Introduction
Chronic human immunodeficiency virus (HIV) infection
is characterized by dysregulated immune responses. In addition to
CD4+ T cells, macrophages may be major reservoirs of
HIV-1 infection (1–3), and HIV accessory proteins have been
reported to influence macrophage immune activity (4).
Recepteur d'origine nantais (RON) also termed MST1R,
is a receptor tyrosine kinase that is closely associated with c-Met
expressed on tissue resident macrophages (5). RON has been reported to regulate
macrophage function and inflammation (6). The RON ligand, macrophage-stimulating
protein (MSP), is a member of the plasminogen-related growth factor
family (7). MSP acts to inhibit
the release of proinflammatory mediators, and enhances expression
of genes associated with the resolution of inflammation. MSP
stimulation of RON is reported to reduce the release of nitric
oxide (NO), interleukin (IL)-12 and tumor necrosis factor (TNF)-α
(8–11), and increase the expression of
scavenger receptor A, IL-1Rα and arginase (12). Deletion of RON impairs resistance
to pathogens, and reduces autoimmune and pathogenic inflammatory
conditions responses in animal models (13–15).
As active transcription of HIV-1 is enhanced by inflammation,
activation of RON likely acts to reduce HIV transcription. In
addition, RON was recently reported to directly repress HIV-1
transcription by targeting RNA polymerase II (16). Overexpressing RON in monocytes
reduced HIV-1 proviral transcription, and was reported to reduce
the binding of nuclear factor-κB (NF-κB) to the HIV-1 long terminal
repeat (17).
Several HIV proteins have previously been reported
to influence macrophage immune activity (4). HIV-encoded transactivator tat
primarily acts to enhance HIV-1 transcription by increasing
polymerase activity (18–20) and recruiting coactivators including
to the HIV long terminal repeat (LTR) (21–23).
However, tat was also reported to reduce cell-surface RON in
HIV-infected monocytic cells by specifically tagging RON for
proteasome degradation (24,25).
RON expression has been reported to be altered during chronic
inflammation induced by HIV-1 infection of the brain (16,17,24).
However, the related mechanisms remain unclear.
The aim of this study was to examine the influence
of HIV infection on the expression and function of RON in the
peripheral blood of HIV-1-infected patients, and to investigate the
role of RON in the HIV-1 infection of a T cell line. It was
demonstrated that peripheral levels of RON were significantly
higher in HIV-1-infected patients than in healthy control patients.
In an in vitro model of HIV infection in the JLTRG T-cell
line, RON expression and its phosphorylation were found to be
downregulated by HIV-1 infection, which was accompanied by reduced
NF-κB phosphorylation. Thus, HIV-1 downregulates the expression and
phosphorylation of RON by targeting the NF-κB pathway.
Materials and methods
Patients and participants
The cases at the First Affiliated Hospital of
Zhejiang University (Hangzhou, China) and the First and Fifth
Affiliated Hospitals of Suzhou University (Suzhou, China) between
February 2011 and December 2013 were retrospectively reviewed. This
study was approved by the Ethics Committee of the The First
Affiliated Hospital of Soochow University (Suzhou, China). One
hundred and four HIV-1-infected individuals and 37 healthy donors
were enrolled in this study. Consent of the blood donors or their
guardians was obtained in a manner consistent with the policies of
the appropriate local institutions. HIV-1 infection was confirmed
by a positive immunoblot and acquired immune deficiency syndrome
(AIDS) was diagnosed based on the CDC classification (26). Of the 104 HIV-1 positive patients,
82 met WHO criteria (27) for
highly active anti-retroviral therapy (HAART) initiation and
received a stable antiretroviral regimen. In total, 22 were
seropositive, but did not meet WHO criteria for HAART initiation.
Healthy control participants (n=37) were also recruited and were
age-, gender-, and ethnicity-matched. A short medical history was
obtained from all healthy control donors to ensure that they did
not have an infectious disease in the past 3 months. Peripheral
blood samples (5 ml) from healthy, HIV-negative individuals and
HIV-1-positive patients were drawn into a syringe containing EDTA
and stored at −80°C.
Measurement of viral load and lymphocyte
counts
Whole blood was treated with the red blood cell
lysis buffer to lyse the red blood cells, and then centrifuged at
1,500 × g for 5 min. The supernatant was discarded, and pellets
were re-suspended in 200 μl phosphate-buffered saline. The
resultant cells were incubated with mouse fluorescein
isothiocyanate (FITC)-conjugated CD4 monoclonal antibody (cat. no.
6603850; 1:10; Beckman Coulter, Brea, CA, USA) at room temperature
for 1 h, and analyzed using a flow cytometer.
Isolated lymphocytes from whole blood cells were
stained with a PC5-conjugated CD4-directed monoclonal antibody
(cat. no. A07752; 1:10; CD4-PC5; Beckman Coulter) and staining was
analyzed on a FACS Calibur cell analyzer (Becton Dickinson, USA).
Flow cytometry data were analyzed using WINMDI software version 2.8
(The Scripps Institute, San Diego, CA, USA).
Measurement of RON and MSP in peripheral
blood
The peripheral level of RON and MSP in blood samples
was measured with a dual antibody switch enzyme-linked
immunosorbent assay (ELISA) using the RON-directed mouse anti-Zt/G4
and 2F2 monoclonal antibodies (1:200; provided by Professor Wang,
Texas Tech University Health Sciences Center, Amarillo, TX, USA) as
described previously (28–31) and human MSP/MST1 α Chain MAb (Clone
45904), mouse IgG1 (R&D Systems, Inc., Minneapolis, MN,
USA).
Cell culture
The JLTRG cell line was a gift from the National
Institutes of Health, (Baltimore, MD, USA), and the H9/HTLV-IIIB
(human T cell line infected with HIV III) cell line was purchased
from the American Type Culture Collection (Mannassas, VA, USA). The
HeLa, L02, MRC, 293T, Huvee, Wish and Sup T1 cell lines were
provided by First Affiliated Hospital, Zhejiang University School
of Medicine (Hangzhou, China). All cell lines were cultured in
RPMI-1640 (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal calf serum (Gibco, Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, 100 mg/ml streptomycin and
0.2 M L-glutamine at 37°C and 5% CO2.
HIV infection of the JLTRG cell line
JLTRG cells (1×106) were cultured in a 10
cm culture dish, and co-cultured with H9/HTLV-IIIB cells to achieve
a ratio of 10:1. After 24, 48, 72 and 96 h, infection was assessed
by fluorescence microscopy (Olympus IX81, Tokyo, Japan). RON, MSP
and NF-κB content was assessed by western blotting, as described
below.
Immunofluorescence
JLTRG cells were cultured on slides (Lab-Tek Chamber
Slide system, Thermo Fisher Scientific, Inc.) and fixed with 4%
paraformaldehyde at 4°C for 20 min and incubated with RON directed
monoclonal antibody (2F2; 1:200; provided by Professor Wang) at
room temperature for 1 h. The cells were then further incubated
with anti-mouse FITC-conjugated secondary antibody (cat. no.
sc-3699; 1:10; Santa Cruz Biotechnology Inc., CA, USA) for 30 min.
Fluorescence was observed and photographed by a fluorescence
microscope (Olympus IX81).
Immunoprecipitation and western
blotting
Cells were incubated with lysis buffer (cat. no.
9803, Cell Signaling Technology Inc., Beverly, MA, USA) for 30 min
at 4°C. Insoluble material was then removed by centrifugation at
8,000 x g for 20 min at 4°C, and the concentration of protein in
each lysate was determined using a bicinchoninic protein assay kit
(Pierce Biotechnology, Rockford, IL, USA) with bovine serum albumin
(BSA; Sigma-Aldrich, St. Louis, MO, USA) as the standard.
Immunoprecipitation and western immunoblotting was conducted as
previously described (29). In
brief, 20 μg of protein lysate was mixed with 2X sodium
dodecyl sulfate (SDS) loading buffer containing DTT and incubated
at 100°C for 10 min before resolving by SDS-polyacrylamide gel
electrophoresis. Proteins were transferred to a polyvinylidene
difluoride membrane and blocked with 5% non-fat dry milk in
phosphate-buffered saline (PBS) with 0.02% v/v Tween-20. The
membrane was incubated with RON-directed monoclonal antibodies
Zt/G4 and 2F2 and anti-p65 (cat. nos. ab16502; 1:1,000; Abcam,
Cambridge, UK) or phospho-p65 polyclonal antibodies (cat. no
ab86299; 1:2,000; Abcam), and antibody binding was detected with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (cat.
no. ab85760; 1:500; Abcam). For detection of β-actin, membranes
were stripped with 100 mM 2-ME, 62.5 mM Tris-HCl (pH 6.7) and 2%
SDS for 30 min at 55°C, and re-probed with β-actin directed mouse
antibody for 1 h at 25°C. Samples were washed four times with PBS
and resuspended in 1 × SDS buffer [50 mM Tris-HCl (pH 6.8), 2% SDS,
0.1% bromphenol blue, 10% glycerol, and 100 mM DTT] Staining was
detected with HRP-conjugated goat anti-mouse secondary antibody
(cat. no. ab19195; 1:1,000; Abcam). Blots were scanned and analyzed
using Image J version 1.45 software (National Institutes of Health,
Bethesda, MD, USA) with protein band densities normalized to
β-actin.
Statistical analysis
Quantitative data is expressed as the mean ±
standard deviation. Single-factor analysis of variance and linear
correlation analysis was conducted using SPSS 17.0 statistical
software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
RON and MSP levels in the serum of
HIV-positive patients
In total, 104 HIV-1-infected individuals and 37 age-
and gender-matched healthy control participants were enrolled. The
mean age of the patients was 47.2±11.3 (25–64) years. There were 62
male and 42 female HIV-1-infected patients; and 10 male and 18
female control participants. Expression levels of RON and MSP in
the peripheral blood of HIV-1 positive patients receiving HAART
(n=22), or not (n=82) and 37 healthy control participants were
determined by ELISA. The level of RON in the peripheral blood was
considerably higher in the plasma of HIV-1 positive patients
undergoing HAART treatment (0.77±0.202 ng/μl) than in
infected patients not undergoing HAART treatment (0.49±0.135
ng/μl) (P<0.01). The levels in these groups were higher
than the level in the healthy control group (0.26±0.036
ng/μl, P<0.05) (Table
I).
 | Table IClinical characteristics of enrolled
participants. |
Table I
Clinical characteristics of enrolled
participants.
| Patient groups
(n) | CD4 cells
(count/mm3) | Viral load
(×107 copies/ml) | RON
(ng/μl) | MSP
(ng/μl) |
|---|
| Untreated (22) | 182.41±91.81a | 0.0786±0.00296 | 0.49±0.135a,b | 0.97±0.29a,c |
| Treated (82) | 213.47±138.06a |
0.0053±0.00244b | 0.77±0.202a | 0.64±0.39a |
| Healthy control
(37) | 768.30±104.12 | – | 0.26±0.036 | 2.08±1.49 |
The MSP level was considerably lower in the plasma
of HIV-1 positive patients undergoing HAART treatment (0.64±0.39
ng/μl) than in infected patients not undergoing HAART
treatment (0.97±0.29 ng/μl) (P<0.05). The levels in these
groups were lower than the levels in the healthy control group
(2.08±1.49 ng/μl, P<0.05) (Table I).
Expression of RON and MSP in a T cell
line
This study aimed to investigate the expression of
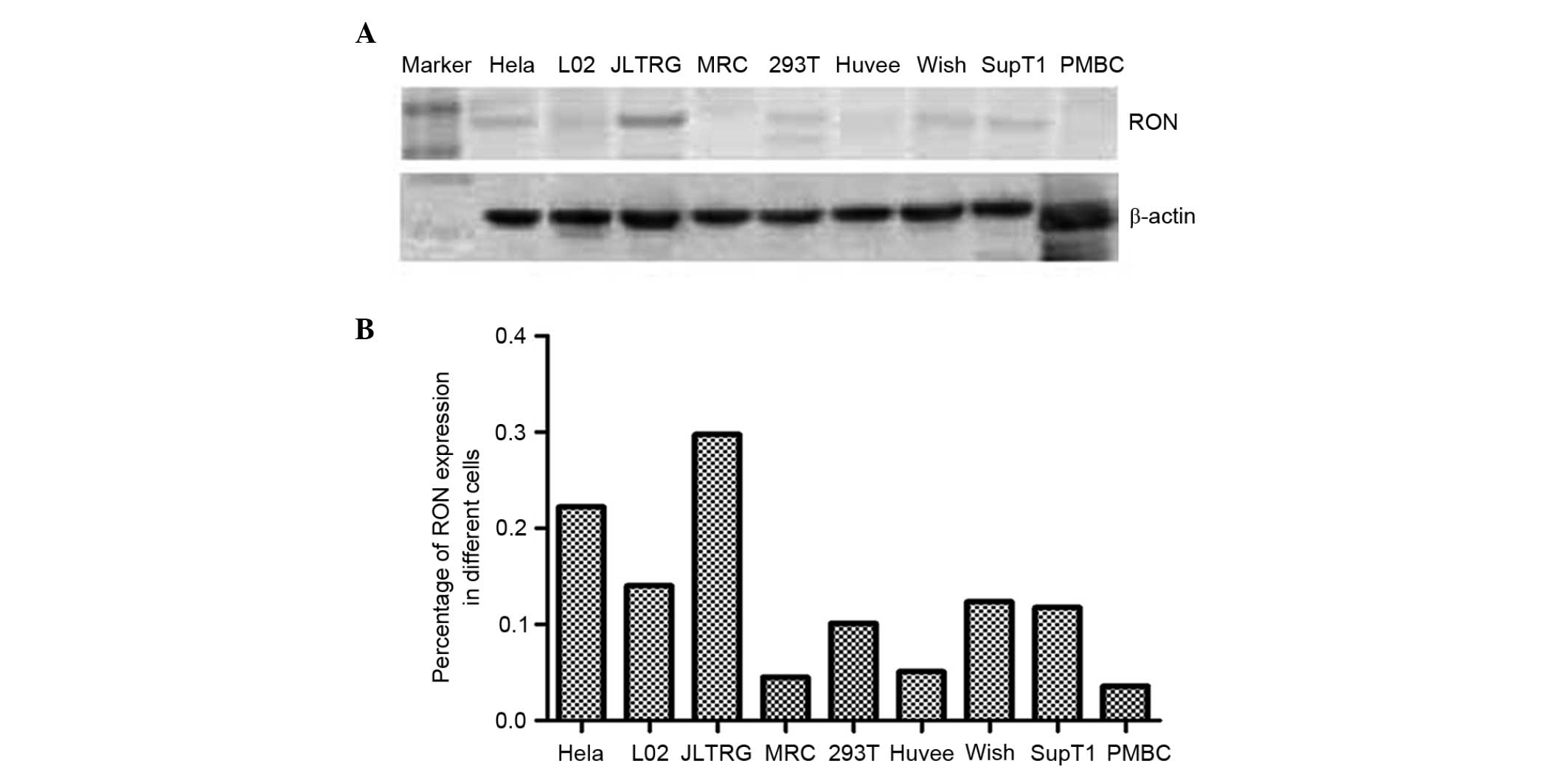
RON and MSP in a range of human cell lines, and found RON to be
strongly expressed in only JLTRG cells (Fig. 1). JLTRG cells are a Jurkat T
cell-based cell line, which express CD4 and CXCR4, and have been
stably transfected with an LTR-green fluorescence protein (GFP)
construct in order to report HIV-1 infection. These cells can be
infected with X4-tropic HIV-1 and in the presence of HIV-1 tat,
expression of enhanced GFP acts as a quantitative marker of HIV-1
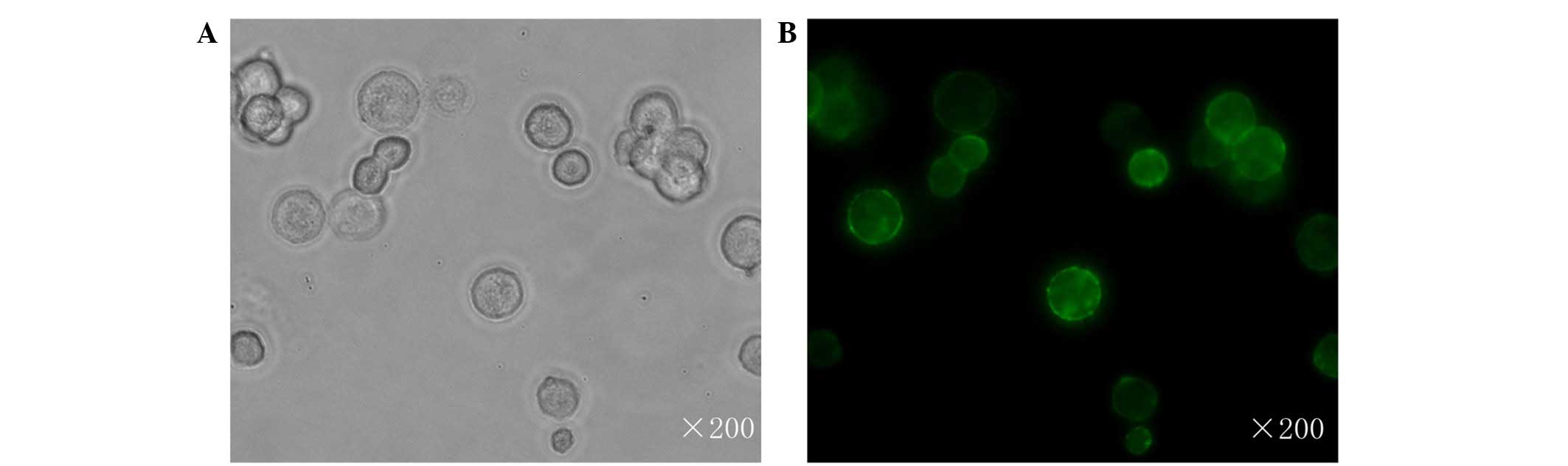
infection (32) The subcellular
location of RON in JLTRG cells was assessed by immunofluourescent
staining, and found it to be localized to the cell membrane
(Fig. 2).
HIV induces regulation of RON
expression
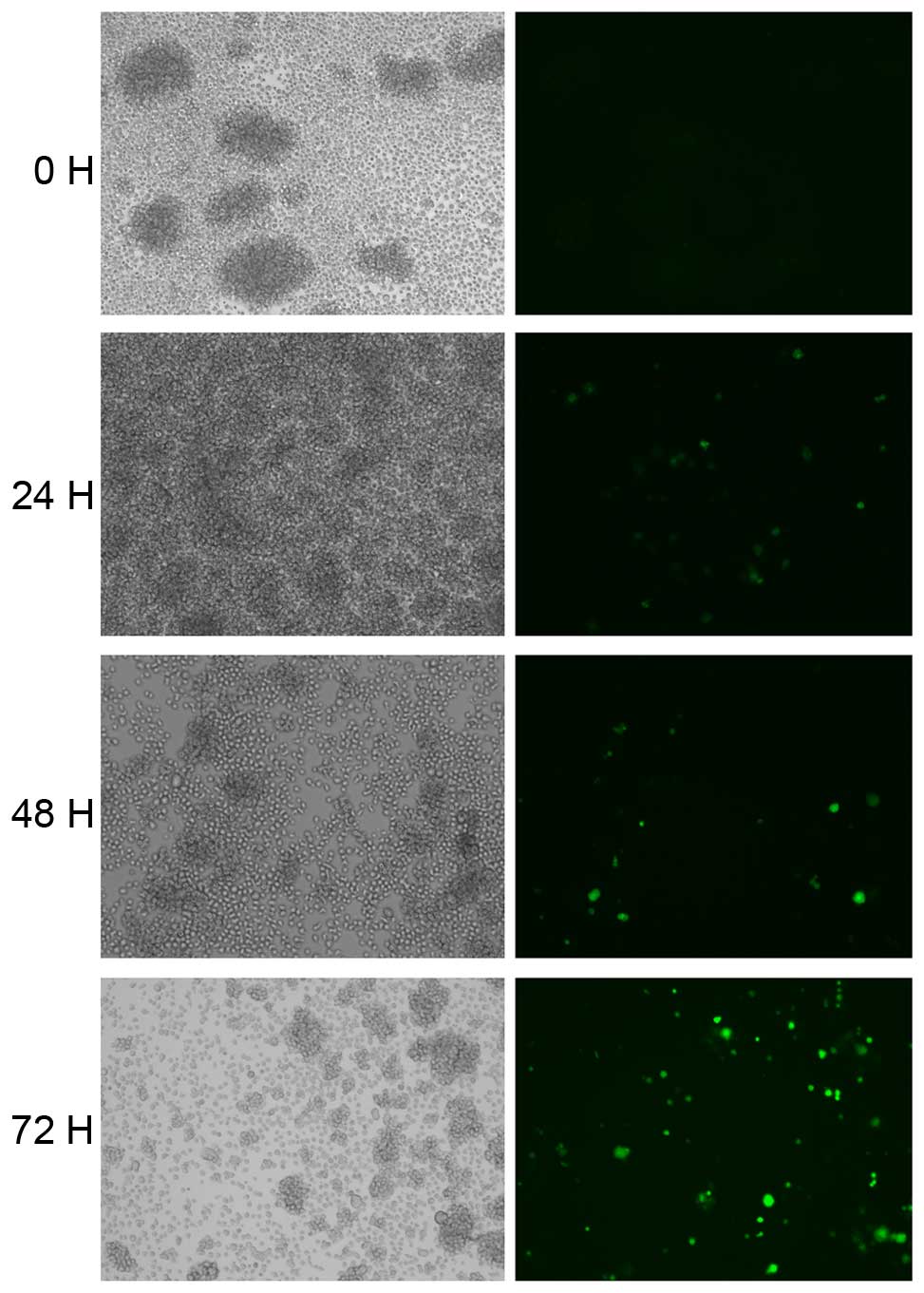
To assess whether HIV-1 can affect the expression of
RON and MSP JLTRG cells were infected by co-culture with
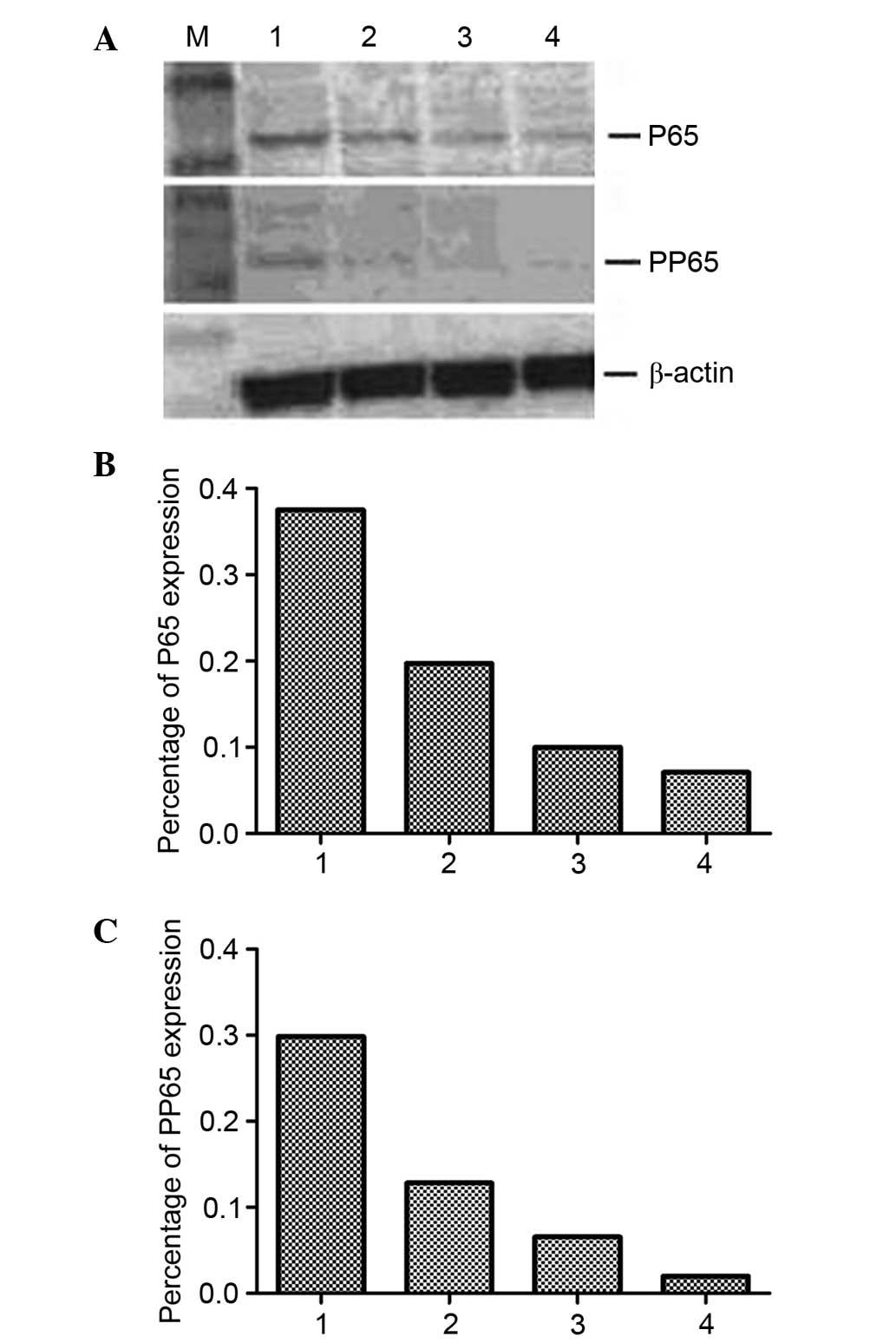
H9/HTLV-IIIB cells (Fig. 3). The
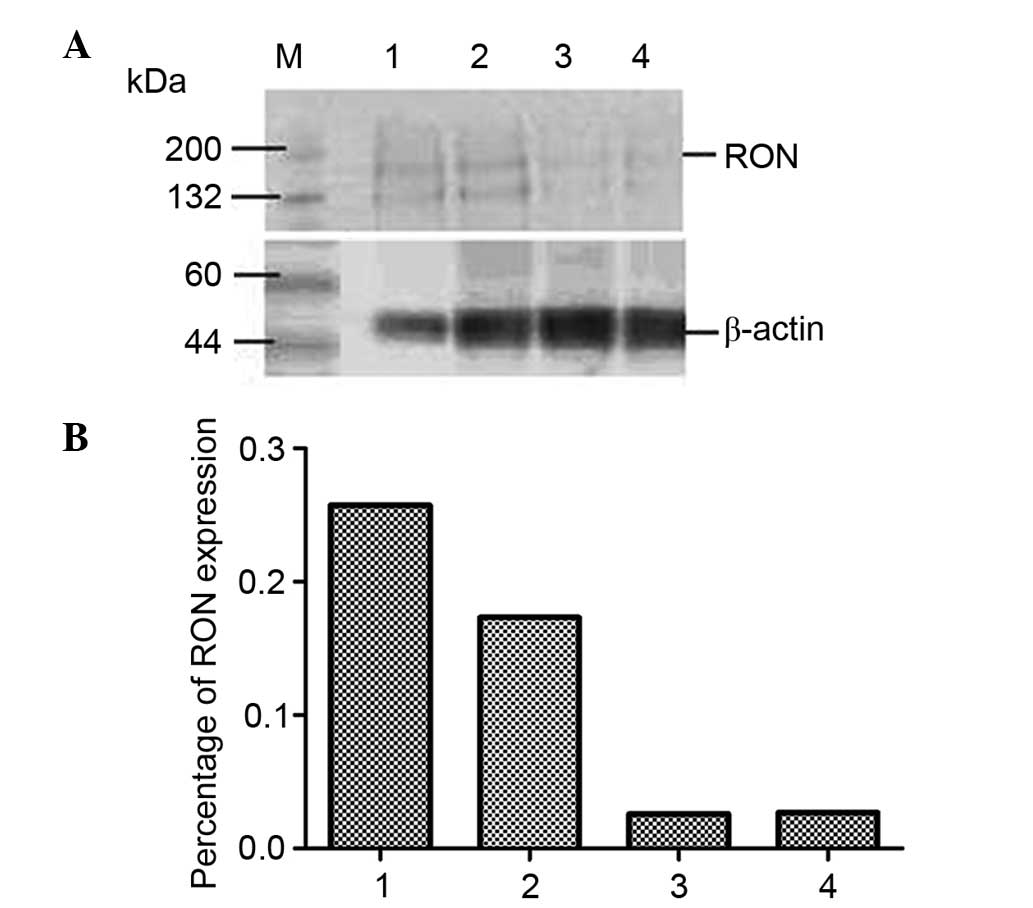
RON content of JLTRG cells cultured alone or co-cultured with
H9/HTLV-IIIB cells was assessed by western blot analysis (Fig. 4). RON expression was demonstrated
to be downregulated by HIV-1 infection in JLTRG cells. NF-κB
content of JLTRG cells cultured alone or co-cultured with
H9/HTLV-IIIB cells (10:1) was assessed by western blot analysis.
HIV-1 infection of JLTRG cells also reduced NF-κB phosphorylation
(Fig. 5).
Discussion
Recently, several studies have suggested a direct
interaction between RON and HIV-1 tat (16,17,24).
This study aimed to determine the circulating level of RON in
patients with HIV-1 that were, or were not receiving HAART therapy.
It was demonstrated that circulating levels of RON were
significantly higher in HIV-1 infected patients than healthy
control patients, and higher in patients that were receiving HAART
therapy than those who were not. The converse was true of the RON
ligand MSP. Circulating levels of MSP were significantly lower in
HIV-1-infected patients compared with healthy control patients, and
lower in patients that were receiving HAART therapy than those who
were not.
HIV-1 tat was reported to downregulate RON, and RON
expression has been reported to be altered during chronic
inflammation induced by HIV-1 infection of the brain (16,17,24,25).
In a small study of brain tissues increased RON was detected in all
seven HIV seronegative patients, but in six of nine patients with
AIDS, reduced RON protein was detected (17). While depressed circulating RON was
observed HIV-1 positive patients not receiving HAART compared with
treated patients, in patients receiving HAART the RON level was
significantly recovered. These findings support the consensus that
HIV-1 tat specifically downregulates RON, likely enhancing the
inflammatory environment under which HIV-1 replication thrives. The
low peripheral level of RON of healthy controls likely reflects the
lack of inflammation, and low level of inflammatory cells in the
circulation, in comparison to the brain tissues (17).
This study aimed to investigate the mechanism by
which HIV-1 downregulates RON. Thus the T-cell line, JLTRG, with
high basal RON expression was investigated. HIV-1 infection of
JLTRG cells reduced RON expression and its phosphorylation, which
was accompanied by reduced NF-κB phosphorylation. Thus, it was
concluded that HIV-1 downregulates the expression and
phosphorylation of RON by targeting the NF-κB pathway in T cells.
In addition, the downregulation of RON expression on the HIV-1
infected T cell surface may contribute to the increase in the
circulating levels of RON in HIV-1-infected patients, probably via
the release of the degraded RON on the T cell surface into the
peripheral blood, which will be investigated in our future
studies.
It was previously reported that HIV-1 tat mediates
degradation of RON in HIV-1-infected monocytes (24), and that RON represses HIV-1
transcription by targeting RNA polymerase II processivity in a
monocytic cell line (16).
Overexpressing RON in monocytes/macrophages demonstrates that RON
inhibits HIV-1 proviral transcription in part by decreasing the
binding activity of NF-κB to the HIV-1 LTR (16,17).
Consistent with the present findings in the JLTRG T cell line, RON
expression decreased basal levels of NF-κB in a monocyte cell line,
in addition to binding to the HIV provirus LTR and reducing
efficient HIV-1 transcription (16).
These findings indicate that RON may influence the
capacity of HIV to establish a latent reservoir in macrophages and
T cell subsets, and influence the development of inflammatory
microenvironments that favor HIV-1 replication. HIV-1 has thus
evolved to specifically target RON, and may reduce RON activity by
a range of mechanisms. This study reported that the downregulation
of RON phosphorylation in a HIV-1-infected T cell line is
accompanied by reduced NF-κB phosphorylation. In conclusion the
present study determined that HIV-1 infection modulated the RON
function and thus provided a permissive environment for HIV-1 and
other opportunistic microbes.
Acknowledgments
This study was supported by the Natural Science
Youth Foundation of Jiangsu Province (Grant no. BK20130271) and the
National Science and Technology Major Project (Grant no.
2012ZX10002004-008).
References
|
1
|
Meltzer MS, Nakamura M, Hansen BD, Turpin
JA, Kalter DC and Gendelman HE: Macrophages as susceptible targets
for HIV infection, persistent viral reservoirs in tissue and key
immunoregulatory cells that control levels of virus replication and
extent of disease. AIDS Res Hum Retroviruses. 6:967–971.
1990.PubMed/NCBI
|
|
2
|
Balestra E, Perno CF, Aquaro S, Panti S,
Bertoli A, Piacentini M, Forbici F, D'Arrigo R, Calió R and Garaci
E: Macrophages: A crucial reservoir for human immunodeficiency
virus in the body. J Biol Regul Homeost Agents. 15:272–276.
2001.PubMed/NCBI
|
|
3
|
Kedzierska K and Crowe SM: The role of
monocytes and macrophages in the pathogenesis of HIV-1 infection.
Curr Med Chem. 9:1893–1903. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fantuzzi L, Belardelli F and Gessani S:
Monocyte/macrophage-derived CC chemokines and their modulation by
HIV-1 and cytokines: A complex network of interactions influencing
viral replication and AIDS pathogenesis. J Leukoc Biol. 74:719–725.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leonis MA, Thobe MN and Waltz SE:
Ron-receptor tyrosine kinase in tumorigenesis and metastasis.
Future Oncol. 3:441–448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Correll PH, Morrison AC and Lutz MA:
Receptor tyrosine kinases and the regulation of macrophage
activation. J Leukoc Biol. 75:731–737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang MH, Julian FM, Breathnach R, Godowski
PJ, Takehara T, Yoshikawa W, Hagiya M and Leonard EJ: Macrophage
stimulating protein (MSP) binds to its receptor via the MSP beta
chain. J Biol Chem. 272:16999–17004. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang MH, Cox GW, Yoshimura T, Sheffler LA,
Skeel A and Leonard EJ: Macrophage-stimulating protein inhibits
induction of nitric oxide production by endotoxin- or
cytokine-stimulated mouse macrophages. J Biol Chem.
269:14027–14031. 1994.PubMed/NCBI
|
|
9
|
Chen YQ, Fisher JH and Wang MH: Activation
of the RON receptor tyrosine kinase inhibits inducible nitric oxide
synthase (iNOS) expression by murine peritoneal exudate
macrophages: Phosphatidylinositol-3 kinase is required for
RON-mediated inhibition of iNOS expression. J Immunol.
161:4950–4959. 1998.PubMed/NCBI
|
|
10
|
Liu QP, Fruit K, Ward J and Correll PH:
Negative regulation of macrophage activation in response to
IFN-gamma and lipopolysaccharide by the STK/RON receptor tyrosine
kinase. J Immunol. 163:6606–6613. 1999.PubMed/NCBI
|
|
11
|
Morrison AC, Wilson CB, Ray M and Correll
PH: Macrophage-stimulating protein, the ligand for the stem
cell-derived tyrosine kinase/RON receptor tyrosine kinase, inhibits
IL-12 production by primary peritoneal macrophages stimulated with
IFN-gamma and lipopolysaccharide. J Immunol. 172:1825–1832. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morrison AC and Correll PH: Activation of
the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase
by macrophage-stimulating protein results in the induction of
arginase activity in murine peritoneal macrophages. J Immunol.
168:853–860. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Correll PH, Iwama A, Tondat S, Mayrhofer
G, Suda T and Bernstein A: Deregulated inflammatory response in
mice lacking the STK/RON receptor tyrosine kinase. Genes Funct.
1:69–83. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leonis MA, Toney-Earley K, Degen SJ and
Waltz SE: Deletion of the Ron receptor tyrosine kinase domain in
mice provides protection from endotoxin-induced acute liver
failure. Hepatology. 36:1053–1060. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsutsui S, Noorbakhsh F, Sullivan A,
Henderson AJ, Warren K, Toney-Earley K, Waltz SE and Power C:
RON-regulated innate immunity is protective in an animal model of
multiple sclerosis. Ann Neurol. 57:883–895. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klatt A, Zhang Z, Kalantari P, Hankey PA,
Gilmour DS and Henderson AJ: The receptor tyrosine kinase RON
represses HIV-1 transcription by targeting RNA polymerase II
processivity. J Immunol. 180:1670–1677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee ES, Kalantari P, Tsutsui Section S,
Klatt A, Holden J, Correll PH, Power Section C and Henderson AJ:
RON receptor tyrosine kinase, a negative regulator of inflammation,
inhibits HIV-1 transcription in monocytes/macrophages and is
decreased in brain tissue from patients with AIDS. J Immunol.
173:6864–6872. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Griffin GE, Leung K, Folks TM, Kunkel S
and Nabel GJ: Activation of HIV gene expression during monocyte
differentiation by induction of NF-kappa B. Nature. 339:70–73.
1989. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harrich D, Garcia J, Wu F, Mitsuyasu R,
Gonazalez J and Gaynor R: Role of SP1-binding domains in in vivo
transcriptional regulation of the human immunodeficiency virus type
1 long terminal repeat. J Virol. 63:2585–2591. 1989.PubMed/NCBI
|
|
20
|
Henderson AJ, Connor RI and Calame KL:
C/EBP activators are required for HIV-1 replication and proviral
induction in monocytic cell lines. Immunity. 5:91–101. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agbottah E, Deng L, Dannenberg LO, Pumfery
A and Kashanchi F: Effect of SWI/SNF chromatin remodeling complex
on HIV-1 Tat activated transcription. Retrovirology. 3:482006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahmoudi T, Parra M, Vries RG, Kauder SE,
Verrijzer CP, Ott M and Verdin E: The SWI/SNF chromatin-remodeling
complex is a cofactor for Tat transactivation of the HIV promoter.
J Biol Chem. 281:19960–19968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tréand C, du Chéné I, Brès V, Kiernan R,
Benarous R, Benkirane M and Emiliani S: Requirement for SWI/SNF
chromatin-remodeling complex in Tat-mediated activation of the
HIV-1 promoter. Embo J. 25:1690–1699. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kalantari P, Harandi OF, Hankey PA and
Henderson AJ: HIV-1 Tat mediates degradation of RON receptor
tyrosine kinase, a regulator of inflammation. J Immunol.
181:1548–1555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng T, Shao X, Li J, et al: Human
immunodeficiency virus (HIV)-1 Tat downregulates the
phosphorylation of recepteur d'origine nantais (RON) receptor
tyrosine kinase induced by macrophage-stimulating protein. African
Journal of Biotechnology. 10:16610–16616. 2013.
|
|
26
|
Schneider E, Whitmore S, Glynn KM,
Dominguez K, Mitsch A and McKenna MT; Centers for Disease Control
and Prevention (CDC): Revised surveillance case definitions for HIV
infection among adults, adolescents, and children aged <18
months and for HIV infection and AIDS among children aged 18 months
to <13 years - United States, 2008. MMWR Recomm Rep. 57:1–12.
2008.PubMed/NCBI
|
|
27
|
Hargreaves N and Scano F: Guidelines for
implementing collaborative TB and HIV programme activities.
2003
|
|
28
|
Lu Y, Yao HP and Wang MH: Multiple
variants of the RON receptor tyrosine kinase: Biochemical
properties, tumorigenic activities and potential drug targets.
Cancer Lett. 257:157–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu Y, Yao HP and Wang MH: Significance of
the entire C-terminus in biological activities mediated by the RON
receptor tyrosine kinase and its oncogenic variant RON160. J Exp
Clin Cancer Res. 27:552008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao HP, Luo YL, Feng L, Cheng LF, Lu Y, Li
W and Wang MH: Agonistic monoclonal antibodies potentiate
tumorigenic and invasive activities of splicing variant of the RON
receptor tyrosine kinase. Cancer Biol Ther. 5:1179–1186. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guin S, Yao HP and Wang MH: RON receptor
tyrosine kinase as a target for delivery of chemodrugs by antibody
directed pathway for cancer cell cytotoxicity. Mol Pharm.
7:386–397. 2010. View Article : Google Scholar
|
|
32
|
Ochsenbauer-Jambor C, Jones J, Heil M,
Zammit KP and Kutsch O: T-cell line for HIV drug screening using
EGFP as a quantitative marker of HIV-1 replication. Biotechniques.
40:91–100. 2006. View Article : Google Scholar : PubMed/NCBI
|