Introduction
Breast cancer is the most common type of cancer and
leading cause of cancer-related mortality in females with an
estimated 1.7 million cases and 521,900 related fatalities in 2012
(1). Breast cancer is the most
common type of cancer and was the leading cause of cancer mortality
in women in 2008, and it is estimated that breast cancer will
affect five million women worldwide over the subsequent decade
(1). Over 60% of breast cancers
are estrogen receptor (ER)-positive, and their development can be
stimulated by estrogen and inhibited by ER antagonists, including
tamoxifen.
Tamoxifen, broadly used in ER-positive breast cancer
prevention and treatment, was approved as the first line
anti-estrogen therapy in 1999 by the US Food and Drug
Administration (2). Endocrine
therapy with tamoxifen for five years has resulted in a 9.2%
absolute reduction in mortality at 15 years, with a 34% reduction
in the breast cancer mortality rate per year (3). However, the emergence of resistant
cancer cells limits its therapeutic effectiveness (4,5).
Thus, tamoxifen is typically administered in combination with other
drugs, reducing the incidence of the development of drug resistance
(6,7).
Previously, researchers have taken an increasing
interest in combination therapy by associating anti-cancer herbal
medicine with chemotherapeutic agents, and have demonstrated
significant successes (8–10). Berberine, an isoquinoline plant
alkaloid isolated from Coptidis rhizome, regulates multiple targets
and may be a promising natural agent with chemotherapeutic
potential based on its effect on the expression of various proteins
(11–14). Berberine has previously been used
to enhance cancer therapy sensitization and to assist chemo-therapy
by modulating multiple pathways (15–17).
These molecular targets of berberine are also involved in growth
maintenance and resistance acquisition in tamoxifen-resistant
breast cancer. Thus, a combination of berberine with tamoxifen may
be an interesting option in the treatment of patients with
ER-positive breast cancer.
The current study evaluated the individual and
combined effects of berberine and tamoxifen in breast cancer MCF-7
and tamoxifen-resistant MCF-7/TAM cells. The results of the present
study demonstrated that berberine does not abolish the anti-tumor
effects of tamoxifen, and it induces cell G1 arrest, apoptosis, and
significantly enhances the growth inhibition effect of tamoxifen in
these cells. The mechanisms underlying these effects involve the
regulation of important cell cycle and apoptosis-associated
proteins.
Materials and methods
Materials
MCF-7 human breast cancer cells were provided by the
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences (Shanghai, China). Berberine (Sigma-Aldrich, St. Louis,
MO, USA) and tamoxifen (Sigma-Aldrich) were freshly dissolved in
dimethyl sulfoxide and methanol, respectively, and stored in the
dark at 4°C. The final concentration of vehicle in the culture did
not exceed 0.1% (v/v). RPMI 1640 medium and fetal bovine serum
(FBS) were purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay reagent was purchased from Promega Corporation
(Madison, WI, USA). Anti-P21 (goat-anti-rabbit polyclonal; cat. no.
10355-1-AP), cyclin D1 (goat-anti-mouse monoclonal; cat. no.
60186-1-Ig), B-cell CLL/lymphoma 2 (Bcl-2; goat-anti-rabbit
polyclonal; cat. no. 12789-1-AP), Bcl-2 associated X protein (Bax;
goat-anti-rabbit polyclonal; cat. no. 23931-1-AP), β-actin
(goat-anti-mouse monoclonal; cat. no. 60008-1-Ig) antibodies were
purchased from Protein-Tech Group, Inc. (Chicago, IL, USA).
Anti-mouse and anti-rabbit horseradish peroxidase (HRP)-conjugated
(cat. nos. A0428 and A0423, respectively) secondary antibodies were
purchased from Beyotime Institute of Biotechnology (Haimen,
China).
Establishment of MCF-7/TAM cells
To establish tamoxifen-resistant MCF-7 cell
resistant to tamoxifen, MCF-7 cells were exposed to 100 nM
tamoxifen in culture. Following 6 months of continuous expose to
tamoxifen, these cells exhibited proliferation in medium plus
tamoxifen comparable to that of parental cells without tamoxifen,
suggesting the development of resistance to the
proliferation-inhibitory properties of tamoxifen.
Cell culture and treatment
MCF-7 and tamoxifen-resistant MCF-7/TAM cells were
cultured in RPMI 1640 medium supplemented with penicillin (100
U/ml), streptomycin (100 µg/ml) and 10% FBS. To maintain
tamoxifen resistance, MCF-7/TAM cells were cultured in a medium
containing 100 nM of tamoxifen. All cells were maintained in
humidified 37°C incubators with 5% CO2.
MTS assay of cell proliferation
The effects of berberine, tamoxifen and their
combination on MCF-7 and MCF-7/TAM cell viability were determined
by MTS assay. The cells were seeded in 96-well plates at a density
of 1×104 cells/well and were cultured for 24 h. Cells
were treated with increasing doses of berberine (0, 20, 40, 80, 120
and 160 µM) for 24, 48 and 72 h, respectively. MCF-7 cells
were exposed to different concentrations of tamoxifen (0.5, 1, 2, 4
and 8 µM) with or without 20 µM berberine for 48 h
and MCF-7/TAM cells were exposed to different concentrations of
tamoxifen (1, 2, 4, 8 and 16 µM) with or without 20
µM berberine for 48 h. Subsequently, MCF-7 and MCF-7/TAM
cells were then exposed to different concentrations of tamoxifen
with or without 20 µM berberine for 48 h. Subsequently, 20
µl MTS was added to each well and incubated for 4 h at 37°C.
The absorbance was examined at 490 nm using an enzyme-linked
immunosorbent detector (BioTek Synergy2; BioTek Instruments, Inc.,
Winooski, VT, USA).
Flow cytometry analysis of cell cycle
distribution
Cells were plated in 6-well plates and cultured for
24 h. Then MCF-7 and MCF-7/TAM cells were treated with tamoxifen (1
µM), berberine (20 µM), their combination (20
µM berberine and 1 µM tamoxifen) or vehicle.
Following treatment for 48 h, cells were collected, washed twice
with phosphate-buffered saline (PBS), fixed with ice cold 70%
ethanol at −20°C over-night, centrifuged and resuspended in 300
µl PBS containing 0.5 mg/ml propidium iodide (PI;
eBioscience, San Diego, CA, USA) and 0.1 mg/ml of Rnase A
(eBioscience, San Diego, CA, USA). Subsequent to incubation for 30
min at 37°C, samples were evaluated using the Cytomics FC 500 flow
cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Flow cytometry analysis of apoptosis
Briefly, following the described treatment with
berberine and tamoxifen, floating and adherent cells (detached
using trypsin) were collected, washed twice in cold PBS, and then
suspended in binding buffer (BB (BB, BD Pharmingen, San Diego, CA,
USA). Cells (1×105 in 100 µl BB) were then
stained using 2.5 µl Annexin V-fluorescein isothiocyanate
(FITC; BD Pharmingen) and 20 µl 25X PI solution. Cells were
incubated in the dark at room temperature for 15 min, diluted using
additional 400 µl BB and analyzed with Cytomics FC 500 flow
cytometer (Beckman Coulter, Inc.).
Western blot analysis
Cells were treated as described, then placed on ice
and washed twice with cold PBS. Cells were then scraped into a
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology) supplemented with proteinase inhibitor phenyl methyl
sulfonyl fluoride (Beyotime Institute of Biotechnology) and
incubated for 30 min on ice. Following centrifugation at 12,000 × g
for 15 min, the supernatants were used for protein concentration
determination according to a Pierce bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc.). Equal amounts of
proteins (20 µg) were fractionated on SDS-polyacrylamide
gels (10%) and transferred electrophoretically onto polyvinylidene
fluoride (EMD Millipore, Billerica, MA, USA) membranes. Membranes
were blocked with blocking buffer at room temperature (Beyotime
Institute of Biotechnology) and probed overnight with primary mouse
anti-cyclin D1 (1:1,000), P21 (1:500), Bax (1:2,000), Bcl-2
(1:1,000), and β-actin (1:1,000) antibodies at 4°C. Subsequently,
membranes were incubated with the HRP-conjugated secondary antibody
(1:1,000) for 1 h at room temperature, and the signal was detected
using a quantitative chemiluminescent WesternBright Quantum kit
(Advansta, Inc., Menlo Park, CA, USA).
Statistical analysis
Data analysis was performed with the Student's
t-test for paired comparison and one-way analysis of
variance followed by Tukey's test using GraphPad Prism 4 software
(GraphPad Software, Inc., La Jolla, CA, USA). The data are
presented as the mean ± standard deviation and are representative
of three individual experiments. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of berberine on the growth of
MCF-7 and MCF-7/TAM cells
To determine the concentration- and time-dependent
effects of berberine exposure, MCF-7 and tamoxifen-resistant
MCF-7/TAM cells were treated with increasing doses of berberine for
24, 48 and 72 h. Cell proliferation changes were evaluated by MTS
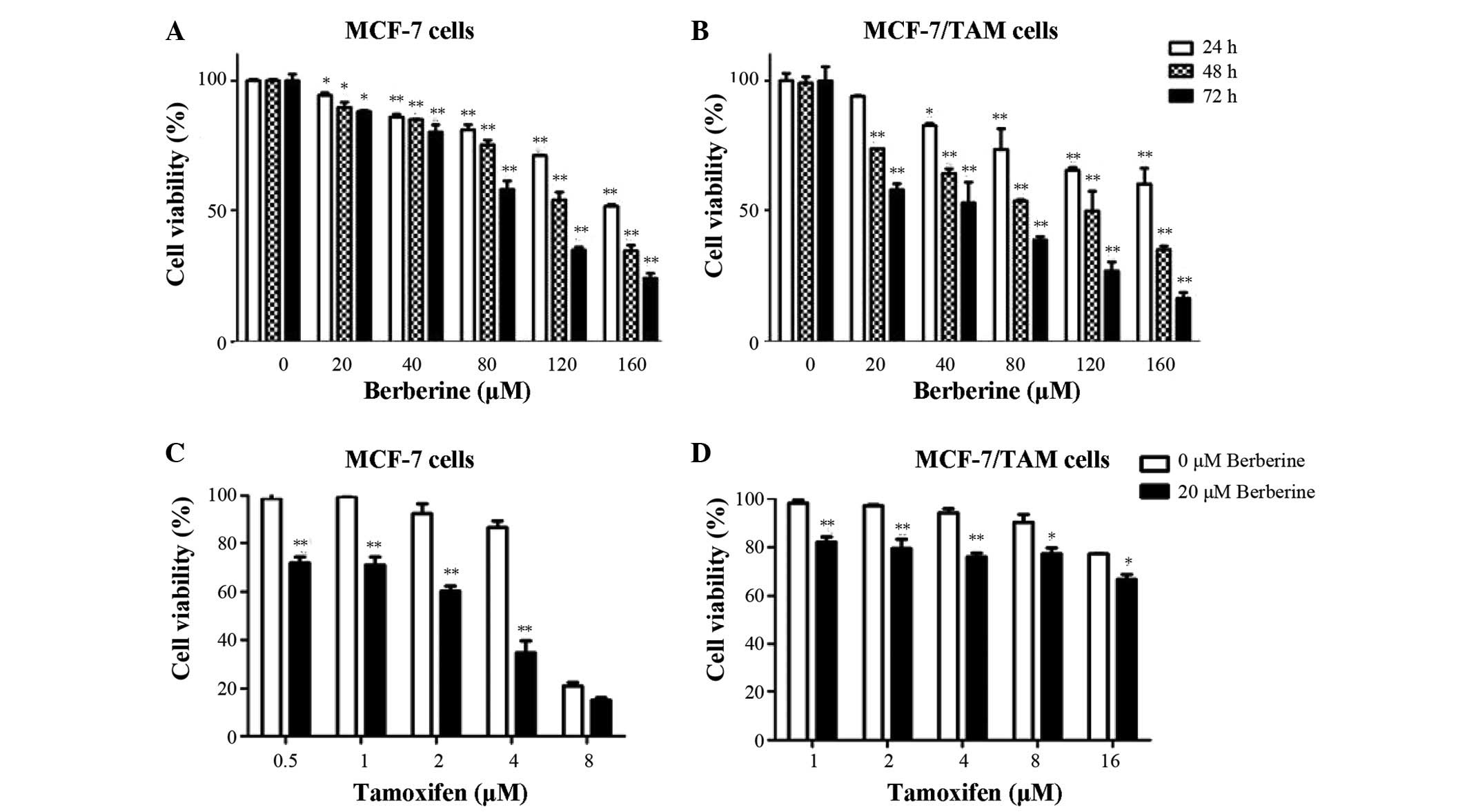
assay. As presented in Fig. 1A and
B, berberine demonstrated dose- and time-dependent
anti-proliferative activity in tamoxifen-sensitive MCF-7 and
resistant MCF-7/TAM cells. The IC50 values of berberine
for MCF-7 and MCF-7/TAM cells were approximately 130.3 and 99.7
µM, respectively. MCF-7/TAM cells were more sensitive to
berberine compared with MCF-7 cells. Collateral sensitivity is a
phenomenon that when a cell population is resistant to certain
drugs, it is more sensitive to others. In the subsequent combined
studies, 20 µM berberine was used, which is also quite
selective as it was previously described to be much less cytotoxic
against a non-tumorigenic breast cancer cell (w). The results of
the present study indicate that berberine exerts potent
anti-proliferation activity in MCF-7 breast cancer cells, whether
or not they are resistant to tamoxifen.
Co-treatment of berberine and tamoxifen
reduces cell viability in MCF-7 and MCF-7/TAM cells
To determine whether combined treatment of tamoxifen
and berberine exerts an enhanced anti-cancer effect on breast
cancer cells, cell proliferation was determined by MTS assay in
MCF-7 and MCF-7/TAM cells treated with tamoxifen alone, or combined
with berberine for 48 h. As demonstrated in Fig. 1C and D, the combined treatment of
tamoxifen and berberine resulted in synergistic inhibitory effects
on MCF-7 and MCF-7/TAM cells. When used alone, tamoxifen (1
µM) induced a 0.87±0.24% inhibitory effect on MCF-7 cell
viability, whereas combined use with 20 µM berberine
resulted in an inhibitory effect of 29±3.25% (P<0.01). Berberine
also significantly enhanced 1 µM tamoxifen-induced cell
proliferation inhibition from 1.87±1.24% to 17.87±2.05% (P<0.01)
in MCF-7/TAM cells. These results clearly demonstrated that
berberine increased the sensitivity of MCF-7 and MCF-7/TAM cells to
tamoxifen compared with tamoxifen alone.
Co-treatment of berberine and tamoxifen
induces cell cycle arrest in MCF-7 and MCF-7/TAM cells
To assess whether combined treatment can induce cell
cycle arrest in MCF-7 and MCF-7/TAM cells, the cell cycle
distribution was examined by flow cytometry analysis. As
demonstrated in Fig. 2A, tamoxifen
marginally increased the number of MCF-7 cells in the G1 phase.
Berberine alone or in combination with tamoxifen significantly
increased the number of MCF-7 cells in G1 phase compared with
control or tamoxifen alone, respectively (P<0.01). Similar
results were also observed in the MCF-7/TAM cells. As demonstrated
in Fig. 2B, 89.07±1.26% of the
cells treated with berberine and tamoxifen combined were in the G1
phase, which was significantly higher compared with the
corresponding percentage of tamoxifen-treated cells (73.29%±1.14;
P<0.01), berberine-treated cells (83.93±2.60%; P<0.05) or
vehicle control treated cells (63.69±1.61%; P<0.01). The results
suggested that co-treatment of tamoxifen and berberine produced an
increase in the number of cells in G1 phase compared with tamoxifen
alone, clearly demonstrating an effect on G1 cell cycle arrest.
Co-treatment of berberine and tamoxifen
induced cell apoptosis in MCF-7 and MCF-7/TAM cells
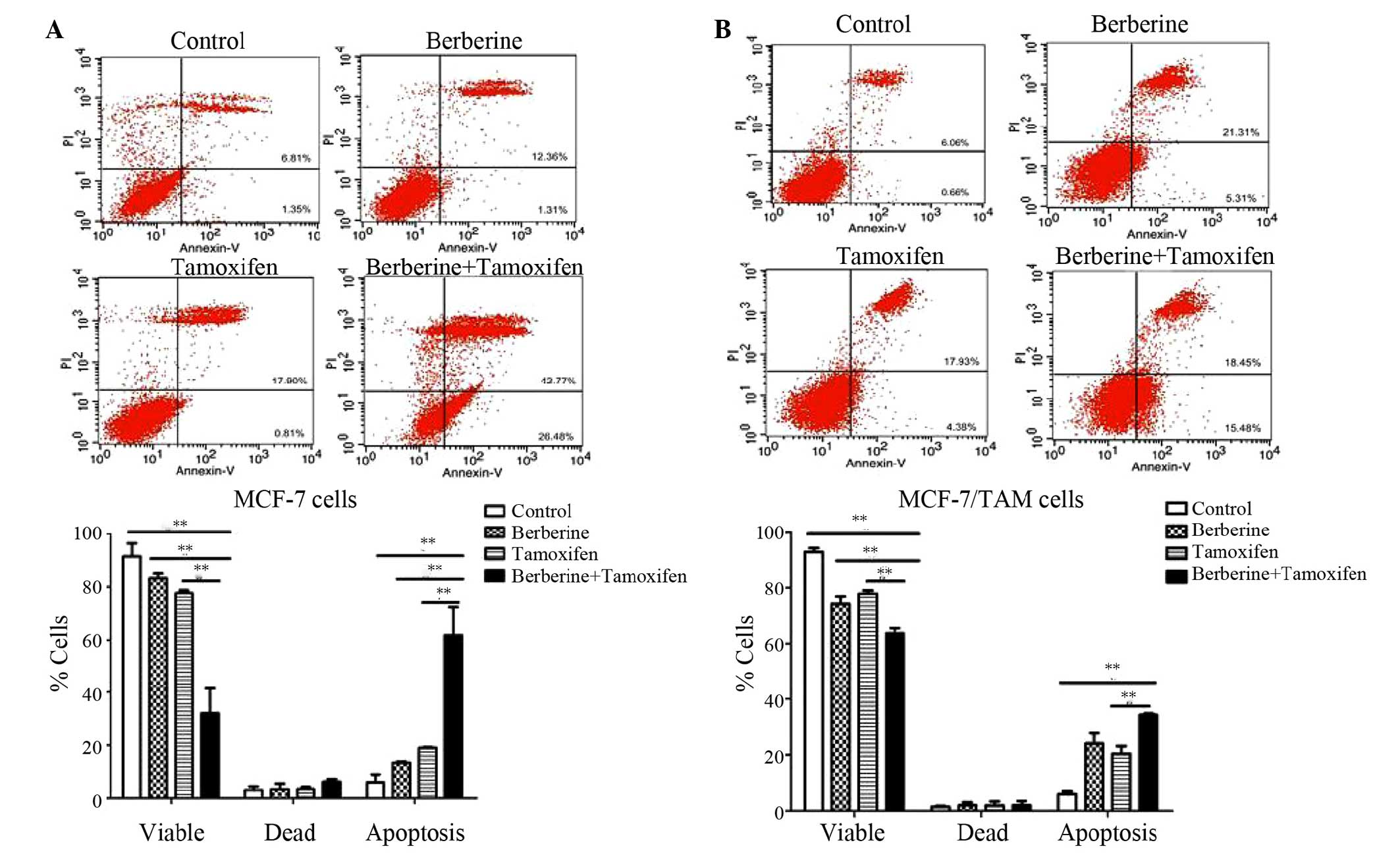
To determine whether the cell growth inhibition was
mediated by apoptosis, cells were stained with Annexin V-FITC/PI
following treatment with berberine and tamoxifen. As demonstrated
in Fig. 3, each compound treatment
produced an observable increase in the percentage of apoptotic
cells. It was demonstrated that 20 µM berberine and 1
µM tamoxifen increased apoptosis to 61.8±7.47% compared with
18.9±0.17% in tamoxifen only-treated MCF-7 cells (P<0.01).
Furthermore, the number of apoptotic MCF-7/TAM cells was increased
in the berberine + tamoxifen group (20.4%±1.95) compared with the
tamoxifen only group (34.2%±0.32; P<0.01) after 48 h treatment.
The results demonstrated that co-treatment is more effective in
activating stimulating compared with tamoxifen alone.
Effects of berberine and tamoxifen on P21
and cyclin D1 protein expression
To assess the cell G1 arrest mechanism induced by
the combined treatment, western blot analysis of the cell
cycle-associated proteins, P21 and cyclin D1, was performed. As
demonstrated in Fig. 4, berberine
treatment alone efficiently induced the protein expression levels
of P21 in MCF-7 and tamoxifen-resistant MCF-7/TAM cells compared
with control, whereas tamoxifen alone only marginally increased the
levels of P21 in the cell lines. Tamoxifen in combination with
berberine markedly increased the tamoxifen-induced level of P21 in
MCF-7 and MCF-7/TAM cells. No change was observed in the protein
expression level of cyclin D1 in any of the treatment groups. These
results suggested that upregulation of P21 may be involved in cell
cycle arrest by tamoxifen and berberine in MCF-7 and MCF-7/TAM
cells.
Effects of berberine and tamoxifen on Bax
and Bcl-2 protein expression
The apoptotic mechanism involved in the cell death
response to berberine and tamoxifen was further examined by
monitoring the expression levels of anti-apoptotic protein, Bcl-2,
and the pro-apoptotic protein, Bax, via western blotting. As
demonstrated in Fig. 5, combined
treatment efficiently inhibited the protein levels of Bcl-2 in
MCF-7 and tamoxifen-resistant MCF-7/TAM cells compared with the
control and tamoxifen groups. Notably, a marked increase in Bax
expression was observed in the MCF-7/TAM cells combined treatment
group, whereas no change in Bax protein expression was observed in
MCF-7 cells. Together, these results demonstrated that tamoxifen +
berberine-induced apoptosis in breast cancer cells may be mediated
by Bax/Bcl-2 upregulation.
Discussion
Breast cancer remains a worldwide public health
concern, and a major cause of morbidity and mortality among
females. Endocrine therapies have permitted important progress for
the treatment of ER-positive breast cancer. Despite of being a
powerful selective ER antagonist, innate or acquired resistance to
tamoxifen is a major problem for anti-estrogen therapy (4,5).
Thus, it is necessary to develop combined treatments to enhance the
efficacy of tamoxifen.
Natural compounds from plants are increasingly being
considered as potential anti-cancer agents. Berberine, an
isoquinoline alkaloid, has previously been demonstrated to regulate
multiple targets and used to enhance targeted therapy sensitization
for cancer chemotherapy. For example, doxorubicin and cisplatin,
with the combination of berberine, exhibited a higher cytotoxic
effect compared with monotherapy in in vivo and in
vitro studies (8,15). Berberine has also previously been
reported to regulate the expression of cell cycle and
apoptosis-associated proteins, including cyclin D1, P21 and Bcl-2.
These targets are also understood to be involved in tamoxifen
sensitivity and resistance in breast cancer treatment (19–21).
It is speculated that berberine may potentially enhance the
efficacy of tamoxifen. The results of the current study
demonstrated that berberine exerted anti-proliferative effects on
MCF-7 and MCF-7/TAM cells, and the co-treatment with berberine and
tamoxifen significantly reduced the viability of MCF-7 and
MCF-7/TAM cells compared with the tamoxifen applied separately.
The induction of cell cycle arrest is considered as
one of the potential mechanisms of inhibition of cancer
development. In order to determine the potential molecular
mechanism underlying the synergistic anti-cancer effects of
berberine and tamoxifen, the cell cycle distribution was analyzed.
The results of the current study indicated that co-treatment
significantly induced cell G1 phase arrest in MCF-7 and
tamoxifen-resistant MCF-7/TAM cells. The cyclin-dependent kinase
inhibitor P21 is involved in the growth and development of breast
cancer. It has previously been reported that berberine induced G1
phase arrest by p53-dependent upregulation of P21 (20). P21 has previously been implicated
in mediating the sensitivity to tamoxifen in MCF-7 breast cancer
cells (22). A previous study
demonstrated that loss of P21 function increased tamoxifen
resistance (19). The results of
the current study demonstrated that berberine + tamoxifen increased
the protein expression level of p21 in MCF-7 and MCF-7/TAM cells
compared with tamoxifen alone, suggesting that there was functional
inactivation of p21 and thus, interference with the effectiveness
of tamoxifen.
Apoptosis is crucial in the response of cancer to
chemotherapy. The Bcl-2/Bax ratio is important in determining
whether a cell will undergo apoptosis or survive (22). The present study demonstrated that
the induction of apoptosis in human breast cancer cells by
co-treatment was potentially mediated by upregulating the Bax/Bcl-2
ratio. It was demonstrated that berberine significantly enhanced
tamoxifen-induced apoptosis. Bcl-2, an anti-apoptotic protein, has
previously been reported to be overexpressed in 28–80% of patients
with breast cancer (23,24). Therefore, the current study
determined whether combinational treatment could regulate the ratio
of Bax/Bcl-2. It has been reported that downregulation of Bcl-2 is
sufficient to enhance sensitivity to tamoxifen in human breast
cancer cells. It has been reported that the downregulation of Bcl-2
is sufficient to enhance sensitivity to tamoxifen in human breast
cancer cells (25). These findings
suggested that Bax/Bcl-2 upregulation may function as a point of
convergence for inducing cell apoptosis. The present study
demonstrated that the induction of apoptosis in human breast cancer
cells by berberine and tamoxifen co-treatment was potentially
mediated by upregulating the Bax/Bcl-2 ratio.
In the present study, the combined effects of
berberine and tamoxifen on growth inhibition were evident. This
finding is based on the fact that co-treatment with berberine and
tamoxifen significantly reduced the viability of breast cancer
cells compared to the tamoxifen treatment alone, Additionally,
co-treatment induced cell cycle arrest and increased apoptotic cell
death in the current study. The tamoxifen-resistant MCF-7/TAM
breast cancer cells exhibited similar responses to
tamoxifen-sensitive MCF-7 cells, indicating that berberine in
combination with tamoxifen enhanced the growth inhibition of breast
cancer cells independent of tamoxifen resistance. These results
demonstrated that berberine may be a powerful adjuvant for
anti-cancer therapy, particularly when combined with tamoxifen for
ER-positive breast cancer treatment. The findings of the present
study provide novel evidence for the importance and the
effectiveness of combinational drug treatment in anti-cancer
chemotherapy. However, in vivo study and further mechanistic
analyses are necessary to confirm the effects of this
combination.
Acknowledgments
This research was supported by the National
Scientific Foundation of China (grant nos. 81473284 and 81102511),
the Natural Science Foundation of Chongqing (grant no.
cstc2011jjA10004), and the Funds for Outstanding Young Scholars in
Chongqing Medical University (grant nos. CYYQ201301 and
CYYQ201401).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cuzick J, Powles T, Veronesi U, Forbes J,
Edwards R, Ashley S and Boyle P: Overview of the main outcomes in
breast-cancer prevention trials. Lancet. 361:296–300. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY,
Zhu Y, Skaar TC, Gomez B, O'Brien K, Wang Y, et al: Antiestrogen
resistance in breast cancer and the role of estrogen receptor
signaling. Oncogene. 22:7316–7339. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lewis JS and Jordan VC: Selective estrogen
receptor modulators (SERMs): Mechanisms of anticarcinogenesis and
drug resistance. Mutat Res. 591:247–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang M, Huang O, Zhang X, Xie Z, Shen A,
Liu H, Geng M and Shen K: Curcumin induces cell death and restores
tamoxifen sensitivity in the antiestrogen-resistant breast cancer
cell lines MCF-7/LCC2 and MCF-7/LCC9. Molecules. 18:701–720. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Charalambous C, Pitta CA and Constantinou
AI: Equol enhances tamoxifen's anti-tumor activity by induction of
caspase-mediated apoptosis in MCF-7 breast cancer cells. BMC
Cancer. 13:2382013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mittal A, Tabasum S and Singh RP:
Berberine in combination with doxorubicin suppresses growth of
murine melanoma B16F10 cells in culture and xenograft.
Phytomedicine. 21:340–347. 2014. View Article : Google Scholar
|
|
9
|
Zhao Q, Wang J, Zou MJ, Hu R, Zhao L,
Qiang L, Rong JJ, You QD and Guo QL: Wogonin potentiates the
antitumor effects of low dose 5-fluorouracil against gastric cancer
through induction of apoptosis by down-regulation of NF-kappaB and
regulation of its metabolism. Toxicol Lett. 197:201–210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Notarbartolo M, Poma P, Perri D, Dusonchet
L, Cervello M and D'Alessandro N: Antitumor effects of curcumin,
alone or in combination with cisplatin or doxorubicin, on human
hepatic cancer cells. Analysis of their possible relationship to
changes in NF-kB activation levels and in IAP gene expression.
Cancer Lett. 224:53–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wen CJ, Wu LX, Fu LJ, Yu J, Zhang YW,
Zhang X and Zhou HH: Genomic screening for targets regulated by
berberine in breast cancer cells. Asian Pac J Cancer Prev.
14:6089–6094. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Liu Q, Liu Z, Li B, Sun Z, Zhou H,
Zhang X, Gong Y and Shao C: Berberine, a genotoxic alkaloid,
induces ATM-Chk1 mediated G2 arrest in prostate cancer cells. Mutat
Res. 734:20–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu B, Hu M, Liu K and Peng J: Cytotoxicity
of berberine on human cervical carcinoma HeLa cells through
mitochondria, death receptor and MAPK pathways, and in-silico
drug-target prediction. Toxicol In Vitro. 24:1482–1490. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu B, Zhao J, Xu L, Xu Y, Wang X and Peng
J: Identification of molecular target proteins in berberine-treated
cervix adenocarcinoma HeLa cells by proteomic and bioinformatic
analyses. Phytother Res. 26:646–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Youn MJ, So HS, Cho HJ, Kim Y, Lee JH,
Sohn JS, Kim YK, Chung SY and Park R: Berberine, a natural product,
combined with cisplatin enhanced apoptosis through a
mitochondria/caspase-mediated pathway in HeLa cells. Biol Pharm
Bull. 31:789–795. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin TH, Kuo HC, Chou FP and Lu FJ:
Berberine enhances inhi-bition of glioma tumor cell migration and
invasiveness mediated by arsenic trioxide. BMC Cancer. 8:582008.
View Article : Google Scholar
|
|
17
|
Fan LX, Liu CM, Gao AH, Zhou YB and Li J:
Berberine combined with 2-deoxy-d-glucose synergistically enhances
cancer cell proliferation inhibition via energy depletion and
unfolded protein response disruption. Biochim Biophys Acta.
1830:5175–5183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patil JB, Kim J and Jayaprakasha GK:
Berberine induces apoptosis in breast cancer cells (MCF-7) through
mitochondrial-dependent pathway. Eur J Pharmacol. 645:70–78. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abukhdeir AM, Vitolo MI, Argani P, De
Marzo AM, Karakas B, Konishi H, Gustin JP, Lauring J, Garay JP,
Pendleton C, et al: Tamoxifen-stimulated growth of breast cancer
due to p21 loss. Proc Natl Acad Sci USA. 105:288–293. 2008.
View Article : Google Scholar :
|
|
20
|
Liu Z, Liu Q, Xu B, Wu J, Guo C, Zhu F,
Yang Q, Gao G, Gong Y and Shao C: Berberine induces p53-dependent
cell cycle arrest and apoptosis of human osteosarcoma cells by
inflicting DNA damage. Mutat Res. 662:75–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Larsen MS, Bjerre K, Giobbie-Hurder A,
Lænkholm AV, Henriksen KL, Ejlertsen B, Lykkesfeldt AE and
Rasmussen BB: Prognostic value of Bcl-2 in two independent
populations of estrogen receptor positive breast cancer patients
treated with adjuvant endocrine therapy. Acta Oncol. 51:781–789.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv L, Zheng L, Dong D, Xu L, Yin L, Xu Y,
Qi Y, Han X and Peng J: Dioscin, a natural steroid saponin, induces
apoptosis and DNA damage through reactive oxygen species: A
potential new drug for treatment of glioblastoma multiforme. Food
Chem Toxicol. 59:657–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Callagy GM, Pharoah PD, Pinder SE, Hsu FD,
Nielsen TO, Ragaz J, Ellis IO, Huntsman D and Caldas C: Bcl-2 is a
prognostic marker in breast cancer independently of the Nottingham
prognostic index. Clin Cancer Res. 12:2468–2475. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cardoso F, Paesmans M, Larsimont D,
Durbecq V, Bernard-Marty C, Rouas G, Dolci S, Sotiriou C, Piccart
MJ and Di Leo A: Potential predictive value of Bcl-2 for response
to tamoxifen in the adjuvant setting of node-positive breast
cancer. Clin Breast Cancer. 5:364–369. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu C, Kong X, Wang H, Zhang N, Kong X,
Ding X, Li X and Yang Q: MTDH mediates estrogen-independent growth
and tamoxifen resistance by down-regulating PTEN in MCF-7 breast
cancer cells. Cell Physiol Biochem. 33:1557–1567. 2014. View Article : Google Scholar : PubMed/NCBI
|