require lower limb amputations,
predominantly (50%) due to infection (5).
Diabetic wounds are associated with deficient
circulation and altered carbohydrate metabolism. Angiogenesis, the
formation of new blood vessels from pre-existing vessels, serves a
key role in the wound healing process, particularly during the
proliferative phase, which involves the migration and proliferation
of endothelial cells, and their differentiation into a tube-like
structure. Ameliorative angiogenesis improves the wound healing
process by facilitating delivery of oxygen and nutrients to the
wounds whereas impaired angiogenesis has been demonstrated to be
key in the impaired healing of diabetic wounds. Hence, promoting
angiogenesis is a potential therapy to enhance wound healing in
patients with diabetes (6).
Vascular endothelial growth factor (VEGF) is a
signaling protein that stimulates vasculogenesis and angiogenesis.
VEGF is an important factor in restoring the oxygen supply to
tissues when blood circulation is inadequate. The normal function
of VEGF is to create new blood vessels during embryonic development
or bypass blocked vessels following injury (7–9). A
close relationship between the reactive oxygen species induced VEGF
signaling pathway and vascular disease in diabetes has been
demonstrated in previous studies (10).
Herbal remedies have been widely used to accelerate
the wound-healing process since ancient times, particularly in
China. In recent years, the study of the use of alternative
therapies and natural remedies in wound management has rapidly
increased. However, their putative active compounds, molecular
mechanisms of action and side effects remain to be fully
elucidated. Dracorhodin perchlorate (Dra) is a key active
ingredient isolated from the fruit of Daemonorops draco, a
type of dragon's blood. Dragon's blood has long been used as a
traditional Chinese medicine to improve blood circulation, prevent
hemorrhages, heal wounds and as an antiseptic (11,12).
It has been reported that Dra is able to ameliorate the process
leading to insulin resistance, enhance insulin sensitivity and
inhibit high plasma lipid levels and reduce intestinal carbohydrate
absorption (13). Previous studies
have demonstrated a complex effect of Dra on human cells (14–18).
Dra has been indicated to induce apoptosis in different cell lines
(19). However, the mechanism of
Dra facilitating wound healing and whether Dra may be used to
relieve vessel damage in patients with diabetic ulcers remains to
be fully elucidated.
The present study aimed to investigate the effect of
Dra on angiogenesis and the associated signaling pathways in the
human umbilical vein endothelial cells (HUVEC) under high-glucose
stimulation.
Materials and methods
Cell culture and treatment
HUVECs were purchased from the Shanghai Bioleaf
Biotech Co., Ltd. (Shanghai, China). HUVECs were cultured in M119
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 5% fetal bovine serum (Gibco; Thermo Fisher
Scienitific, Inc.) and 100 µg/ml streptomycin plus 100 IU/ml
penicillin in the condition of 5% CO2 atmosphere at
37°C.
The experiment was designed in two groups: Low
glucose concentration (LG), 5.5 mM glucose; high glucose
concentration (HG): 25 mM glucose. For Dra treatment, 7.5 µM
Dra (National Institutes for Food and Drug Control, Beijing, China)
was added to the medium. Following 48 h of culture, cells were
harvested and used for experiments.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
HUVECs in the logarithmic phase were seeded, 5,000
cells/well in 96-well plate. Following culture in Dra and LG or HG
48 h at 37°C, MTT solution (5 mg/ml) in 1X PBS was added directly
to the medium in each well, and the plate was then incubated at
37°C for 4 h. The medium was then aspirated and replaced with
dimethyl sulfoxide followed by the measurement of the optical
density of each well at 540 nm.
Tube formation
HUVECs were seeded into 96-well plates at 8000
cells/well on 50 µl Matrigel (BD BioSciences, Franklin
Lakes, NJ, USA). Fresh media containing Dra and LG or HG was
subsequently added. Tubular structures were photographed following
6 h. The total tube length formation was measured for
quantification of angiogenesis using Image-Pro Plus software,
version 6.0 (Media Cybernetics, Rockville, MD, USA).
Cell migration assay
For the migration assay, 6 well Transwell chambers
(8.0 µm pores; Corning Incorporated, Corning, NY, USA) were
coated with 50 µl Matrigel (BD Biosciences). A total of
105 cells/well of HUVECs in the logarithmic phase were
transferred into the upper chamber. Following 4 h of incubation at
37°C, upper chambers containing Dra and LG or HG were set into the
lower chambers. Three wells were used for each experiment.
Following 16 h of incubation, cells were fixed with methanol and
stained with crystal violet. The number of migrated cells was
counted in five randomly selected fields. Each experiment was
repeated in triplicate.
Enzyme-linked immunosorbent assay
(ELISA)
HUVECs were seeded in 6 well plates at a density of
1×106cells/well and were treated with Dra and LG or HG
for 48 h. The levels of VEGF in the supernatants were measured
using an ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA)
according to the manufacturer's instructions. The absorbance values
were read within 30 min using an automatic microplate
spectrophotometer (Anthos Zenyth 340st; Anthos Labtec Instruments
GmbH, Salzburg, Austria) at 450 nm (A450). The average A450 values
were calculated for each set of reference standards, controls and
samples. A standard curve was constructed by plotting the mean
absorbance obtained for each reference standard against its
concentration using Excel software (Office 2010; Microsoft
Corporation, Redmond, WA, USA). The concentration of each sample
was then determined using the standard curve.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol Reagent
(GenStar Biosolutions, Beijing, China), and the concentration of
RNA was determined by absorbance at 260 and 280 nm. A total of 2 mg
total RNA was reverse transcribed to cDNA using a PrimeScript™ RT
reagent kit with gDNA Eraser (Takara Bio, Inc., Otsu, Japan). qPCR
was conducted to measure mitogen-activated protein kinase 6
(MAPK6), RAS p21 protein activator 1 (RASA1) and VEGF gene
expression. The 20 µl reaction mixture contained 7.4
µl nuclease-free water, 2 µl cDNA, 0.1 µl (10
µM) each primer, 0.4 µl ROX Reference Dye (50X) and
10.0 µl SYBR® Premix Ex Taq (Takara Bio, Inc.).
The thermal cycling conditions for PCR was as follows: 95°C for 30
sec, 40 cycles of 95°C for 15 sec and 60°C for 34 sec. PCR
amplification was conducted using specific primers for VEGF
(forward, 5′-ACG ATC GAT ACA GAA ACC ACG-3′, and reverse, 5′-CTC
TGC GCA GAG TCT CCT CT-3′), RASA1 (forward, 5′-GGG AGG CCG GTA TTA
TAA CAG-3′, and reverse, 5′-CCA ACG TTT TCC TTT GCC C-3′) and MAPK6
(forward, 5′-GAA TGG CAA ATC TGG CTC AAT T-3′, and reverse, 5′-ACA
GTC CTC CCC ACC ACT CA-3′). Actin primers (forward, 5′-TTG CGT TAC
ACC CTT TCT TG-3′, and reverse, 5′-TCA CCT TCA CCG TTC CAG TT-3′)
were used as an internal control. The PCR products were measured
using and ABI 7500 Fast Real-Time PCR Detection System (Applied
Biosystems, Thermo Fisher Scientific, Inc.). The 2−ΔΔCq
method was used to analyze the relative expression of each
gene.
Western blotting
Following culture with Dra and LG or HG, total
protein was extracted using a total protein extraction kit (Qiagen,
Inc., Valencia, CA, USA) and the concentration was measured using a
bicinchoninic acid protein assay kit (Beijing Transgen Co., Ltd.,
Beijing, China). Samples (10 µl) were loaded onto 10% sodium
dodecyl sulfate polyacrylamide gel electrophoresis. Subsequently
the proteins were transferred to a polyvinylidene fluoride membrane
(EMD Millipore, Billerica, MA, USA) and blocked in 2.5% non-fat
dried milk for 1 h at room temperature. Following this, the
membrane was incubated with antibodies against VEGF (1:3,000; cat.
no. ab36844; Abcam, Cambridge, UK), Ras (1:3,000; cat. no. ab52939;
Abcam), MAPK6 (1:5,000; cat. no. ab53277; Abcam) and actin
(1:6,000; cat. no. ab8226; Abcam) overnight at 4°C. Following
washing three times with Tris-buffered saline 0.5% Tween 20 (TBST),
membranes were incubated with goat anti-rabbit or mouse
peroxidase-conjugated secondary antibodies (1:5,000; cat. nos.
A0545 and SAB3701132, respectively; Sigma-Aldrich, St. Louis, MO,
USA). The membrane was washed three times with TBST, and the
signals were detected by Fusion SOLO chemiluminescence system
(Analis sa/nv, Namur, Belgium).
Statistical analysis
Statistical testing was performed using SPSS
software, version 12.0 (SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard deviation of the mean of the
indicated number of experiments. Statistical comparison between
experimental group and control was performed using one-way analysis
of variance and followed by Student–Newman–Keuls test to examine
differences between groups or chi-square test (for percentage).
P<0.05 was considered to indicate a statistically significant
difference.
Results
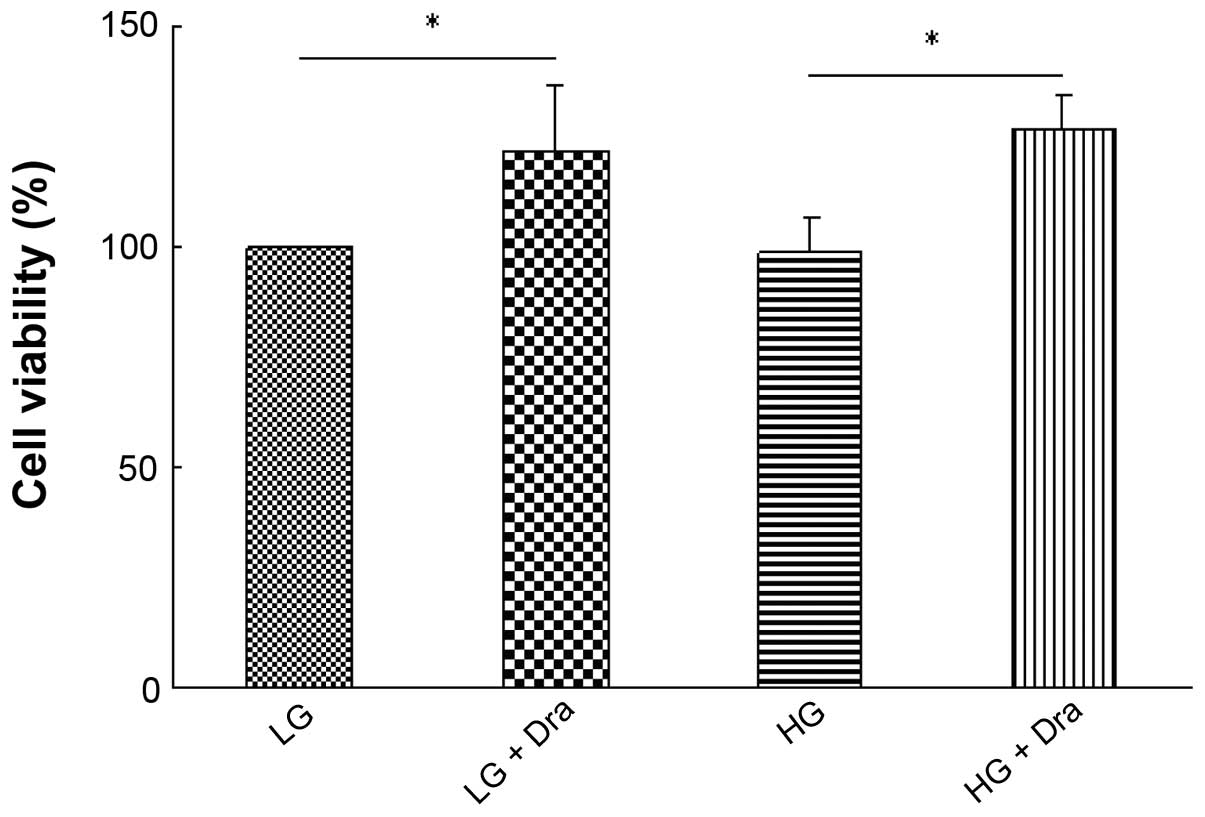
Effect of Dra on HUVEC viability
To investigate the mechanism by which Dra may
promote wound healing, an MTT assay was conducted to measure the
viability of HUVECs under different concentrations of Dra. As
presented in Fig. 1, following
culture with 5.5 mM glucose concentration (LG), 5.5 mM glucose
concentration plus 7.5 µM Dra (LG + Dra), 25 mM glucose (HG)
and 25 mM glucose plus 7.5 µM Dra (HG + Dra) for 48 h, Dra
increased the cell viability under LG and HG concentrations.
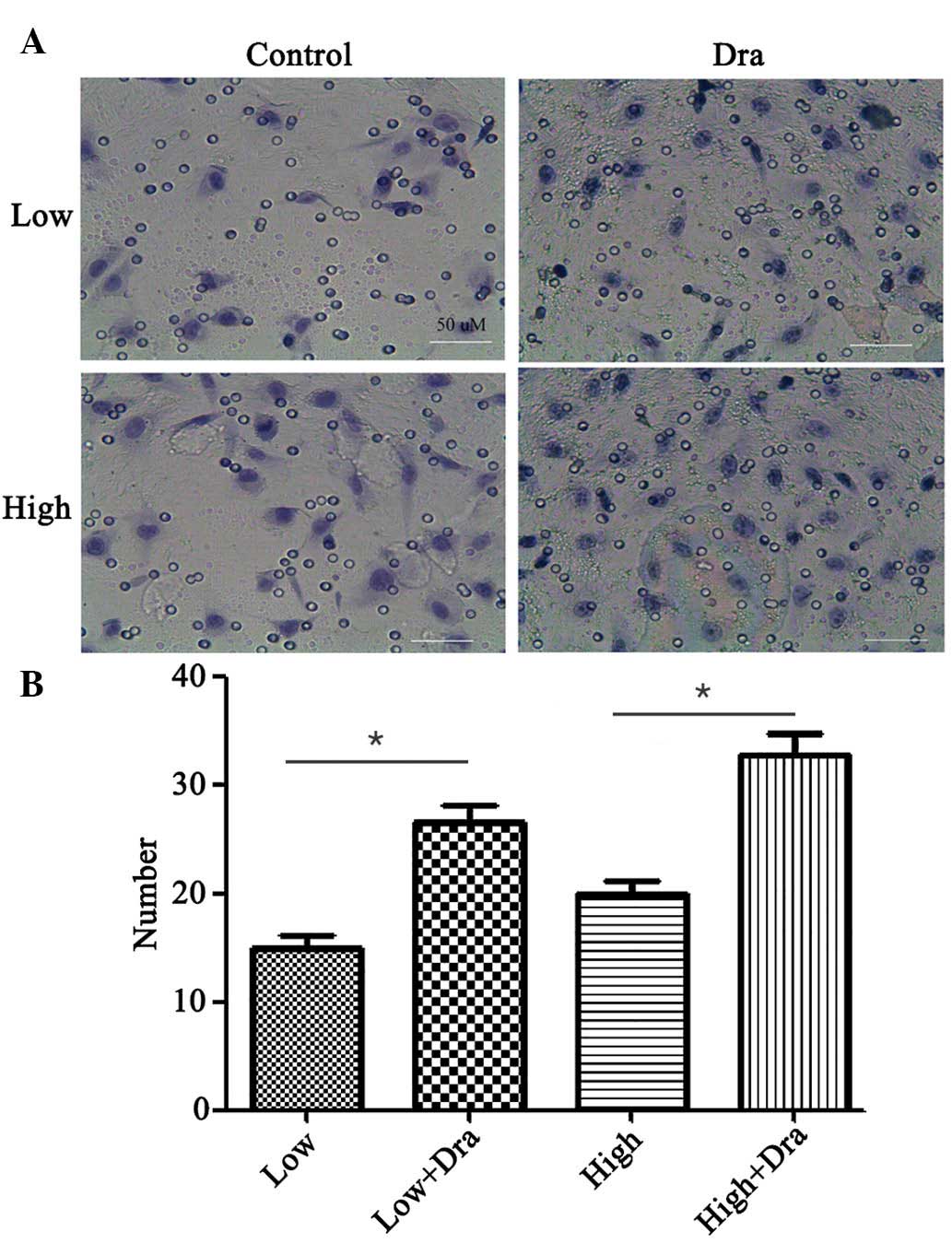
Dra facilitates HUVECs cell
migration
Cell migration is suggested to serve a key role
during wound repair. To investigate this, a Transwell assay was
conducted using Dra and different glucose concentrations (Fig. 2A). This indicated an increased in
the number of migrated cells following Dra treatment with both LG
and HG concentrations. The cell numbers were calculated and are
presented in Fig. 2B.
Effects of Dra on tube formation of
HUVECs
Endothelial cell proliferation and migration are
involved in angiogenesis which leads to subsequent vascular
structure formation. Therefore, a tube formation assay was
performed to evaluate the ability of Dra to facilitate endothelial
cell differentiation during angiogenesis. As presented in Fig. 3A, when HUVECs were cultured on
Matrigel in the absence of Dra, HG was observed to reduce tube
formation. However, when incubated with Dra, more complex and
branched tubular structures were formed.
The results indicate that Dra may induce tube
formation in HG concentrations and reduce tube formation in LG
concentrations (Fig. 3B). Further
analysis revealed the effect of the Dra treatment on the tube
diameter under LG and HG concentrations. As presented in Fig. 3C, compared with the HG
concentration, LG concentration induced the formation of narrower
and more filamentous tubes. However, increased branched tubular
structure was formed following Dra treatment under both low and
high glucose concentration.
Dra enhances the production of VEGF in
HUVECs
In order to determine whether Dra treatment
increased VEGF secretion by HUVECs, the VEGF levels were measured
in the HUVECs using ELISA. HG treatment resulted in a reduction in
VEGF secretion (Fig. 4). Following
Dra treatment, the secretion of VEGF was induced in both the LG and
HG concentrations. Additionally, the ratio of VEGF increasing under
low glucose concentration was significant compared with the ratio
under high glucose concentration (Fig.
4).
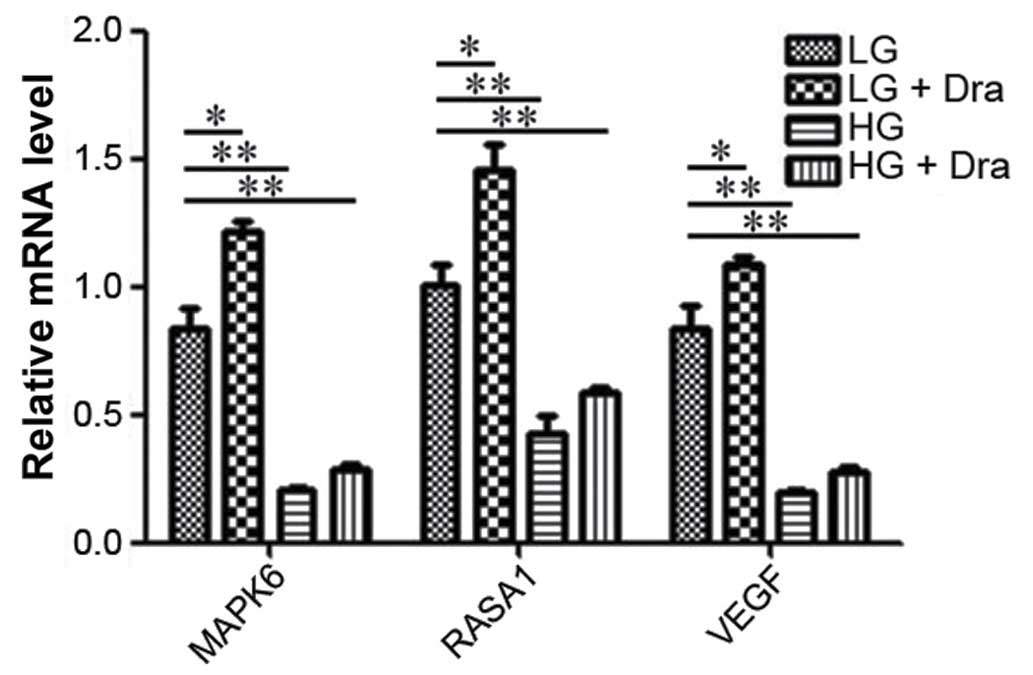
MAPK signaling pathway analysis
The VEGF receptor (R)-Ras-MAPK signaling pathway
serves an important role in the regulation of angiogenesis.
Therefore, VEGF, Ras and MAPK protein expression levels were
analyzed by western blotting (Fig.
5) and RT-qPCR (Fig. 6). The
expression levels Ras protein and of VEGF, Ras and MAPK mRNA were
suppressed by the HG concentration as shown in Figs. 5 and 6. These results were consistent with
capillary growth suppression in diabetic ulcers (8). In addition, Dra was able to induce
the expression levels of VEGF, Ras and MAPK under both LG and HG
concentrations, with a greater effect observed in the LG
concentration.
Discussion
Wound healing is a complicated process. There are at
least three stages during the process of wound healing:
Inflammation, proliferation and remodeling. The proliferation phase
is characterized by granulation tissue formation, which primarily
contains newly generated blood vessels, fibroblasts and collagen
fibers. Endothelial cell and fibroblasts are considered to serve an
essential role in the wound healing process in addition to
angiogenesis. Angiogenesis during wound repair involves new blood
vessel growth from endothelial cells, and serves a dual function of
providing essential nutrients and oxygen to the wound site and
promoting granulation tissue formation. However, in diabetic ulcer
cases, healing impairment is caused by a number of physiological
factors, with reduced angiogenesis being a main factor. Inhibition
of angiogenesis delays the wound healing process. Poor vasculature
in diabetic wounds has been observed in diabetic mice, suggesting
that dysfunction of the endothelium is a key factor in the
pathogenesis of angiogenesis in diabetic ulcers (20). Therefore, agents that promote
angiogenesis may improve diabetic wound healing.
Debridement, offloading and infection control are
the main treatments for diabetic foot ulcers, and new treatments
continue to be introduced. However, the majority of treatments are
only effective for mild and moderate wounds, and the risk of
amputation remains. Therefore, integration of traditional Chinese
medicines with conventional treatments may aid in treating diabetic
foot ulcers. Dragon's blood has long been used as a traditional
Chinese medicine for improving blood circulation, inhibiting
platelet aggregation and thrombus formation and healing wounds.
Furthermore, dragon's blood has been indicated to have additional
bioactivities, including alleviating insulin resistance,
antimicrobial, anti-inflammatory and antioxidant effects (17,21,22).
Considering its predominant effect on circulation, the current
study postulated that the effects of dragon's blood on chronic
wounds induced by diabetes, may be attributed to the promotion of
angiogenesis. Therefore, the effects of Dra, one of the main
biological components of dragon's blood that can facilitate wound
healing, were evaluated in angiogenesis, and the underlying
mechanisms were explored.
Using an in vitro HG concentration in
cultured HUVECs, it was possible to investigate the main steps of
angiogenesis, in addition to the signaling pathway through which
Dra may function. HUVECs are frequently used as in vitro
model systems for various physiological and pathological processes,
in particular in angiogenesis research. The proliferation,
migration and formation of tubular structure by endothelial cells
are indicators for the development of new blood vessels from the
preexisting vascular bed in angiogenesis. The present study
indicated that Dra was able to promote angiogenesis through
increasing the proliferation, migration and tube formation of
HUVECs, which are the initial stages of angiogenesis. The MTT assay
indicated that Dra treatment was able to induce proliferation in
HUVECs under both LG and HG concentrations. Furthermore, the
migration of HUVECs was observed to be facilitated by Dra, in
particular at the HG concentration, in addition to tube formation
activity. These results are consistent with previous studies
(23). However, further analysis
revealed that Dra promoted the formation of thick tubes with large
diameters under the different glucose concentrations. More highly
branched tubular structures were observed to form following Dra
treatment at both LB and HG concentrations.
It is notable that numerous types of cytokines and
growth factors are responsible for inflammation,
re-epithelialization and the formation of granulation tissue in the
wound healing process. For example, VEGF is an important angiogenic
cytokine, and promotes wound healing by stimulating proliferation
and the migration of endothelial cells through the extracellular
matrix. In addition, VEGF promotes the secretion of active growth
factors and cytokines necessary for wound repair (23). In the present study, the production
of VEGF by HUVECs was measured, and under both LG and HG, Dra was
able to increase VEGF secretion.
VEGF activates various signaling pathways including
phosphatidylinositol 3-kinase/Akt, protein kinase C and MAPK
cascades. The Ras/MAPK signaling pathway is a signaling system that
controls fundamental cellular processes including cell
proliferation, differentiation, survival and migration (24). Receptor tyrosine kinases and their
ligands often act as the inducers of this signaling system. The
VEGFR/Ras/MAPK signaling pathways have been shown to be crucial
downstream signaling targets involved in VEGF-induced angiogenesis,
and both RAS and MAPK serve an important role in it. Therefore, the
current study aimed to determine whether Dra treatment led to the
activation of MAPK pathway by measuring Ras and MAPK expression
levels. It was observed that in accordance with the VEGF
expression, the mRNA expression levels of Ras and MAPK were
downregulated in HG concentrations and upregulated by Dra
treatment. In addition, the western blotting results indicated that
VEGF, Ras and MAPK signaling were all activated by Dra treatment in
HUVECs under HG conditions. From the results of the present study,
it may be concluded that the effects of Dra on endothelial cells
such as producing VEGF and activating the Ras/MAPK signaling
pathway, are the cornerstones of Dra-mediated promotion of
angiogenesis.
In conclusion, the current study indicates that
promoting angiogenesis may be a potential mechanism for the
therapeutic effects of dragon's blood on diabetic wounds. The
function of Dra was observed to involve the activation of the
Ras/MAPK signaling pathway in endothelial cells. The current study
indicates that Dra, a traditional Chinese medicine isolated from
dragon's blood, may hold potential as a clinical treatment for
wound healing and blood vessel damage.
References
|
1
|
Bedell AJ: Retinal Vessel Proliferation in
Diabetes. Trans Am Ophthalmol Soc. 43:271–276. 1945.PubMed/NCBI
|
|
2
|
Cholst MR, Levitt LM and Handelsman MB:
Small vessel dysfunction in patients with diabetes mellitus. II.
Retinal vessel response in diabetics following priscoline. Am J Med
Sci. 224:39–41. 1952. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colwell JA and Lopes-Virella MF: A review
of the development of large-vessel disease in diabetes mellitus. Am
J Med. 85(5A): 113–118. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pedersen J and Olsen S: Small-vessel
disease of the lower extremity in diabetes mellitus. On the
pathogenesis of the foot-lesions in diabetics. Acta Med Scand.
171:551–559. 1962. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kavitha KV, Tiwari S, Purandare VB,
Khedkar S, Bhosale SS and Unnikrishnan AG: Choice of wound care in
diabetic foot ulcer: A practical approach. World J Diabetes.
5:546–556. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Falanga V: Wound healing and its
impairment in the diabetic foot. Lancet. 366:1736–1743. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walshe TE and D'Amore PA: The role of
hypoxia in vascular injury and repair. Annu Rev Pathol. 3:615–643.
2008. View Article : Google Scholar
|
|
8
|
Cooper ME, Vranes D, Youssef S, Stacker
SA, Cox AJ, Rizkalla B, Casley DJ, Bach LA, Kelly DJ and Gilbert
RE: Increased renal expression of vascular endothelial growth
factor (VEGF) and its receptor VEGFR-2 in experimental diabetes.
Diabetes. 48:2229–2239. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qaum T, Xu Q, Joussen AM, Clemens MW, Qin
W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD
and Adamis AP: VEGF-initiated blood-retinal barrier breakdown in
early diabetes. Invest Ophthalmol Vis Sci. 42:2408–2413.
2001.PubMed/NCBI
|
|
10
|
Advani A, Connelly KA, Advani SL, Thai K,
Zhang Y, Kelly DJ and Gilbert RE: Role of the eNOS-NO system in
regulating the antiproteinuric effects of VEGF receptor 2
inhibition in diabetes. BioMed Res Int. 2013:2014752013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang Y, Chang TC, Lee JJ, Chang NC, Huang
YK, Choy CS and Jayakumar T: Sanguis draconis, a dragon's blood
resin, attenuates high glucose-induced oxidative stress and
endothelial dysfunction in human umbilical vein endothelial cells.
Scientific-World Journal. 2014:4232592014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Lin S, Xiao D, Zheng X, Gu Y and
Guo S: Evaluation of the Wound Healing Potential of Resina Draconis
(Dracaena cochinchinensis) in Animal Models. Evid Based Complement
Alternat Med. 2013:7098652013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang P, Li J, Tang X, Zhang J, Liang J
and Zeng G: Dracorhodin perchlorate induces apoptosis in primary
fibroblasts from human skin hypertrophic scars via participation of
caspase-3. Eur J Pharmacol. 728:82–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu JH, Zheng GB, Liu CY, Zhang LY, Gao HM,
Zhang YH, Dai CY, Huang L, Meng XY, Zhang WY and Yu XF: Dracorhodin
perchlorate induced human breast cancer MCF-7 apoptosis through
mitochondrial pathways. Int J Med Sci. 10:1149–1156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu Q, Sun E, Xu FJ, Zhang ZH and Jia XB:
Simultaneous content determination of notoginsenoside R1,
ginsenoside Rg1, Re, Rb1 and dracorhodin in ZJHX rubber paste by
double wavelength HPLC. Zhongguo Zhong Yao Za Zhi. 38:2793–2797.
2013.In Chinese.
|
|
16
|
Rasul A, Ding C, Li X, Khan M, Yi F, Ali M
and Ma T: Dracorhodin perchlorate inhibits PI3K/Akt and NF-κB
activation, upregulates the expression of p53, and enhances
apoptosis. Apoptosis. 17:1104–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He Y, Ju W, Hao H, Liu Q, Lv L and Zeng F:
Dracorhodin perchlorate suppresses proliferation and induces
apoptosis in human prostate cancer cell line PC-3. J Huazhong Univ
Sci Technolog Med Sci. 31:215–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia MY, Wang MW, Cui Z, Tashiro SI,
Onodera S, Minami M and Ikejima T: Dracorhodin perchlorate induces
apoptosis in HL-60 cells. J Asian Nat Prod Res. 8:335–343. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang P, Li J, Tang X, Zhang J, Liang J
and Zeng G: Dracorhodin perchlorate induces apoptosis in primary
fibroblasts from human skin hypertrophic scars via participation of
caspase-3. Eur J Pharmacol. 728:82–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roy DC, Mooney NA, Raeman CH, Dalecki D
and Hocking DC: Fibronectin matrix mimetics promote full-thickness
wound repair in diabetic mice. Tissue Eng Part A. 19:2517–2526.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu S, Li Z and Qian G: Quantitative
determination of dracorhodin in Daemonorops draco B1. and
traditional Chinese medicines containing Daemonorops draco B1. by
HPLC. Hua Xi Yi Ke Da Xue Xue Bao. 28:450–453. 1997.In Chinese.
|
|
22
|
Xia M, Wang D, Wang M, Tashiro S, Onodera
S, Minami M and Ikejima T: Dracorhodin perchlorate induces
apoptosis via activation of caspases and generation of reactive
oxygen species. J Pharmacol Sci. 95:273–283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Corral CJ, Siddiqui A, Wu L, Farrell CL,
Lyons D and Mustoe TA: Vascular endothelial growth factor is more
important than basic fibroblastic growth factor during ischemic
wound healing. Arch Surg. 134:200–205. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kolch W: Meaningful relationships: The
regulation of the Ras/Raf/MEK/ERK pathway by protein interactions.
Biochem J. 351:289–305. 2000. View Article : Google Scholar : PubMed/NCBI
|