Introduction
Silicosis is a fibrotic lung disease caused by
inhalation of free crystalline silicon dioxide or silica.
Occupational exposure to respirable crystalline silica dust
particles occurs in many industries (1). To date, there is no general therapy
for the treatment of this disease. In 1997, the International
Agency for Research on Cancer upgraded crystalline silica to a
Group 1 human carcinogen (2).
Despite extensive research efforts, the exact mechanism of
silicosis remains to be fully elucidated (3).
Silica (SiO2) dust is the critical
pathogenic factor in the initiation of silicosis (4), and the alveolar epithelial cells of
the lung are the primary target cells, particularly in the early
stages of the disease. However, the underlying molecular mechanism
of epithelial cell damage induced by SiO2 remains to be
elucidated (5). Aquaporins (AQPs),
of which there are 13 subtypes (AQP0-AQP12), are cell membrane
transport proteins associated with water permeability. AQPs are
widely distributed in animals and plants and their important role
in water transport has been extensively investigated (6–8).
AQP1 was first reported in 1992 (9), and has been demonstrated to be
ubiquitously involved in the water balance of the respiratory
system (10–13). In addition, AQP4 regulates the
exchange of fluid between the alveolar space and alveolar
epithelium barrier and has a significant compensational role in
pulmonary liquid clearance in the event of sodium transport damage
in acute lung injury (14,15). However, a limited number of studies
have investigated the involvement of AQP1 and AQP4 in occupational
pulmonary diseases, such as silicosis (16,17).
Therefore, in the present study, human A549 type II alveolar
epithelial cells cultured in vitro were stimulated with
SiO2 dust to investigate possible mechanisms of AQP1 and
AQP4 in the pathological process of silicosis. This may provide an
experimental basis to identify effective targets for the prevention
and control of silicosis.
Materials and methods
Reagents
Crystalline silica (MIN-U-SIL5®;
SiO2 purity, 99.2%; median particle diameter, 1.6
µm) was purchased from U.S. Silica (Frederick, MD, USA).
RPMI-1640 culture medium was supplied by HyClone; GE Healthcare
Life Sciences (Logan, UT, USA). Fetal bovine serum (FBS) and 0.25%
trypsin were purchased from Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). TRIzol® Reagent for total RNA
extraction was purchased from Invitrogen; Thermo Fisher Scientific,
Inc. The Moloney Murine Leukemia Virus Reverse Transcriptase RNase
H Minus Point Mutant kit and SYBR Green quantitative polymerase
chain reaction (qPCR) reagent kit were products of Promega
Corporation (Madison, WI, USA). Anti-β-actin rabbit polyclonal
antibody (ab8227) was purchased from Abcam (Cambridge, MA, USA) and
anti-AQP1 (sc-20810) and anti-AQP4 (sc-20812) rabbit polyclonal
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Horseradish peroxidase-conjugated sheep
anti-rabbit IgG secondary antibody (A0208) was obtained from
Beyotime Institute of Biotechnology (Haimen, China). SP
Immunohistochemical kit (SP-9000) was a product of Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
Enhanced chemiluminescence (ECL) substrate kit was obtained from
PerkinElmer, Inc. (Waltham, MA, USA). Cell Counting Kit-8 (CCK-8)
was obtained from Beyotime Institute of Biotechnology. Triton™
X-100 was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Experimental cells and design
Human A549 type II alveolar epithelial cell lines
(purchased from the Cell Center of the Institute of Basic Medical
Sciences, Peking Union Medical College (Beijing, China) were
cultured in RPMI-1640 medium containing 10% FBS in a 37°C and 5%
CO2 incubator. The cultured cells were divided into four
groups: Control (untreated A549 cells); SiO2-stimulated
(50 mg/ml SiO2 composite suspension-treated A549 cells);
AQP1 inhibitor [A549 cells were treated with 0.2 mmol/l mercury
(II) chloride (HgCl2) for 3 min and then
SiO2-stimulated]; and AQP4 inhibitor [A549 cells were
treated with 100 µmol/l 2-(nicotinamide)-1,3,4-thiadiazole
(TGN-020) for 2 h and then SiO2-stimulated] (13).
Detection of SiO2 influence on
the growth of A549 cells by CCK-8 assay
A549 cells were transferred into 96-well plates at
5,000 cells/well. Following acclimatization in 100 µl
RPMI-1640 containing 0.4% FBS for 24 h, cells were stimulated with
mixed suspensions of SiO2 particles for 24 h (10, 25, 50
or 100 mg/ml in RPMI-1640 containing 10% FBS). Cell counting kit-8
reaction solutions (20 µl) were subsequently added to the
wells (6 wells/group). Cell growth was measured by absorbance at
450 nm on a microplate reader 1 h later. The dose of 50 mg/ml
SiO2 was thus selected as the stimulus in subsequent
experiments.
Reverse transcription (RT)-qPCR
analysis
A549 cells were transferred to 6-well plates at
20,000 cells/well for 24 h. Cells were treated as described in the
previous section (3 wells/group). Total cellular RNA extraction was
performed using TRIzol® reagent according to the
manufacturer's instructions. RNA was reverse transcribed to
complementary (c) DNA by incubation with reverse transcriptase at
42°C for 50 min. qPCR analysis was performed in a Roter-Gene 3000
Sequence Detection System (Qiagen Pty Ltd., Melbourne, Australia)
using SYBR Green PCR Master Mix. PCR amplification was performed on
0.5 µl of cDNA using the following gene-specific primers:
AQP1 forward, 5′-TGACCCGCTCGGACTTACT-3′ and reverse,
3′-CTTCTGGACCCATGCTGTG-5′; AQP4 forward,
5′-CATCTCCCTTTGCTTTGGACTC-3′ and reverse,
3′-CAGATAGAGGATTCCTGCTCCAA-5′; and β-actin forward,
5′-CACCCCCACTGAAAAAGATGA-3′ and reverse,
5′-CATCTTCAAACCTCCATGACG-3′. The following conditions were used: A
1 min pre-denaturing step at 95°C; and cycles of 15 sec at 95°C and
20 sec at 59°C. A total of 40 cycles were performed to prevent
saturation of the reaction. Melting curve analysis revealed a
single sharp peak with the correct melting temperature. GAPDH was
used as an internal control, and AQP1 and AQP4 mRNA expression was
normalized against GAPDH expression. Relative quantification by the
2−ΔΔCq method was performed by comparing to control
groups (18).
Western blotting
A549 cells were transferred to 6-well plates at
20,000 cells/well for 24 h. Cells were treated as described in the
previous section (3 wells/group). Proteins were extracted from
cells using radioimmunoprecipitation assay buffer (catalog no.
P0013; Beyotime Institute of Biotechnology, Haimen, China), and
quantified by a bicinchoninic acid assay (catalog no. P0012;
Beyotime Institute of Biotechnology). A total of 30 µg
extracted protein was loaded onto a 12% SDS-PAGE gel, subjected to
electrophoresis at 120 V for 90 min and transferred to a
nitrocellulose membrane (Whatman; GE Healthcare Life Sciences).
Following blocking with 5% skim milk in Tris-buffered saline with
0.1% Tween (TBST) at room temperature for 2 h, the membranes were
incubated with primary antibodies at 4°C overnight (anti-rat AQP1
and AQP4; 1:800). Membranes were washed in TBST three times, and
incubated with the HRP-conjugated secondary antibody at room
temperature for 2 h (anti-rat antibodies; 1:1,000). Protein bands
were visualized using ECL. All western blotting densitometry data
were normalized to β-actin using Image Lab software version 4.0
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunocytochemical staining
A549 cells were cultured for 24 h in 24-well plates
at 5,000 cells/well; sterile coverslips were placed in each well in
advance to allow cells to adhere during growth. The cells were
fixed in 1% paraformaldehyde for 15 min and rinsed three times by
phosphate-buffered saline, and incubated with 0.1% Triton X-100 in
phosphate-buffered saline at room temperature for 5 min. Normal
goat serum blocking fluid (taken from the SP-9000
Immunohistochemical kit; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) was added for 15 min, and cells were
subsequently incubated with primary antibodies recognizing AQP1 and
AQP4 (1:100) overnight, followed by the biotinylated secondary
antibody and finally the S-A/HRP reagent taken from the SP-9000
Immunohistochemical kit; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) for 15 min. Immunoreactivity was
visualized with DAB (Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd.). Brown staining was considered to indicate a positive
result. Staining was visualized using an optical microscope at
magnification, ×20 (Olympus Corporation, Tokyo, Japan). Cells that
were stained a brown color were considered positive.
Immunocytochemistry was quantified using Image-Pro Plus analysis
software version 5.0 (Media Cybernetics, Inc., Rockville, MD, USA)
to detect positive cells and automatically determine the optical
density and percentage per field, which were used to calculate the
expression levels of AQP1 and AQP4 protein in A549 cells. All
sections were incubated under identical conditions with the same
concentration of antibodies therefore the immunostaining was
comparable among the various experimental groups.
Statistical analysis
All experiments were performed three times and all
statistical analyses were performed using SPSS version 17.0 (SPSS
Inc., Chicago, IL, USA). Statistical comparisons were performed
using a Student's t-test for unpaired samples and a one-way
analysis of variance for multiple comparisons. Post hoc comparisons
were performed using the Least Significant Difference test when
equal variances were assumed or with Dunnett's test when equal
variances were not assumed. Data are expressed as the mean ±
standard error. P<0.05 was considered to indicate a
statistically significant difference.
Results
Influence of various concentrations of
SiO2 on the growth of A549 cells
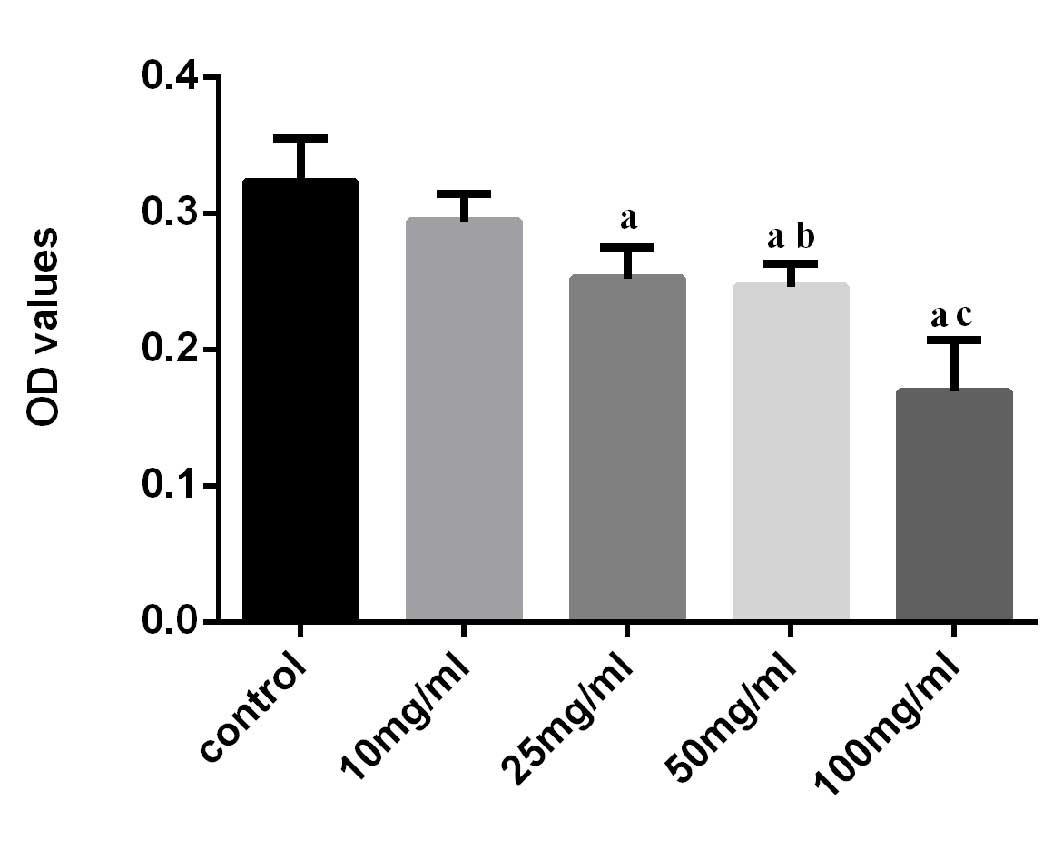
CCK-8 assay absorbance of A549 cells reduced
following stimulation with various concentrations of
SiO2, suggesting cell growth suppression (Fig. 1). The suppression was similar for
SiO2 concentrations of 25 and 50 mg/ml, at 78 and 76% of
the control, respectively. Although 100 mg/ml SiO2
produced the greatest suppression of cell activity, it led to
increased cell death and influenced the detection of mRNA and
protein of AQP1 and AQP4. Therefore 50 mg/l was selected as the
stimulus concentration for the subsequent experiments.
The expression levels of AQP1 and AQP4
mRNA in A549 cells exposed to SiO2
Following SiO2 stimulation, the AQP1 mRNA
expression levels increased rapidly compared with the control group
(0.5 and 1 h, both P<0.001 vs. control group), and peaked at 2 h
(P<0.001 vs. control group) post SiO2 treatment
(Fig. 2). From 2 h onwards there
was a gradual decrease, and this difference (2 vs. 4 and 8 h) was
statistically significant (both P<0.001). Following exposure to
SiO2 for 8 h, the mRNA expression levels of AQP1
returned to those of the control group. Pretreatment of cells with
AQP1 inhibitor prior to stimulation with SiO2 for 2 h
resulted in significantly decreased AQP1 mRNA expression levels
compared with the SiO2-stimulated group at 2 h
(P<0.001). The AQP4 mRNA expression levels in A549 cells
followed a similar pattern (0.5, 1 and 2 h, P<0.001 vs. control
group; 1 vs. 2 h P=0.023; 1 vs. 4 h, P<0.001; 1 vs. 8 h,
P<0.001; Fig. 2).
Western blot analysis of AQP1 and AQP4
protein expression levels in A549 cells exposed to
SiO2
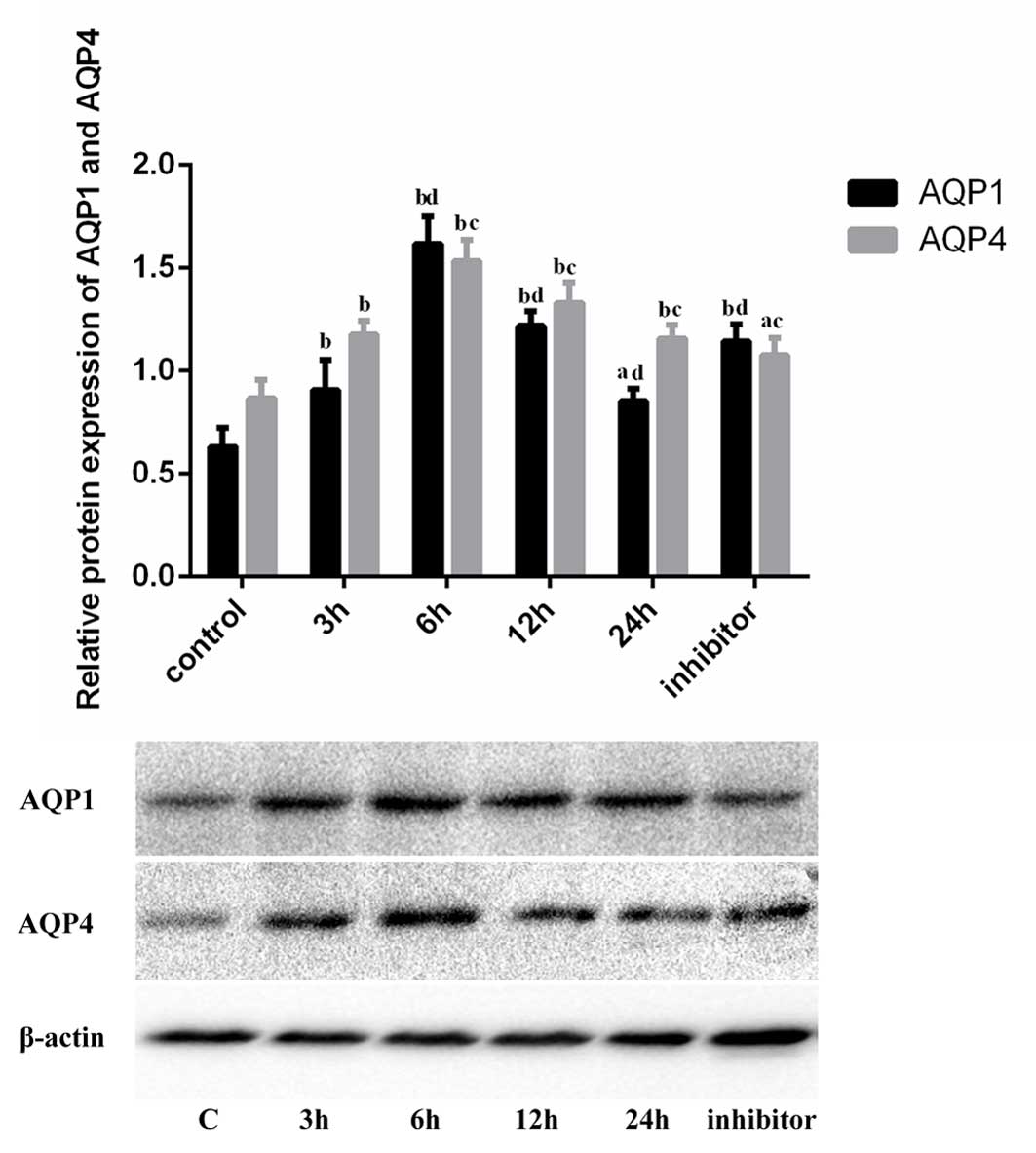
Western blotting (Fig.
3) revealed that SiO2 treatment led to an increase
in the expression levels of AQP1 protein in A549 cells. The
increase was observed at 3 h of stimulation and peaked at 6 h.
Protein expression levels declined from 12 h; however, at 24 h they
remained greater than in the control group (P=0.021). Following
pretreatment with inhibitor, AQP1 protein expression levels were
increased compared with those of the control group (P<0.001);
however, they were reduced compared with the
SiO2-stimulated group at 6 h (P<0.001). AQP4 protein
expression levels were increased in the SiO2-stimulated
groups compared with the control group (3 h, P=0.001), and peaked
at 6 h (P<0.001). Following pretreatment with inhibitor, AQP4
protein expression levels remained increased compared with those of
the control group (P=0.014); however, they were reduced compared
with the SiO2-stimulated group at 6 h (P<0.001).
Immunocytochemistry staining
evaluation
AQP1 and AQP4 immunocytochemistry staining products
appeared brown (Fig. 4) and were
expressed in the cytoplasm and nucleus. The immunocytochemistry
quantitative analysis revealed that AQP1 protein expression levels
were increased compared with untreated cells at 3 (P=0.004), 6
(P<0.001) and 12 h (P<0.001) following SiO2
exposure and gradually declined from 6 h until similar to control
levels at 24 h (P=0.153). Following pretreatment with inhibitor,
AQP1 expression levels decreased ~29% compared with the
SiO2-stimulated group at 6 h (P<0.001). The AQP4
protein expression levels in A549 cells followed a similar pattern
(Fig. 4).
 | Figure 4Immunocytochemistry staining and
evaluation (A) AQP1 and (B) AQP4 immunocytochemistry staining
products were brown and expressed in the cytoplasm and nucleus of:
A, control cells; b, cells stimulated by SiO2 for 3 h;
c, cells stimulated by SiO2 for 6 h; d, cells stimulated
by SiO2 for 12 h; e, cells stimulated by SiO2
for 24 h; and f, cells pretreated with inhibitor. (C) AQP1 and AQP4
expression levels in A549 cells followed similar patterns,
increasing at 3, 6 and 12 h following SiO2 exposure
compared with the control and gradually declining from 6 h until
similar to control levels at 24 h. Following pretreatment with
inhibitor (at 0 h and detected at 6 h), expression levels declined
~29% compared with the SiO2-stimulated group at 6 h.
aP<0.05 and bP<0.01 vs. control; and
cP<0.05 and dP<0.01 vs. 6 h
SiO2 stimulation. AQP, aquaporin; OD, optical
density. |
Discussion
The water balance of the body is important for the
maintenance of health. The AQP family is crucial in this balance
and is closely associated with the development of various diseases.
Therefore, AQPs have attracted attention as targets for the
treatment of numerous diseases (19). To date, 13 AQP isoforms
(AQP0-AQP12) have been identified in mammals and are expressed in
diverse tissues (20–22), and 6 AQPs (AQP1, AQP3, AQP4, AQP5,
AQP8 and AQP9) are expressed in the lung and airways (23,24).
AQP1 is expressed in the microvascular endothelia, AQP3 and AQP4 in
the airway epithelia and AQP5 in type I alveolar epithelial cells,
submucosal gland acini and a subset of airway epithelial cells
(10). Although AQP8 and AQP9
protein expression has been observed in the lung,
immunolocalization of these proteins has not yet been demonstrated
(25,26). AQP1 is the primary water channel
responsible for water transport through numerous epithelia and
endothelia (10). In addition, a
previous study suggested important roles for this protein in gas
permeation, angiogenesis, cell proliferation and migration
(27). The expression of AQP1
protein may be mechanistically involved in the airway inflammation
and pathogenesis of chronic obstructive pulmonary disease (COPD)
(28), and expression of AQP1
increased markedly in pulmonary fibrosis, suggesting that AQP1 may
be important in the progression of this disease (12). However, in our previous study it
was demonstrated that the expression of AQP1 increased first and
then declined in the lung tissue of silicosis rats, suggesting that
AQP1 may potentially be involved in the pathogenesis of silicosis
(17). This phenomenon also
existed in rat lung infection and endotoxin-induced lung injury,
and a study observed mouse lung AQP-1 and AQP-5 were downregulated
(29). This may be due to the
disorder of water balance caused by silica dust. AQP4 is primarily
distributed in the airway epithelium, alveolar endothelial and
epithelial cells (29). A previous
study revealed that AQP4 expression was significantly increased in
cells from patients with asthma (30). However, in other respiratory
diseases, including COPD, patients also demonstrated decline of
AQP4 mRNA expression (31). This
varying expression of AQP4 may be due to the nature and different
stages of these diseases. In addition, AQP4 has been associated
with the tumorigenesis and migration of lung adenocarcinoma,
potentially promoting lung adenocarcinoma cancer cell migration via
adjustment of E-cadherin protein expression levels (32).
In pulmonary diseases, lung epithelial cells are
highly susceptible to become targets for damage (33). In the development of silicosis,
SiO2 is the initiating factor, and upon entering the
body it affects alveolar epithelial cells, particularly in the
acute phase of lung injury. Notably, at this stage the inflammatory
response is pronounced and increased vascular permeability and
pulmonary edema follow (29). The
pathway for the transport of water across the endothelial and
epithelial lung barrier remains to be fully elucidated; however,
AQPs have been described as critical in water removal from the lung
extracellular space (30). The
present study was designed to observe the alteration of AQP1 and
AQP4 expression levels in A549 cells exposed to SiO2, to
indicate whether a water imbalance within lung epithelial cells
exists in acute lung injury resulting from SiO2
stimulation.
The results of the present study demonstrated that
the expression levels of AQP1 and AQP4 mRNA increased in A549 cells
following exposure to SiO2, and that this increased
expression was inhibited by pretreatment with specific inhibitors
of AQP1 and AQP4, HgCl2 and TGN-020, respectively.
Notably, by immunocytochemistry, inhibitor reduced AQP protein
expression to control levels, whereas by western blot analysis, it
did not. This may be due to differential sensitivity of various
methods and measurement software. The alterations in AQP1 and AQP4
expression levels may therefore be associated with SiO2
stimulation and these proteins may contribute to A549 cell damage.
Alterations in AQP1 and AQP4 expression levels may induce the
imbalance of lung epithelial water transportation. This may
exacerbate acute inflammatory damage of lung tissue, which may then
gradually develop into chronic hyperplastic lesions, ultimately
resulting in silicosis fibrosis. However, the specific underlying
mechanism remains to be elucidated.
The acute inflammatory damage stage induced by
SiO2 may be crucial for the early occurrence and
development of silicosis (1).
Early application of specific inhibitors of water channel proteins
may thus reverse the imbalance of lung epithelial water
transportation, and decrease or even prevent the inflammatory
reaction caused by SiO2 stimulation and so reduce the
degree of silicosis fibrosis (34). Therefore, AQP1 and AQP4 may become
novel targets for silicosis prevention and early treatment
(35–37).
Although HgCl2 and TGN-020 act as
specific inhibitors for AQP1 and AQP4 and effectively inhibit their
expression (35,38), the results of the present study
suggest that they have specific cell toxicity. The identification
and development of novel water channel protein inhibitors is
therefore required for use in the prevention and treatment of
silicosis.
There are numerous AQPs in lung tissue, and in the
present study only the expression of AQP1 and AQP4 was
investigated; therefore, the expression of other AQPs and their
roles in silicosis requires additional investigation. Research into
the role of AQPs in the pathogenesis of silicosis is at an early
stage; however, novel therapeutic agents targeting AQP have been
identified (39–41). Additional studies are necessary to
clarify the causal association between AQP1 and AQP4 upregulation
and the development of silicosis, and to determine whether AQP1 and
AQP4 are suitable targets for silicosis treatment or
prevention.
In conclusion, the results of the present study
demonstrated that the expression of AQP1 and AQP4 increased when
exposed to SiO2, and that the specific inhibitors
HgCl2 and TGN-020 inhibited this
SiO2-stimulated increase. This suggests that AQP1 and
AQP4 may contribute to A549 cell damage induced by SiO2.
AQP1 and AQP4 may thus be involved in the initiation and
development of silicosis.
References
|
1
|
Leung CC, Yu IT and Chen W: Silicosis.
Lancet. 379:2008–2018. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guha N, Straif K and Benbrahim-Tallaa L:
The IARC monographs on the carcinogenicity of crystalline silica.
Med Lav. 102:310–320. 2011.PubMed/NCBI
|
|
3
|
Zhang H, Yin G, Jiang H and Zhang C:
High-dose N-acetylcysteine decreases silica-induced lung fibrosis
in the rat. J Int Med Res. 41:1179–1186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steenland K and Ward E: Silica: A lung
carcinogen. CA Cancer J Clin. 64:63–69. 2014. View Article : Google Scholar
|
|
5
|
Chu L, Wang T, Hu Y, Gu Y, Su Z and Jiang
H: Activation of Egr-1 in human lung epithelial cells exposed to
silica through MAPKs signaling pathways. Plus One. 8:e689432013.
View Article : Google Scholar
|
|
6
|
Ishibashi K, Kondo S, Hara S and Morishita
Y: The evolutionary aspects of aquaporin family. Am J Physiol Regul
Integr Comp Physiol. 300:R566–R576. 2011. View Article : Google Scholar
|
|
7
|
Li G, Santoni V and Maurel C: Plant
aquaporins: Roles in plant physiology. Biochim Biophys Acta.
1840:1574–1582. 2014. View Article : Google Scholar
|
|
8
|
Sahdeva R and Singh B: Insights into
structural mechanisms of gating induced regulation of aquaporins.
Prog Biophys Mol Biol. 114:69–79. 2014. View Article : Google Scholar
|
|
9
|
Preston GM and Agre P: Isolation of the
cDNA for erythrocyte integral membrane of 28 kilodaltons: Member of
an ancient channel family. Proe Natl Acad Sci USA. 88:11110–11114.
1991. View Article : Google Scholar
|
|
10
|
Verkman AS: Role of aquaporins in lung
liquid physiology. Respir Physiol Neurobiol. 159:324–330. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benga G: The first discovered water
channel protein, later called aquaporin 1: Molecular
characteristics, functions and medical implications. Mol Aspects
Med. 33:518–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao X, Wang G, Zhang W, Peng Q, Xue M and
Jinhong H: Expression of pulmonary aquaporin 1 is dramatically
upregulated in mice with pulmonary fibrosis induced by bleomycin.
Arch Med Sci. 9:916–921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang QY, Fu JH and Xue XD: Expression and
function of aquaporin-1 in hyperoxia-exposed alveolar epithelial
type II cells. Exp Ther Med. 8:493–498. 2014.PubMed/NCBI
|
|
14
|
Ebeling G, Bläsche R, Hofmann F, Augstein
A, Kasper M and Barth K: Effect of P2X7 receptor knockout on AQP-5
expression of type I alveolar epithelial cells. PLoS One.
9:e1002822014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Warth A, Muley T, Meister M, Herpel E,
Pathil A, Hoffmann H, Schnabel PA, Bender C, Buness A, Schirmacher
P and Kuner R: Loss of aquaporin-4 expression and putative function
in non-small cell lung cancer. BMC Cancer. 11:1612011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hao XH, Wang HL, Liu HL, Zhang L and Li
YH: Effect of curcumin on expression of aquaporin-1 in lung tissue
of silicotic rats. Zhongguo Zhi Ye Yi Xue. 39:471–474. 2012.In
Chinese.
|
|
17
|
Zhang L, Hao XH, Wang HL, Zhao JY and Liu
HL: Expression and significance of aquaporin 1 in lung tissue of
silicotic rats. Zhongguo Gongye Yixue Zazhi. 25:91–93. 1612012.In
Chinese.
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Day RE, Kitchen P, Owen DS, Bland C,
Marshall L, Conner AC, Bill RM and Conner MT: Human aquaporins:
Regulators of transcellular water flow. Biochim Biophys Acta.
1840:1492–1506. 2014. View Article : Google Scholar
|
|
20
|
Matsuzaki T, Tajika Y, Tserentsoodol N,
Suzuki T, Aoki T, Hagiwara H and Takata K: Aquaporins: A water
channel family. Anat Sci Int. 77:85–93. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishibashi K: Aquaporin subfamily with
unusual NPA boxes. Biochim Biophys Acta. 1758:989–993. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takata K, Matsuzaki T and Tajika Y:
Aquaporins: Water channel proteins of the cell membrane. Prog
Histochem Cytochem. 39:1–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singha O, Kengkoom K, Chaimongkolnukul K,
Cherdyu S, Pongponratn E, Ketjareon T, Panavechkijkul Y and
Ampawong S: Pulmonary edema due to oral gavage in a toxicological
study related to aquaporin-1, -4 and -5 expression. J Toxicol
Pathol. 26:283–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alimit A, Hasan B, Lu W, Qin W, Wushouer
Q, Zhong N and Upur H: Changes in water channel aquaporin 1 and
aquaporin 5 in the small airways and the alveoli in a rat asthma
model. Micron. 45:68–73. 2013. View Article : Google Scholar
|
|
25
|
Elkjaer ML, Nejsum LN, Gresz V, Kwon TH,
Jensen UB, Frøkiaer J and Nielsen S: Immunolocalization of
aquaporin-8 in rat kidney, gastrointestinal tract, testis, and
airways. Am J Physiol Renal Physiol. 281:F1047–F1057. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsukaguchi H, Weremowicz S, Morton CC and
Hediger MA: Functional and molecular characterization of the human
neutral solute channel aquaporin-9. Am J Physiol. 277:F685–F696.
1999.PubMed/NCBI
|
|
27
|
La Porta C: AQP1 is not only a water
channel: It contributes to cell migration through
Lin7/beta-catenin. Cell Adh Migr. 4:204–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calero C, López-campos JL, Izouierdo LG,
Sánchez-Silva R, López-Villalobos JL, Sáenz-Coronilla FJ,
Arellano-Orden E, Montes-Worboys A and Echevarría M: Expression of
aquaporins in bronchial tissue and lung parenchyma of patients with
chronic obstructive pulmonary disease. Multidiscip Respir Med.
9:292014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen Y, Wang X, Wang Y, Wang X, Chen Z,
Jin M and Bai C: Lipopolysaccharide decreases aquaporin 5, but not
aquaporin 3 or aquaporin 4, expression in human primary bronchial
epithelial cells. Respirology. 17:1144–1149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jardim MJ, Dailey L, Silbajoris R and
Diaz-Sanchez D: Distinct microRNA expression in human airway cells
of asthmatic donors identifies a novel asthma-associated gene. Am J
Respir Cell Mol Biol. 47:536–542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu B, Yang L and Chen T: Relationship
between AQP4 mRNA expression in bronchial epithelium of patients
with COPD and the airway inflammation. Xi'an Jiaotong Daxue Xuebao
(Yexueban). 29:656–658. 6782008.In Chinese.
|
|
32
|
McCoy E and Sontheimer H: Expression and
function of water channels (aquaporins) in migrating malignant
astrocytes. Glia. 55:1034–1043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grainge CL and Davies DE: Epithelial
injury and repair in airways diseases. Chest. 144:1906–1912. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie Y, Wen X, Jiang Z, Fu H, Dai L and Han
H: Correlation between exression of aquaporin 4 and migration
ability and growth of lung adenocarcinoma cells. Huazhong Keji
Daxue Xuebao (Yixueban). 40:682–685. 2011.In Chinese.
|
|
35
|
Mola MG, Nicchia GP, Svelto M, Spray DC
and Frigeri A: Automated cell-based assay for screening of
aquaporin inhibitors. Anal Chem. 81:8219–8229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Verkman AS, Anderson MO and Papadopoulos
MC: Aquaporins: Important but elusive drug targets. Nat Rev Drug
Discov. 13:259–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jullienne A and Badaut J: Molecular
contributions to neurovascular unit dysfunctions after brain
injuries: Lessons for target-specific drug development. Future
Neurol. 8:677–689. 2013. View Article : Google Scholar
|
|
38
|
Moon HG, Zheng Y, An CH, Kim YK and Jin Y:
CCN1 secretion induced by cigarette smoking extracts augments IL-8
release from bronchial epithelial cells. Plos One. 8:e681992013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
von Bülow J: Aquaporins-water channels in
the cell membrane and therapeutic targets. Med Monatsschr Pharm.
36:86–94; quiz 95–96. 2013.In German.
|
|
40
|
Xie Y, Wen X, Jiang Z, Fu HQ, Han H and
Dai L: Aquaporin 1 and aquaporin 4 are involved in invasion of lung
cancer cells. Clin Lab. 58:75–80. 2012.PubMed/NCBI
|
|
41
|
Frede J, Fraser SP, Oskay-Özcelik G, Hong
Y, Ioana Braicu E, Sehouli J, Gabra H and Djamgoz MB: Ovarian
cancer: Ion channel and aquaporin expression as novel targets of
clinical potential. Eur J Cancer. 49:2331–2344. 2013. View Article : Google Scholar : PubMed/NCBI
|