Introduction
Idiopathic pulmonary fibrosis (IPF) is a complex
disease, which remains to be fully elucidated, and is associated
with high mortality and morbidity rates, of which the median
survival rate following diagnosis is 2–5 years (1,2). The
number of individuals succumbing to pulmonary fibrosis-associated
mortality in the USA has increased by 50% in just 10 years
(1992–2003) (3). Previous findings
have identified certain mechanisms involving fibrosis, including
transforming growth factor-β (TGF-β), Wnt ligands, toll-like
receptor-mediated signaling and type 2 immune responses (4–7).
Although studies have been performed over decades (5–7), the
etiology of the disease remains to be elucidated, the mechanism of
fibrosis remains unclear and disease-modifying therapies show only
poor efficacy.
Interleukin (IL) -33/ST2 signaling, a novel pathway,
has been investigated in several fibrotic diseases, including
scleroderma, progressive systemic sclerosis and liver fibrosis, in
mice and humans (8–10). In the present study, the IL-33/ST2
signaling pathway was examined in a mouse model of lung
fibrosis.
Materials and methods
Regents and instruments
Bleomycin hydrochloride powder (cat. no. H20040205)
was purchased from Nippon Kayaku Co., Ltd. (Toyko, Japan). Rabbit
anti-mouse myeloid differentiation primary response 88 (MyD88)
polyclonal antibody (cat. no. BA2321; 1:2,000) was purchased from
Boster Biotechnology, Co., Ltd. (Wuhan, China). Rabbit anti-mouse
TRAF6 polyclonal antibody (cat. no. sc-7221; 1:2,000) was purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
remaining antibodies, including goat anti-rabbit horseradish
peroxidase (HRP) -conjugated antibody (1:2,000; CW Biotech Co.,
Ltd., Beijing, China), rabbit anti-mouse IL-33 monoclonal antibody
(cat. no. AF3626; 1:2,000; R&D Systems, Inc. CA, USA) and
rabbit anti-mouse ST2 polyclonal antibody (cat. no. ab72778;
1:2,000; Abcam, Cambridge, UK), were also used in the present
study. Commercial kits, including an enzyme-linked immunosorbent
assay (ELISA) kit (Biotech Co., Ltd, Beijing, China) and Masson's
kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China),
were also used in the present study. Instruments, including a
Typhoon 9400 scanner (GE Healthcare Life Sciences, Bethesda, MD,
USA), a Power Transfer system (model 3550; Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and microplate reader (EL-311; Bio-TEK,
Instruments, Inc), were used for the experimental processes.
Animals
The animals used in the present study were purchased
from the Laboratory Animal Center of Jiangsu University (Zhenjiang,
China). A total of 40 female Kunming strain mice (6 weeks-old,
specific pathogen-free grade), weighing 20±2 g, were maintained at
25°C under a 12-h light/dark cycle with ad libitum access to
rodent chow and water. All experiments were performed in accordance
with the research proposal for the Care and Use of Laboratory
Animals formulated by the Ministry of Science and Technology of
China (11). The present study was
approved by the ethics committee of the First People's Hospital of
Changzhou (Changzhou, China).
Induction of lung injury and pulmonary
fibrosis by BLM and sample harvest
Using a random digit table, the 40 mice were evenly
divided into two groups: Control group (Cont group, n=20) and
experimental group (BLM group; n=20). On day 0, following
anesthesia by intraperitoneal injection with chloral hydrate (0.01
mg/kg; Sigma-Aldrich, St. Louis, MO, USA), the BLM group was
intratracheally administered bleomycin solution at an optimum dose
of 5 mg/kg (body weight) (12),
whereas the animals in the Cont group were administered the same
volume of saline. On days 3, 7, 14 and 28 following modeling, five
randomly selected mice from each group were sacrificed by cervical
dislocation following intraperitoneal injection with 1 mg/kg
pentobarbital sodium (Sigma-Aldrich), and the lung tissues were
harvested. The left lung tissues were fixed with 4%
paraformaldehyde (Sigma-Aldrich), whereas the right samples were
frozen in liquid nitrogen (Tiangen Biotech Co., Ltd., Beijing,
China) for 10 mins and then stored at −70°C.
Histopathological assessment of pulmonary
injury, inflammation and fibrosis
Following fixing with 4% paraformaldehyde, the left
lung tissues were paraffin-embedded (Tiangen Biotech Co., Ltd.) and
4 µm sections were generated. The section were then stained
with hematoxylin and eosin to detect the degree of inflammatory
injury and Masson's trichrome staining was used to detect the
degree of fibrosis. Alveolitis was graded on a scale of 0–3, as
follows: 0, normal pulmonary alveolus morphology without alveolar
inflammation; 1, mild alterations, including a widened alveolar
septum due to inflammatory cell infiltration; 2, moderate
alterations; and 3, severe alterations, including large numbers of
infiltrated inflammatory cells and diffused pathological changes.
Pulmonary fibrosis was graded on a scale of 0–3, as follows: 0,
normal pulmonary tissue without or with only a few filament-like
collagen fibers; 1, slightly increased numbers of collagen fibers
showing a fine bundle shape; 2, moderately increased numbers of
collagen fibers that were fused into a narrow-band shape, with a
disordered alveolar structure; and 3, markedly increased numbers of
collagen fibers, exhibiting a broad-band or lamellar shape, as well
as collapsed or fused pulmonary alveoli and a disordered structure.
According to the method established by Szapiel et al
(13), scores of 0, 1, 2 and 4
were indicative of grades 0, 1, 2 and 3 alveolitis or pulmonary
fibrosis, respectively.
Detection of protein expression levels
using western blotting
The lung tissues from the two groups were lysed in
radioimmunoprecipitation assay buffer (Sigma-Aldrich), which
included protease and phosphatase inhibitors. Proteins were
extracted using the EpiQuik Whole Cell Extraction kit (cat. no.
OP-0003-100; Epigentek Group Inc., Farmingdale, NY, USA) and the
protein concentrations were quantified using the BCA kit (cat. no.
BCA1-1KT; Sigma-Aldrich). Equal quantities of protein (10
µg) were separated by 10% SDS-PAGE and then transferred onto
polyvinylidene fluoride membranes (Tiangen Biotech Co., Ltd.). The
membranes were blocked with 5% skimmed milk powder for 1 h,
followed by incubation with primary antibodies for 2 h at room
temperature. After washing the membranes three times for 5 min each
with tris-buffered saline containing Tween-20, they were incubated
with HRP-conjugated goat anti-rabbit secondary antibody for 1 h at
37°C. β-actin was used as an internal control. Specific bands were
visualized using an ECL-PLUS chemiluminescence system (Beyotime
Institute of Biotechnology, Wuhan, China) and band intensities were
quantified using Land-1D Analyzer software, version 4.0 (Beijing
Sage Creation Science, Co., Ltd., Beijing, China).
ELISA for the detection of serum levels
of IL-4 and IL-13
Blood samples (10 ml) were obtained from the
posterior eyeball veins using heparin-treated glass capillary
tubes, and then stored at −70°C. To detect the optical density
values of IL-4 and IL-13, the supernatants of each group were
obtained by centrifugation at 1,000 × g for 5 min at 4°C, and were
measured using a sandwich ELISA kit, according to the
manufacturer's protocol. From a standard curve, the concentrations
of each cytokine were obtained.
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analyses. Data are presented as the mean ±
standard deviation. Statistical differences between groups were
compared using an Independent Sample t-test. P≤0.05 was
considered to indicate a statistically significant difference.
Results
Pathological changes in lung tissues
The architectures in the lung tissues of the Cont
group were normal with no substantial inflammatory cell
infiltration or fiber collagen proliferation (Fig. 1Aa and Ba). In the BLM group, from
day 3 of model establishment, alveolitis began to deepen, with
inflammatory cells and erythrocytes in the septations and alveoli,
but without evident fiber collagen (Table I). On day 7, the architecture of
the alveoli were severely damaged and inflammation peaked, and this
was accompanied by a small quantity of fiber collagen (Fig. 1Ab and Bb). The inflammation on day
14 was less severe, however, the alveoli were collapsed and fused,
and the septations were widened with extensive fiber collagen
(Fig. 1Ac and Bc). On day 28, the
inflammation was less marked, however, the architecture of the
alveoli were blurred, with wild broad-banded fiber collagen and
diffuse lung fibrosis (Fig. 1Ad and
Bd). Compared with the Cont group, significant differences were
observed in alveolitis on days 3, 7, 14 and 28 (P<0.01), and
grades of fibrosis were significantly different on days 7, 14 and
28 (P<0.01).
 | Table IComparison of the extent of alveolitis
and lung fibrosis. |
Table I
Comparison of the extent of alveolitis
and lung fibrosis.
| Group | Day 3
| Day 7
| Day 14
| Day 28
|
|---|
| Alveolitis | Fibrosis | Alveolitis | Fibrosis | Alveolitis | Fibrosis | Alveolitis | Fibrosis |
|---|
| Cont | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 | 1.00±0.00 |
| BLM | 2.40±0.10a | 1.10±0.10a | 3.50±0.10a | 1.70±0.20a | 2.56±0.15a | 2.63±0.31a | 2.20±0.10a | 3.47±0.25a |
Protein expression levels of IL-33, ST2,
MyD88 and TRAF6
Compared with the Cont group, the protein levels of
ST2, MyD88 and TRAF6 in the BLM group increased from day 3, and
reached a peak on day 28, with significantly higher levels,
compared with the Cont group, on days 3, 7, 14 and 28 (P<0.05;
Fig. 2A and B). The expression of
IL-33 increased initially, and then decreased gradually with a peak
on day 7. Significant differences were observed on days 3 and 7
between the two groups (P<0.05; Fig. 2A and B).
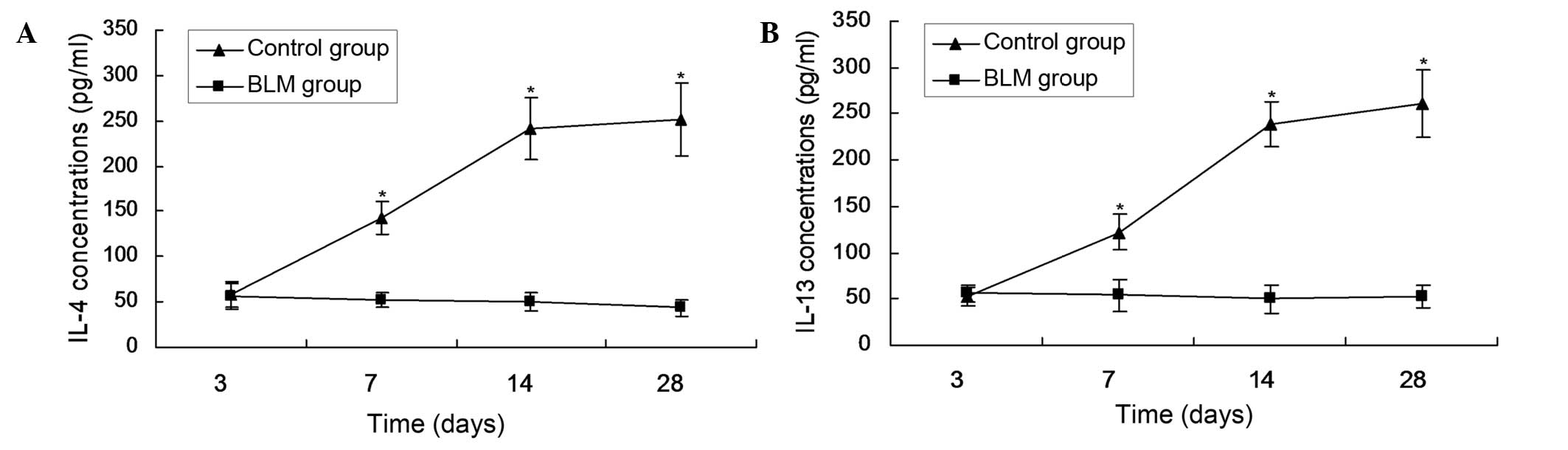
Serum levels of IL-4 and IL-13
The levels of IL-4 and IL-13 in the serum increased
from day 7 and peaked on day 28. The levels were significantly
higher, compared with the Cont group, on days 7, 14 and 28
(P<0.05; Fig. 3).
Discussion
The present study was the first, to the best of our
knowledge, to investigate whether IL-33/ST2 signaling exists in a
murine model of bleomycin-induced pulmonary fibrosis. IL-33, as a
newly described cytokine of the IL-1 family, can also be termed
IL-1F11 according to systematic nomenclature, and has been
identified as DVS27, which shows the closest amino acid sequence
homology to DVS27 of dogs, and as a nuclear factor from high
endothelial venules, which is expressed in the endothelial cell
nuclei (14). IL-33 is produced as
pro-IL-33, a 31-kDa protein, and is cleaved by caspase-1 to form a
mature 18-kDa protein, which acts as a cytokine through its
IL-1-receptor family members (14,15).
IL-33, which contributes to the immigration, differentiation and
maturation of T helper (Th)2 cells, eosinophils and mast cells, is
released passively from damaged epithelial and endothelial cells
(16). In the present study, high
expression levels of IL-33 were observed during the acute early
stage of fibrosis, whereas low expression levels were observed at
the late stage of pulmonary fibrosis. This suggested that IL-33, as
an alarmin, was secreted from the injured cells induced by
bleomycin, and resisted damage and apoptosis (15).
ST2, the most prominent orphan IL-1 receptor, also
termed T1, Fit-1, and DER4, was originally detected as one of the
primary response genes at the initial stage of cell proliferation
in fibroblasts and belongs to the Toll-like receptor (TLR) -IL-1
receptor superfamily (17–19). Schmitz et al first
identified orphan receptor ST2, also termed IL-1R4, as a receptor
for IL-33 via co-immunoprecipitation, and showed that the
combination of IL-33 and ST2, through the downstream molecules of
ST2, MyD88 and TRAF6, can ultimately lead to the activation of
NF-κB and mitogen-activated protein kinases, which are involved in
the control of cellular proliferation and apoptosis (14). Xu et al showed that ST2 gene
products are predominantly expressed in Th2 cells, but not in Th1
cells, and has been recognized as a stable marker of Th2 cells
(20,21). The suggestion that the co-operation
of IL-33 and ST2 promotes the activation of Th2 cells was confirmed
by the findings of the present study that the protein expression
levels of MyD88 and TRAF6 began to increase from day 3 following
model establishment, with a similar trend in the protein level of
ST2. This was accompanied by increases in the levels of IL-4 and
IL-13, two major cytokines of Th2 cells. Netanya et al
confirmed that IL-4 and IL-13 can involve fibrogenesis by
upregulating genes associated with wound healing, specifically,
arginase, collagens, matrix metalloproteinases (MMPs), and tissue
inhibitors of MMP, or by recruiting M2 cells, mast cells,
eosinophils, dendritic cells and myofibroblast, facilitating
excessive tissue repair and tissue fibrosis (22,23).
Therefore, the present study hypothesized that
IL-33, as a 'master switch' of tissue repair that is secreted from
dying or apoptotic cells, activated the IL-33-ST2-MyD88-TRAF6
pathway, amplified Th2-type responses and was involved in the
pulmonary fibrosis process, via its receptor ST2, a stable marker
of Th2 cells. The pathogenesis of IPF has been described in
previous investigations, which revealed that the exuberant
deposition of extracellular matrix and the formation of fibrotic
foci were induced by the repeated injury of alveolar epithelial
cells (24–27). Hayakawa et al (28) showed that soluble ST2 had
antagonistic effects on IL-33 signaling, using murine thymoma EL-4
cells stably expressing ST2L and a murine model of asthma.
Therefore, intervention of the IL-33/ST2 signaling pathway may
offer potential in the therapy of fibrotic diseases, and may be
used as a potential target for IPF.
Acknowledgments
The present study was supported by the Applied Basic
Research of Changzhou Municipal Science and Technology Bureau
(grant no. CJ20130028).
References
|
1
|
Takenaka K, Gemma A, Yoshimura A, Hosoya
Y, Nara M, Hosomi Y, Okano T, Kunugi S, Koizumi K, Fukuda Y, et al:
Reduced transcription of the Smad 4 gene during pulmonary
carcinogenesis in idiopathic pulmonary fibrosis. Mol Med Rep.
2:73–80. 2009.PubMed/NCBI
|
|
2
|
du Bois RM: Idiopathic pulmonary fibrosis:
Present understanding and future options. Eur Respir Rev.
20:132–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Olson AL, Swigris JJ, Lezotte DC, Norris
JM, Wilson CG and Brown KK: Mortality from pulmonary fibrosis
increased in the United States from 1992 to 2003. Am J Respir Crit
Care Med. 176:277–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khalil N, O'Connor RN, Flanders KC and
Unruh H: TGF-beta 1, but not TGF-beta 2 or TGF-beta 3, is
differentially present in epithelial cells of advanced pulmonary
fibrosis: An immunohistochemical study. Am J Respir Cell Mol Biol.
14:131–138. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Villar J, Cabrera N, Valladares F, Casula
M, Flores C, Blanch L, Quilez M, Santana-Rodriguez N, Kacmarek R
and Slutsky A: Activation of the Wnt/b-Catenin signaling pathway by
mechanical ventilation is associated with ventilator-induced
pulmonary fibrosis in healthy lungs. PLoS One. 6:e239142012.
View Article : Google Scholar
|
|
6
|
Zhang Y, Li Y, Li Y, Li R, Ma Y, Wang H
and Wang Y: Chloroquine inhibits MGC803 gastric cancer cell
migration via the Toll-like receptor 9/nuclear factor kappa B
signaling pathway. Mol Med Rep. 11:1366–1371. 2015.
|
|
7
|
Zhang Z, Chen N, Liu JB, Wu JB, Zhang J,
Zhang Y and Jiang X: Protective effect of resveratrol against acute
lung injury induced by lipopolysaccharide via inhibiting the
myd88-dependent Toll-like receptor 4 signaling pathway. Mol Med
Rep. 10:101–106. 2014.PubMed/NCBI
|
|
8
|
Kuroiwa K, Arai T, Okazaki H, Minota S and
Tominaga S: Identification of human ST2 protein in the sera of
patients with autoimmune diseases. Biochem Biophys Res Commun.
284:1104–1108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lax A, Sanchez-Mas J, Asensio-Lopez MC,
Fernandez-Del Palacio MJ, Caballero L, Garrido IP, Pastor-Perez FJ,
Jauzzi JL and Pascual-Figal DA: Mineralocorticoid receptor
antagonists modulate galectin-3 and interleukin-33/ST2 signaling in
left ventricular systolic dysfunction after acute myocardial
infarction. JACC Heart Fail. 3:50–58. 2015. View Article : Google Scholar
|
|
10
|
Marvie P, Lisbonne M, L'Helgoualc'h A,
Rauch M, Turlin B, Preisser L, Bourd-Boittin K, Théret N, Gascan H,
Piquet-Pellorce C and Samson M: Interleukin-33 overexpression is
associated with liver fibrosis in mice and humans. J Cell Mol Med.
14:1726–1739. 2010. View Article : Google Scholar
|
|
11
|
The Ministry of Science and Technology of
the People's Republic of China: Guidance Suggestions for the Care
and Use of Laboratory Animals. http://www.most.gov.cn/eng/pressroom/.
Accessed September 30, 2006.
|
|
12
|
Tao Z and Li HP: Comparison of mouse
pulmonary fibrosis models induced by bleomycin at different doses.
Zhongguo zuzhi gongcheng yanjiu yu linchuang kangfu. 13:1214–1218.
2009.In Chinese.
|
|
13
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI
|
|
14
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an Interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baekkevold ES, Roussigné M, Yamanaka T,
Johansen FE, Jahnsen FL, Amalric F, Brandtzaeg P, Erard M,
Haraldsen G and Girard JP: Molecular characterization of NF-HEV, a
nuclear factor preferentially expressed in human high endothelial
venules. Am J Pathol. 163:69–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Borish L and Steinke JW: Interleukin-33 in
Asthma: How big of a role does it play? Curr Allergy Asthma Rep.
11:7–11. 2011. View Article : Google Scholar :
|
|
17
|
Tominaga S: A putative protein of a growth
specific cDNA from BALB/c-3T3 cells is highly similar to the
extracellular portion of mouse interleukin 1 receptor. FEBS Lett.
258:301–304. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yanagisawa K, Tsukamoto T, Takagi T and
Tominaga S: Murine ST2 gene is a member of the primary response
gene family induced by growth factors. FEBS Lett. 302:51–53. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bergers G, Reikerstorfer A, Braselmann S,
Graninger P and Busslinger M: Alternative promoter usage of the
Fos-responsive gene Fit-1 generates mRNA isoforms coding for either
secreted or membrane-bound proteins related to the IL-1 receptor.
EMBO J. 13:1176–1188. 1994.PubMed/NCBI
|
|
20
|
Xu D, Chan WL, Leung BP, Huang Fp, Wheeler
R, Piedrafita D, Robinson JH and Liew FY: Selective expression of a
stable cell surface molecule on type 2 but not type 1 helper T
cells. J Exp Med. 187:787–794. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Löhning M, Stroehmann A, Coyle AJ, Grogan
JL, Lin S, Gutierrez-Ramos JC, Levinson D, Radbruch A and Kamradt
T: T1/ST2 is preferentially expressed on murine Th2 cells,
independent of interleukin 4, interleukin 5, and interleukin 10,
and important for Th2 effector function. Proc Natl Acad Sci USA.
95:6930–6935. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sandler NG, Mentink-Kane MM, Cheever AW
and Wynn TA: Global gene expression profiles during acute
pathogen-induced pulmonary inflammation reveal divergent roles for
Th1 and Th2 responses in tissue repair. J Immunol. 171:3655–3667.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rubartelli A and Lotze MT: Inside,
outside, upside down: Damage-associated molecular-pattern molecules
(DAMPs) and redox. Trends Immunol. 28:429–436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tajima S, Oshikawa K, Tominaga S and
Sugiyama Y: The increase in serum soluble ST2 protein upon acute
exacerbation of idiopathic pulmonary fibrosis. Chest.
124:1206–1214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
du Bois RM: Strategies for treating
idiopathic pulmonary fibrosis. Nat Rev Drug Discov. 9:129–140.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meltzer EB and Noble PW: Idiopathic
pulmonary fibrosis. Orphanet J Rare Dis. 3:82008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Selman M, King TE and Pardo A; American
Thoracic Society; European Respiratory Society; American College of
Chest Physicians: Idiopathic pulmonary fibrosis: Prevailing and
evolving hypotheses about its pathogenesis and implications for
therapy. Ann Intern Med. 134:136–151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hayakawa H, Hayakawa M, Kume A and
Tominaga S: Soluble ST2 blocks interleukin-33 signaling in allergic
airway inflammation. J Biol Chem. 282:26369–26380. 2010. View Article : Google Scholar
|