1. Current status of chronic fatigue
syndrome
Chronic fatigue syndrome (CFS) is a disabling
condition in which the patient is affected by long-term fatigue.
Symptoms, which can lasts for ≥6 months, include tiredness, pain,
breathing problems, depression leading to digestive disturbances,
low-grade fever, difficulty in concentrating, and weakness of the
immune system and muscles (1). One
characteristic of this disease is that the symptoms are not
relieved by increased rest (1).
Another characteristic of CFS is the absence of intervention or
medication that is universally effective in treating the symptoms
(2). Furthermore, CFS causes not
only personal problems, but also economic problems. Several
previous studies have demonstrated that the incidence of CFS is
0.4% in the USA and other countries (3) and 0.26% in Japan (4). CFS is present in 522/100,000 females
and 291/100,000 males in the USA, indicating that CFS incidence in
females is higher compared with in males (2). In addition, economic losses due to
CFS are estimated to be as high as $9.1 billion per annum in the
USA (5) and ¥408 billion per annum
in Japan (4). Therefore, CFS has
an impact on society in general, as well as on the individual
patient.
While patients affected by CFS sometimes suffer from
recognized symptoms, they often experience other social problems,
which makes the disorder difficult to recognize. Research performed
by the Centers for Disease Control and Prevention (Atlanta, GA,
USA) estimates that <20% of patients with CFS in the USA have
been successfully diagnosed (3,6).
Clearly, the establishment of a reliable diagnostic test for CFS
will improve our understanding of this disorder. However, several
obstacles are preventing the accomplishment of this goal. One such
obstacle is the high degree of heterogeneity in the symptoms of
patients with CFS (7). Thus, at
present, CFS is diagnosed on the basis of the presenting symptoms,
coupled with the exclusion of other medical disorders (1). The other predominant barriers to
diagnosing patients with CFS are an absence of biophysical and
biochemical signs that identify the disease, and a lack of
diagnostic laboratory tests (7).
Consequently, typical screening results of blood samples for CFS
are often unrevealing or negative. As a result, CFS diagnosis
requires experience and techniques that can be performed only by
skilled doctors on the basis of exclusion of all other possible
causes of chronic fatigue as listed.
2. CFS abnormalities and research
Despite various data on the psychological,
endocrinological and immunological abnormalities observed among CFS
patients, no clear consensus exists on the symptoms for this
disorder (12). Several
indications of CFS have been reported, including altered levels of
cytokines (13), immunoglobulins
(13), autoantibodies (14), RNase L (15), 2′-5′oligo-adenylate synthetase
(15), melatonin (16), dehydroepiandrostenedione (16), growth hormones (17), acylcarnitine (18), folic acid (19), vitamins (20), amino acids (20), carnitine Coenzyme Q10 (20), fatty acids (20) and minerals (20,21).
Altered cell populations and activity of the immune system have
also been reported (13,22). In addition, alterations in T-cell
phenotype and proliferative response, along with the specific
signature of the NK cell phenotype, have been reported in certain
individuals with CFS (23). Other
homeostatic changes involving the opioid system (24) and arginine vasopressin system
(24) may be associated with CFS.
Furthermore, adrenocorticotropic hormone and the cortisol response
appear to be aberrant among patients with CFS (16).
The severe levels of fatigue and disability
associated with CFS may be associated with peripheral inflammation
and immune activation of blood cells, as is the case with
neuroinflammatory and autoimmune illnesses (25). The mental and physical fatigue
associated with CFS appears to be the consequence of interactions
between multiple systemic and central pathways that take place via
immune-inflammatory and neuroinflammatory networks (26). Such interactions would be supported
by the activation of cytokines and immune cells, which has been
reported in numerous previous studies (27,28).
This background to CFS prompted us to investigate
fundamental abnormalities in patients with CFS and to develop an
objective diagnostic method for this disease. As a result, our
research group has recently explored an approach based on visible
and near-infrared (Vis-NIR) spectroscopy.
3. Vis-NIR spectroscopy for CFS
research
The analysis of Vis-NIR spectra from the sera of
patients with CFS identified certain characteristics that could be
distinguished from the corresponding spectra of healthy donors
(12,29). A chemometrics analysis, termed soft
independent modeling of class analogy (SIMCA), was applied to
develop a multivariate model to discriminate between the Vis-NIR
spectra of patients with CFS and those of healthy individuals
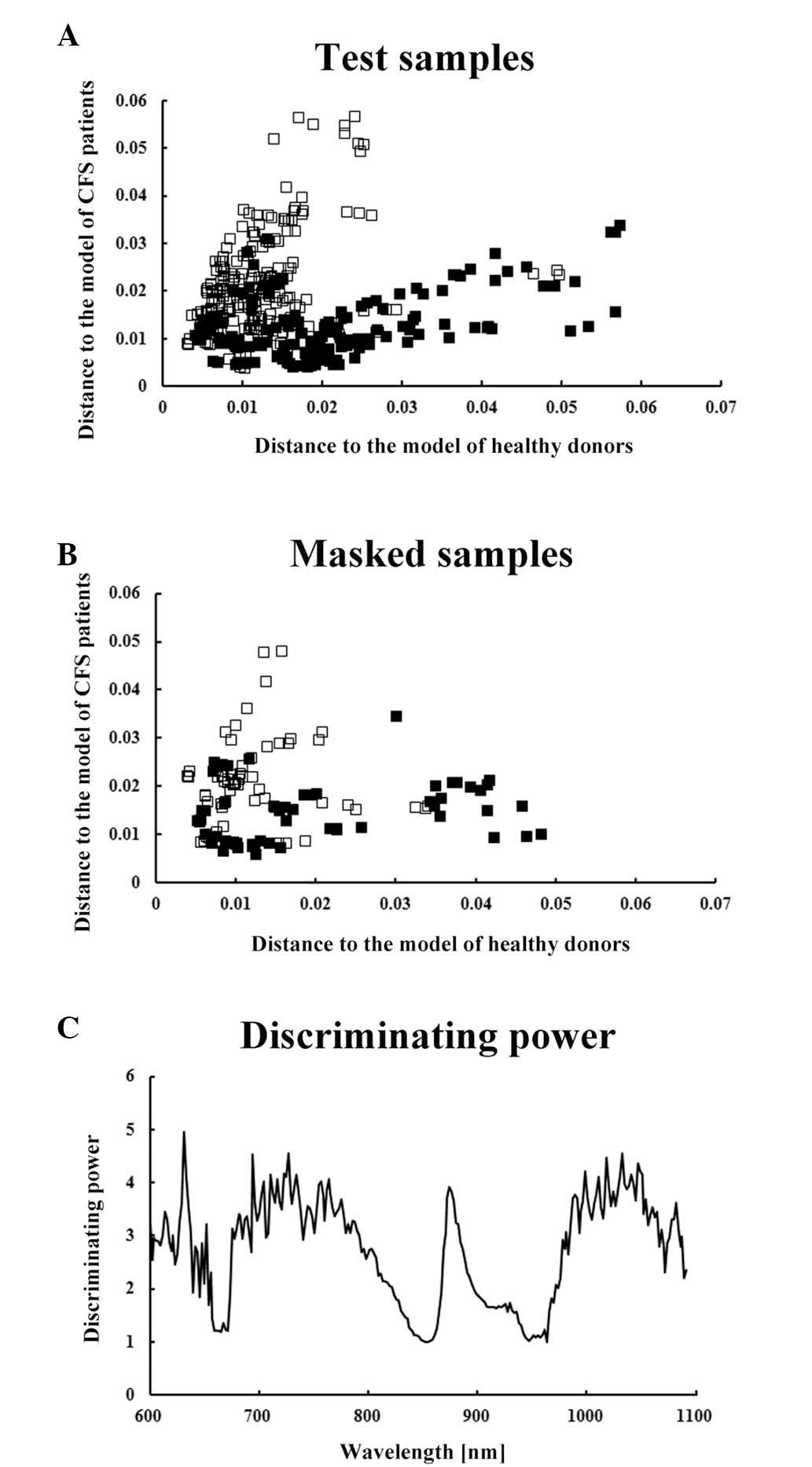
(Fig. 1). As a result, the SIMCA
models correctly predicted 54/54 (100%) healthy subjects and 42/45
(93.3%) patients with CFS based on the analysis of Vis-NIR spectra
from masked serum samples (10).
Subsequently, to develop a non-invasive approach,
Vis-NIR spectroscopic analysis of thumbs was performed (Fig. 2). The SIMCA models were applied to
the Vis-NIR spectra of thumbs from patients with CFS and healthy
individuals (Fig. 3). The model
successfully predicted 51/60 (83.3%) healthy subjects and 42/60
(70%) patients with CFS (11).
The discriminating power of the calibration models
from these previous studies suggested the presence of common
factors among the sera and thumbs of patients with CFS (Figs. 1C and 3C). One of these factors may be
associated with blood flow and energy metabolism, since the Vis-NIR
spectra of thumbs suggested that patients with CFS have a
significantly higher oxyhemoglobin content (Table I), and significantly increased
oxidation of heme a+a3 and copper in cytochrome c
oxidase (33).
 | Table ISpectroscopic comparison of the ratio
of oxyhemoglobin to deoxyhemoglobin in the thumbs of patients with
CFS and healthy controls. |
Table I
Spectroscopic comparison of the ratio
of oxyhemoglobin to deoxyhemoglobin in the thumbs of patients with
CFS and healthy controls.
| Characteristic | Absorbance at 850–760
nm
|
|---|
| Male | Female |
|---|
| Healthy | 0.6137±0.0073 | 0.5005±0.0082 |
| CFS | 0.6517±0.0108 | 0.5352±0.0080 |
| D'Agostino-pearson
test (parametric data or not) | Yes | Yes |
| Unpaired t test
(P-value) | (0.0027)a | (0.0027)a |
| Mann-Whitney test
(P-value) | – | – |
IR spectroscopy is a form of vibrational
spectroscopy. As such, the principal of IR spectroscopy is similar
to that of Vis-NIR spectroscopy (34), although there are certain
differences. The absorption observed in IR is due to fewer
overtones as compared with Vis-NIR, resulting in sharper bands with
higher intensity. Thus, IR spectroscopy is a powerful tool for CFS
research. A previous study using IR spectroscopy found that
fingernails of patients with CFS showed a decrease in α-helix
content and increased β-sheet content compared with those of
healthy individuals, suggesting reduced levels of normal protein
elements of the nail plate (35).
4. Conclusion
As described above, previous studies have
demonstrated the potential of Vis-NIR spectroscopy for the
diagnosis of CFS using serum samples and thumbs. Furthermore,
analysis of the Vis-NIR and IR spectra of sera, thumbs and
fingernails suggests that factors absorbing in this spectral region
are altered in patients with CFS. Combining chemometric analysis of
spectra obtained from CFS samples with analysis of a spectra
database may enable us to identify potential candidates for CFS
biomarkers. Currently, although vast IR spectra databases,
including the KnowItAll Informatics System (Bio-Rad Laboratories,
Inc., Philadelphia, PA, USA), are commercially available, no
similarly large NIR spectra databases exist. Thus, construction of
an NIR spectra database would be necessary for a chemometrics-based
study. Further analysis using such a spectra database would
facilitate the search for biomarkers for CFS and aid our
understanding of the pathophysiology of this disorder.
Although the current review has focused on previous
studies using Vis-NIR spectroscopy to examine CFS, this approach
can be applied to other diseases, including cancer (36,37),
diabetes (38,39), Alzheimer's disease (40) and epilepsy (41,42).
Therefore, the application of Vis-NIR spectroscopy to the diagnosis
and analysis of disease in general is likely to increase. Its
applications include not only diagnosis, but also assessment and
monitoring of diseases (Fig. 4).
In addition, Vis-NIR spectroscopy coupled with quantitative
chemometrics analyses, including partial least squares and
principal component regression, can be used to predict levels of
biochemical constituents, including triglycerides, cholesterols,
urea and lactate in body fluids, including blood (43,44)
and hematocrit in the body (45).
Therefore, this method may contribute to advances in clinical
laboratory testing as well as maintaining human health in the form
of a home testing kit.
Acknowledgments
The present study was supported by Grant-in-Aids for
Scientific Research from the Japan Society for the Promotion of
Science.
References
|
1
|
Fukuda K, Straus SE, Hickie I, Sharpe MC,
Dobbins JG and Komaroff A: The chronic fatigue syndrome: A
comprehensive approach to its definition and study. International
chronic fatigue syndrome study group. Ann Intern Med. 121:953–959.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bested AC, Logan AC and Howe R: Hope and
Help for Chronic Fatigue Syndrome and Fibromyalgia. Cumberland
House; Nashville, TN: 2008
|
|
3
|
Jason LA, Richman JA, Rademaker AW, Jordan
KM, Plioplys AV, Taylor RR, McCready W, Huang CF and Plioplys S: A
community-based study of chronic fatigue syndrome. Arch Intern Med.
159:2129–2137. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuratsune H: Overview of chronic fatigue
syndrome focusing on prevalence and diagnostic criteria. Nihon
Rinsho. 65:983–990. 2007.In Japanese. PubMed/NCBI
|
|
5
|
Reynolds KJ, Vernon SD, Bouchery E and
Reeves WC: The economic impact of chronic fatigue syndrome. Cost
Eff Resour Alloc. 2:42004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reyes M, Nisenbaum R, Hoaglin DC, Unger
ER, Emmons C, Randall B, Stewart JA, Abbey S, Jones JF, Gantz N, et
al: Prevalence and incidence of chronic fatigue syndrome in
Wichita, Kansas. Arch Intern Med. 163:1530–1536. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vernon SD, Whistler T, Aslakson E,
Rajeevan M and Reeves WC: Challenges for molecular profiling of
chronic fatigue syndrome. Pharmacogenomics. 7:211–218. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wold S: Pattern Recognition by means of
disjoint principal components models. Pattern Recognition.
8:127–139. 1976. View Article : Google Scholar
|
|
9
|
Coomans D, Broeckaert I, Derde MP, Tassin
A, Massart DL and Wold S: Use of a microcomputer for the definition
of multivariate confidence regions in medical diagnosis based on
clinical laboratory profiles. Comput Biomed Res. 17:1–14. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakudo A, Kuratsune H, Kobayashi T, Tajima
S, Watanabe Y and Ikuta K: Spectroscopic diagnosis of chronic
fatigue syndrome by visible and near-infrared spectroscopy in serum
samples. Biochem Biophys Res Commun. 345:1513–1516. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakudo A, Kuratsune H, Kato YH and Ikuta
K: Visible and near-infrared spectra collected from the thumbs of
patients with chronic fatigue syndrome for diagnosis. Clin Chim
Acta. 413:1629–1632. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakudo A, Hakariya Y, Kobayashi T,
Sugimoto A and Ikuta K: Possible application of visible and
near-infrared spectral patterns in serum to provide emerging clue
to biomarkers for chronic fatigue syndrome. J IiME. 2:4–7.
2008.
|
|
13
|
Patarca R: Cytokines and chronic fatigue
syndrome. Ann NY Acad Sci. 933:185–200. 2001. View Article : Google Scholar
|
|
14
|
Vernon SD and Reeves WC: Evaluation of
autoantibodies to common and neuronal cell antigens in Chronic
Fatigue Syndrome. J Autoimmune Dis. 2:52005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nijs J and De Meirleir K: Impairments of
the 2-5A synthetase/RNase L pathway in chronic fatigue syndrome. In
vivo. 19:1013–1021. 2005.PubMed/NCBI
|
|
16
|
Cleare AJ: The neuroendocrinology of
chronic fatigue syndrome. Endocr Rev. 24:236–252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allain TJ, Bearn JA, Coskeran P, Jones J,
Checkley A, Butler J, Wessely S and Miell JP: Changes in growth
hormone, insulin, insulinlike growth factors (IGFs), and
IGF-binding protein-1 in chronic fatigue syndrome. Biol Psychiatry.
41:567–573. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuratsune H, Yamaguti K, Takahashi M,
Misaki H, Tagawa S and Kitani T: Acylcarnitine deficiency in
chronic fatigue syndrome. Clin Infect Dis. 18(Suppl 1): S62–S67.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jacobson W, Saich T, Borysiewicz LK, Behan
WM, Behan PO and Wreghitt TG: Serum folate and chronic fatigue
syndrome. Neurology. 43:2645–2647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Werbach MR: Nutritional strategies for
treating chronic fatigue syndrome. Altern Med Rev. 5:93–108.
2000.PubMed/NCBI
|
|
21
|
Maes M, Mihaylova I and De Ruyter M: Lower
serum zinc in Chronic Fatigue Syndrome (CFS): Relationships to
immune dysfunctions and relevance for the oxidative stress status
in CFS. J Affect Disord. 90:141–147. 2006. View Article : Google Scholar
|
|
22
|
Whiteside TL and Friberg D: Natural killer
cells and natural killer cell activity in chronic fatigue syndrome.
Am J Med. 105(Suppl): 27S–34S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Curriu M, Carrillo J, Massanella M, Rigau
J, Alegre J, Puig J, Garcia-Quintana AM, Castro-Marrero J, Negredo
E, Clotet B, et al: Screening NK-, B- and T-cell phenotype and
function in patients suffering from Chronic Fatigue Syndrome. J
Transl Med. 11:682013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parker AJ, Wessely S and Cleare AJ: The
neuroendocrinology of chronic fatigue syndrome and fibromyalgia.
Psychol Med. 31:1331–1345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morris G, Anderson G, Galecki P, Berk M
and Maes M: A narrative review on the similarities and
dissimilarities between myalgic encephalomyelitis/chronic fatigue
syndrome (ME/CFS) and sickness behavior. BMC Med. 11:642013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morris G, Berk M, Galecki P, Walder K and
Maes M: The neuro-immune pathophysiology of central and peripheral
fatigue in systemic immune-inflammatory and neuro-immune diseases.
Mol Neurobiol. 53:1195–1219. 2016. View Article : Google Scholar
|
|
27
|
Dell'Osso L, Bazzichi L, Baroni S,
Falaschi V, Conversano C, Carmassi C and Marazziti D: The
inflammatory hypothesis of mood spectrum broadened to fibromyalgia
and chronic fatigue syndrome. Clin Exp Rheumatol. 33(1 Suppl 88):
S109–S116. 2015.PubMed/NCBI
|
|
28
|
Nijs J, Nees A, Paul L, De Kooning M,
Ickmans K, Meeus M and Van Oosterwijck J: Altered immune response
to exercise in patients with chronic fatigue syndrome/myalgic
encephalomyelitis: A systematic literature review. Exerc Immunol
Rev. 20:94–116. 2014.PubMed/NCBI
|
|
29
|
Sakudo A, Hakariya Y, Kobayashi T and
Ikuta K: Visible and near-infrared (Vis-NIR) spectroscopy:
Introduction and perspectives for diagnosis of chronic fatigue
syndrome. J IiME. 1:8–18. 2007.
|
|
30
|
Heise HM: Applications of near-infrared
spectroscopy in medical sciences. Near-infrared Spectroscopy:
Principles, Instruments, Applications. Siesler HW, Ozaki Y, Kawata
S and Heise HM: Wiley-VCH; Weinheim: pp. 289–333. 2002
|
|
31
|
Matsunaga A, Nomura Y, Kuroda S, Tamura M,
Nishihira J and Yoshimura N: Energy-dependent redox state of heme a
+ a3 and copper of cytochrome oxidase in perfused rat brain in
situ. Am J Physiol. 275:C1022–C1030. 1998.PubMed/NCBI
|
|
32
|
Hoshi Y, Hazeki O and Tamura M: The oxygen
dependency of the redox state of heme and copper in cytochrome
oxidase in vitro. Adv Exp Med Biol. 248:71–76. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sakudo A, Kato YH, Tajima S, Kuratsune H
and Ikuta K: Visible and near-infrared spectral changes in the
thumb of patients with chronic fatigue syndrome. Clin Chim Acta.
403:163–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stuart B: Biological Applications of
Infrared Spectroscopy. John Wiley & Sons Ltd; New York:
1997
|
|
35
|
Sakudo A, Kuratsune H, Kato YH and Ikuta
K: Secondary structural changes of proteins in fingernails of
chronic fatigue syndrome patients from Fourier-transform infrared
spectra. Clin Chim Acta. 402:75–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Simick MK, Jong R, Wilson B and Lilge L:
Non-ionizing near-infrared radiation transillumination spectroscopy
for breast tissue density and assessment of breast cancer risk. J
Biomed Opt. 9:794–803. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu KZ, Shi M, Man A, Dembinski TC and
Shaw AR: Quantitative determination of serum LDL cholesterol by
near-infrared spectroscopy. Vib Spectrosc. 38:203–208. 2005.
View Article : Google Scholar
|
|
38
|
Robinson MR, Eaton RP, Haaland DM, Koepp
GW, Thomas EV, Stallard BR and Robinson PL: Noninvasive glucose
monitoring in diabetic patients: A preliminary evaluation. Clin
Chem. 38:1618–1622. 1992.PubMed/NCBI
|
|
39
|
Gabriely I, Wozniak R, Mevorach M, Kaplan
J, Aharon Y and Shamoon H: Transcutaneous glucose measurement using
near-infrared spectroscopy during hypoglycemia. Diabetes Care.
22:2026–2032. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hock C, Villringer K, Müller-Spahn F,
Hofmann M, Schuh-Hofer S, Heekeren H, Wenzel R, Dirnagl U and
Villringer A: Near infrared spectroscopy in the diagnosis of
Alzheimer's disease. Ann N Y Acad Sci. 777:22–29. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Watanabe E, Nagahori Y and Mayanagi Y:
Focus diagnosis of epilepsy using near-infrared spectroscopy.
Epilepsia. 43(Suppl 9): 50–55. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sokol DK, Markand ON, Daly EC, Luerssen TG
and Malkoff MD: Near infrared spectroscopy (NIRS) distinguishes
seizure types. Seizure. 9:323–327. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hazen KH, Arnold MA and Small GW:
Measurement of glucose and other analytes in undiluted human serum
with near-infrared transmission spectroscopy. Anal Chim Acta.
371:255–267. 1998. View Article : Google Scholar
|
|
44
|
Kobayashi T, Kato YH, Tsukamoto M, Ikuta K
and Sakudo A: Portable visible and near-infrared spectrophotometer
for triglyceride measurements. Int J Mol Med. 23:75–79. 2009.
|
|
45
|
Sakudo A, Kato YH, Kuratsune H and Ikuta
K: Non-invasive prediction of hematocrit levels by portable visible
and near-infrared spectrophotometer. Clin Chim Acta. 408:123–127.
2009. View Article : Google Scholar : PubMed/NCBI
|