Introduction
Nicotinic acetylcholine (ACh) receptors are members
of the pentameric ligand-gated ion channel superfamily, and are
expressed at neuromuscular junctions and within the central and
peripheral nervous system, where they are activated by nicotine and
the endogenous neurotransmitter, ACh. A total of 17 nicotinic ACh
receptor subunits have been identified in vertebrates
(α1–α10, β1–β4, γ, δ
and ε), and these subunits can assemble into a variety of
heteropentameric and homopentameric receptors (1,2).
Of the nicotinic ACh receptor subtypes, the
homopentameric α7 nicotinic ACh receptor is known to be
the most permeable to calcium ions (Ca2+). Calcium
influx through the α7 nicotinic ACh receptor is involved
in increasing cytoplasmic calcium levels, which in turn triggers a
series of calcium-dependent intracellular processes. Following the
suggestion that the α7 nicotinic ACh receptor regulates
inflammation, it has been the focus of intense investigation since
the early 21st century (3).
Consequently, there has been substantial interest in the
identification and characterization of the α7 nicotinic
ACh receptor.
However, the expression of functional recombinant
α7 nicotinic ACh receptors in mammalian cell types,
including human embryonic kidney (HEK) 293 cells, has been
problematic, as the assembly of the α7 nicotinic ACh
receptor is a slow and inefficient process. Individual subunits
require appropriate transmembrane topology and undergo a series of
critical post-translational modifications (4). In addition, to enable folding into
the correct conformation, the receptors require appropriate
inter-subunit interactions. The early steps of receptor folding and
assembly occur within the endoplasmic reticulum, an intracellular
compartment containing several proteins required for efficient
protein folding and post-translational modification (4). Although there have been reports of
the successful functional expression of the recombinant
α7 nicotinic ACh receptor in certain mammalian cell
lines (5–8), measurable levels of functional
receptors have been difficult to achieve in several cell types.
This effect appears to be host-cell dependent (9,10),
as functional α7 nicotinic ACh receptors can be
generated in mammalian cell lines when co-expressed with either
Caenorhabditis elegans resistance to inhibitors of
cholinesterase 3 (CeRIC-3) or its human homolog (hRIC-3).
To the best of our knowledge, the functional
expression of recombinant human α7 nicotinic ACh
receptors in HEK 293 cells co-expressing hRIC-3 has not been
reported. In the present study, heterologously expressed nicotinic
ACh receptors in HEK 293 cells were investigated and the functional
expression levels of recombinant α7 nicotinic ACh
receptors in the HEK 293 cells were examined, in order to aid in
the development of novel pharmaceutical agents.
Materials and methods
Drugs
The drugs used in the present study were purchased
from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise stated.
Fluo-4 AM (1 mM) and Pluronic® F-127 (5%) were prepared
in dimethyl sulfoxide and stored at −20°C. All solutions were
prepared and diluted appropriately prior to experimentation.
Cell culture and transfection
Transfection was performed, as described previously
(10). Expression plasmids (hRIC-3
and hα7) containing complementary DNA sequences for
hRIC-3 and α7 nicotinic ACh receptor subunits,
respectively, were used. The subunits were subcloned into
pcDNA3.1+ (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). HEK 293 cells were cultured at 2×104
cells/ml in Dulbecco's modified Eagle's medium (Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a 5%
CO2 incubator. The medium was renewed every 3 days. The
HEK 293 cells were then transfected with the expression plasmids
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). The transfected cells were incubated for 24 h prior to
obtaining recordings.
Immunohistochemistry
The transfected HEK 293 cells (Cell Bank of Type
Culture Collection of Chinese Academy of Sciences, Shanghai) were
grown on glass chamber slides. The cells were washed twice, (5 min
for each wash) in 0.05 M phosphate-buffered saline (PBS) and fixed
with 4% paraformaldehyde for 15 min at room temperature. Following
washing five times with 0.05 M PBS, the cells were incubated in a
blocking solution [10% normal goat serum (Sigma-Aldrich) and 1%
Triton™ X-100 in PBS] for 1 h at room temperature. The cells were
then incubated for 4 h at 4°C with rabbit anti-human α7
nicotinic ACh receptor polyclonal antibody (cat. no. sc-5544;
Santa-Cruz Biotechnology Inc, Santa Cruz, CA, USA) at a dilution of
1:500 in 0.05 M PBS containing 1% Triton™ X-100 and 2% normal goat
serum. The slides were then washed with 0.05 M PBS four times, (5
min for each wash). The washed sections were then incubated for 2 h
at 37°C with 5% CO2 with a secondary antibody (goat
anti-rabbit Alexa Fluor 488; Molecular Probes, Invitrogen; Thermo
Fisher Scientific, Inc.) at a dilution of 1:1,000 in 10% normal
goat serum/PBS/Triton™ X-100 solution. The cells were then washed
with 0.05 M PBS four times (5 min for each wash) prior to
incubation with 4′,6-diamidino-2-phenylindole (1:15,000) for 5 min.
The slides were stored at 4°C until further use. The cells were
visualized with the fluorescence microscope (Leica DMI4000 B; Leica
Microsystems GmbH, Wetzlar, Germany) and an optical microscope
(BX51, U-TV0.5XC-3; Olympus Corporation, Tokyo, Japan) and camera
(DFC320; Leica Microsystems GmbH).
Western blot analysis
Cell lysates were prepared by incubating the HEK 293
cells with lysis buffer, containing 150 mM NaCl, 5 mM EDTA, 50 mM
Tris (pH 7.4), 0.02% NaN3, 1% Triton™ X-100 and protease
inhibitor cocktail, on ice for 90 min. Protein concentrations were
determined using the Bicinchoninic Acid Protein Assay kit (Beyotime
Institute of Biotechnology, Haimen, China). Equal quantities (5
µg) of total protein were subjected to 12%
SDS-polyacrylamide electrophoresis and were transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% non-fat milk in
Tris-buffered saline prior to western blot analysis. The membranes
wer incubated with the rabbit anti-human α7 nicotinic
ACh receptor polyclonal primary antibody (1:2,000; cat. no.
sc-5544; Santa-Cruz Biotechnology, Inc.) at 4°C overnight, followed
by incubation at 37°C for 2 h with the horseradish
peroxidase-conjugated secondary antibodies (1:5,000; ab6721; Abcam,
Cambridge, MA, USA). The signals were detected using enhanced
chemiluminescence reagent (EMD Millipore). The expression of each
target protein was relative to glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) and was calculated based on the grey
level.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was prepared from the in vitro HEK
293 cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. RT-PCR
analysis was performed using a PrimeScript RT reagent kit and SYBR
Premix Ex Taq II (Tli RNaseH Plus; Takara Bio, Inc., Otsu, Japan),
according to the manufacturer's protocol. The reaction mixture (25
µl) was comprised of 2X Subgreen mix (12.5 µl),
forward and reverse primers (each 10 µM; 1 µl), cDNA
(1 µg; 0.5 µl) and diethylpyrocarbonate-treated
ddH2O (10 µl). The primer sets for reverse
transcription were as follows: RIC-3, forward
5′-TTCAGACTGTATCAAGCGTAGGC-3′ and reverse
5′-TGGATCACACGAGGTAACAGAA-3′; GAPDH, Forward
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse 5′-GCCATCACGCCACAGTTTC-′3.
Cycling was conducted using and ABI 7900 cycling machine (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and the conditions were
as follows: 40 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C
for 30 sec.
Calcium imaging
Changes in cytosolic free calcium concentration were
measured using fluorescence imaging with the
Ca2+-sensitive dye, Fluo-4. The transfected HEK 293
cells were treated with 2 µM Fluo-4 AM for 30 min at 37°C,
in a medium containing 120 mM NaCl, 3 mM KCl, 2 mM
MgCl2, 2 mM CaCl2, 25 mM glucose and 10 mM
HEPES (pH 7.3, adjusted with Tris) prior to imaging. Following
treatment with the dye, the cells were observed under an inverted
microscope (Leica DMI4000 B) and images were captured using a
charge-coupled device camera (Leica DF350, Leica Microsystems
GmbH), as shown in Fig. 1. The
fluorescence intensities of individual cells in regions of interest
were recorded and analyzed using Leica Advanced Florescence
Application software (AF6000; Leica Microsystems GmbH). Nicotine
(10, 30, 100, 300 and 1,000 nM) and adenosine diphosphate (ADP; 10
µM) were applied to the cells by gravity using a
microperfusion apparatus. Between each drug application for 5 sec,
a 15-min washout period with fresh medium was included to allow
clearance of the drug.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analysis was performed using GraphPad Prism
version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Two-tailed unpaired Student's t-tests were used for all
comparisons, unless otherwise indicated.
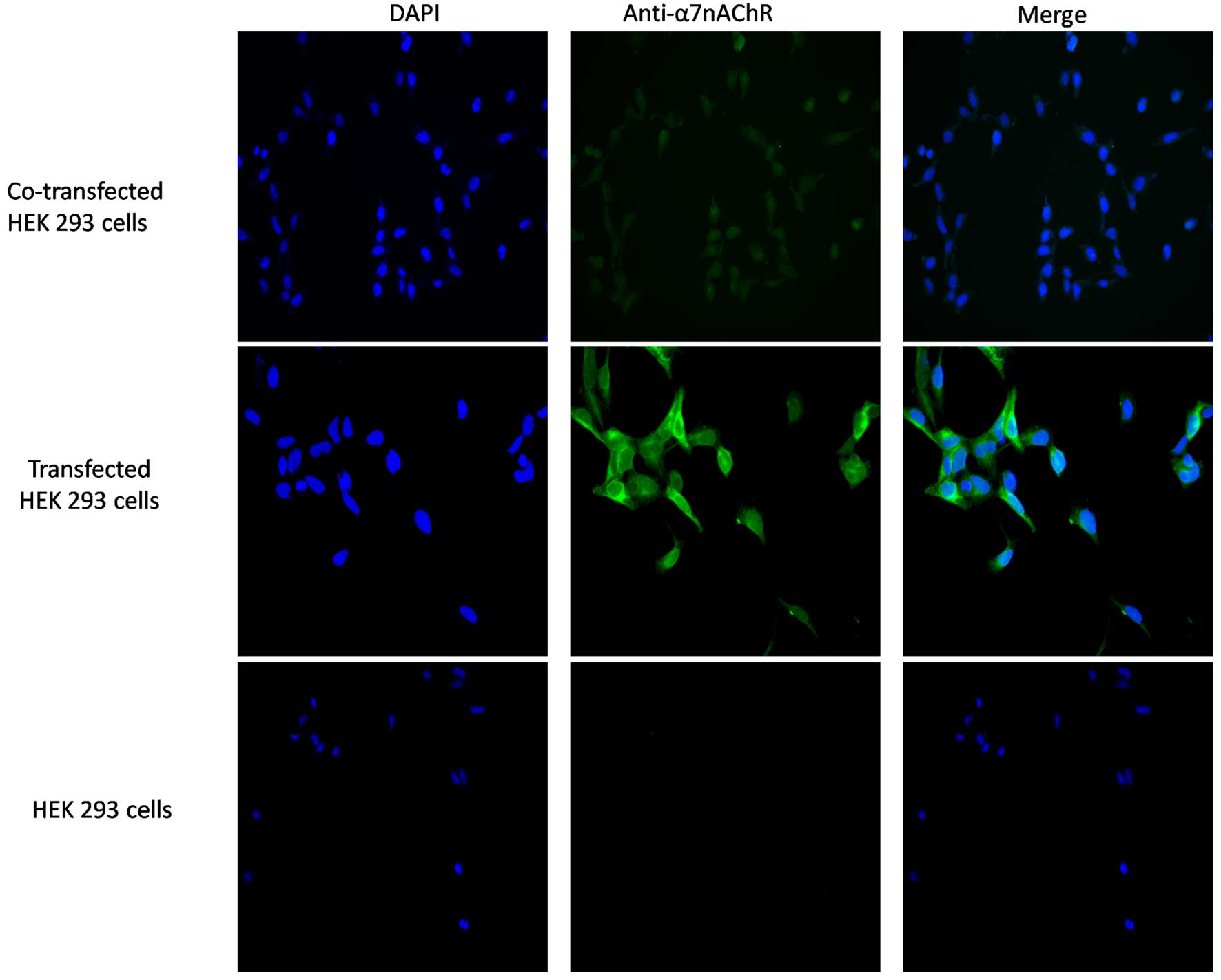
Results
Surface α7 receptors are
detected on the surface of HEK 293 cells expressing the human
α7 receptor and/or co-expressing hRIC-3
To detect the protein expression of α7,
HEK 293 cells were incubated and transfected, and the HEK 293 cells
were treated with antibody directed against the α7
protein. This was followed by incubation with a fluorescent-labeled
secondary antibody. No discernible binding of the
anti-α7 antibody to the HEK 293 control cells was
observed (Fig. 1). Immunostaining
of the HEK 293 cells co-transfected with hRIC-3 revealed binding of
anti-α7 antibodies to the surface of the co-transfected
cells, suggesting that these cells expressed α7
nicotinic ACh receptors on their membrane surface.
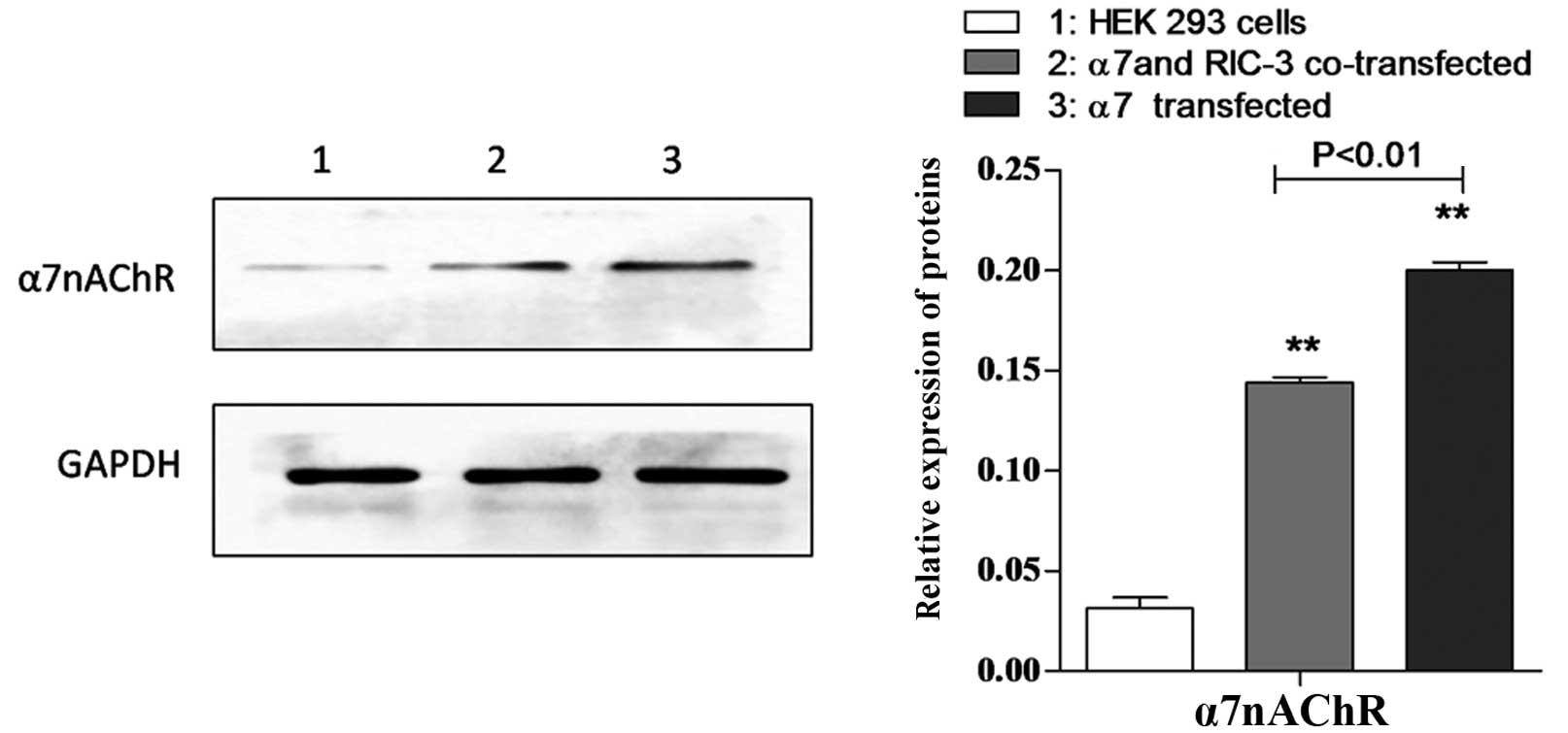
α7 protein is detected in HEK
293 cells expressing human α7 receptors and/or
co-expressing hRIC-3
To detect the protein expression of α7,
the present study examined HEK 293 cells, transfected HEK 293 cells
and co-transfected HEK 293 cells using western blot analysis with
antibody against α7 protein. As shown in Fig. 2, α7 protein was detected
in the transfected and co-transfected HEK 293 cells.
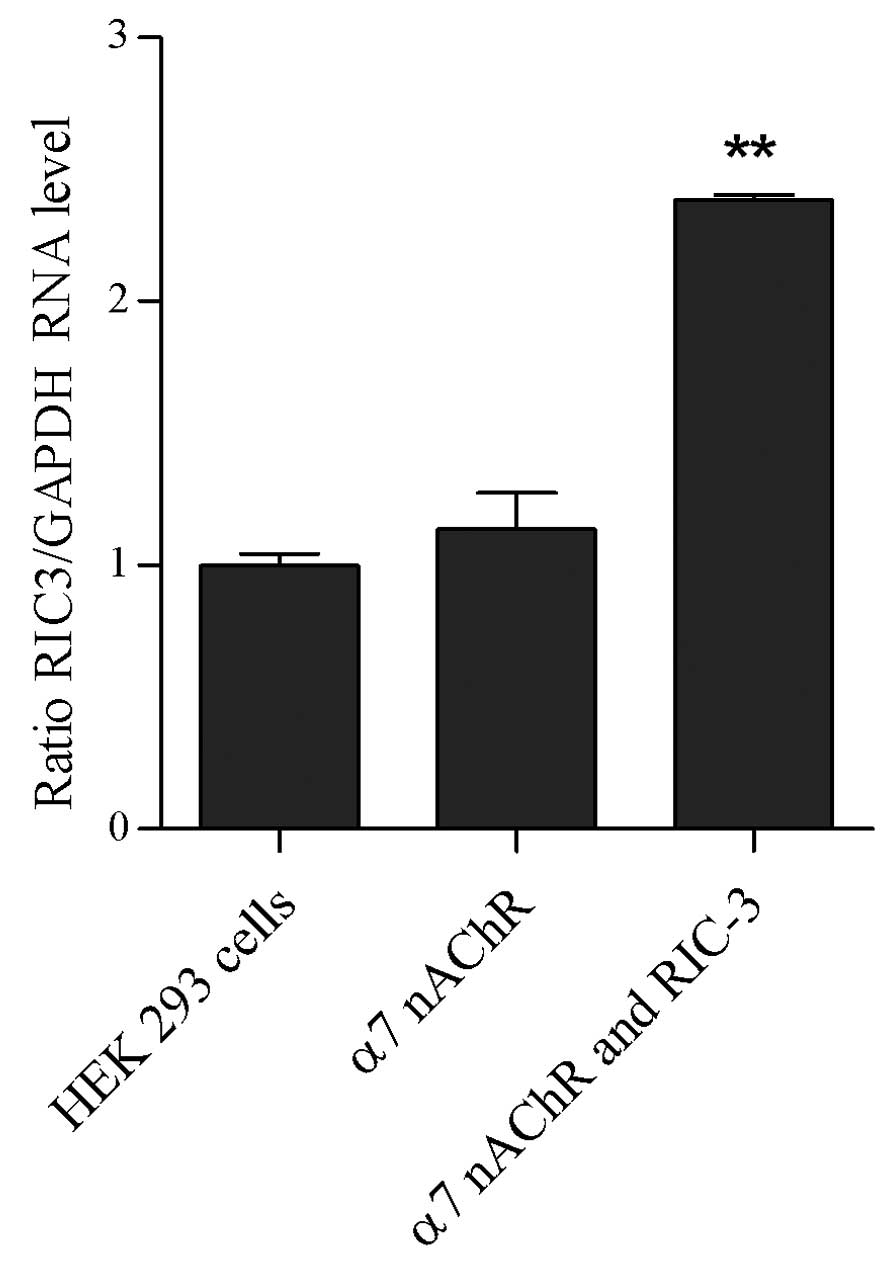
hric3 mRNA is expressed in co-transfected
HEK 293 cells, and is absent in HEK 293 cells and transfected HEK
293 cells
RT-PCR analysis was used to examine the expression
of hric3 in HEK 293 cells, transfected HEK 293 cells and
co-transfected HEK 293 cells. As shown in Fig. 3, hric3 transcripts were detected in
the co-transfected HEK 293 cells only; hric3 transcripts were not
detected in the HEK 293 cells or HEK 293 cells transfected with
α7 nicotinic ACh receptor alone.
Nicotine does not induce calcium
transients in HEK 293 cells expressing human α7
receptors
Subsequently, to analyze the changes in the
concentration of cytosolic free calcium induced by the opening of
the α7 nicotinic ACh receptors, HEK 293 cells expressing
human α7 receptors were treated with various
concentrations of nicotine for 30 sec. Following 15 min of washout
with fresh medium, the cells were treated with 10 µM ADP.
Nicotine did not induce calcium influx in the HEK 293 cells
expressing human α7 receptors at any concentration
(Fig. 4).
 | Figure 4Nicotine does not induce calcium
transients in transfected HEK 293 cells. Fluo-4-loaded transfected
HEK 293 cells were observed under an inverted microscope (Leica
DMI4000 B) and images were captured using a charge-coupled device
camera (Leica DF350). Changes in the fluorescence intensity of the
fluo-4 images were normalized to the intensity of the first image
(ΔF/F0). (A) Nicotine did not induce calcium transients at any
concentration, however, 100 µM ADP induced calcium
transients in the HEK 293 cells. (B) Statistical evaluation of the
data in the graphs in (A). Data are presented as the mean ±
standard error of the mean of responses integrated for 60 sec
following nicotine and ADP treatment in 51, 29, 51, 49 and 44 cells
treated with 10, 30, 100, 300 and 1,000 µM nicotine,
respectively. **P<0.01. Certain graphs show no error
bars, as they are smaller than the symbols. HEK, human embryonic
kidney; Nic, nicotine; ADP, adenosine diphosphate. |
Nicotine induces calcium transients in
HEK 293 cells co-expressing hRIC-3 and human α7
receptors
The present study then analyzed the changes in the
concentration of cytosolic free calcium induced by the opening of
α7 nicotinic ACh receptors in HEK 293 cells transiently
co-expressing hRIC-3 and the human α7 nicotinic ACh
receptor. In these cells, high levels of functional α7
nicotinic ACh receptors were expressed; the activity of these
receptors has been confirmed in a previous study using whole-cell
patch-clamp recording (7).
To assess the effect of nicotine on functional
α7 nicotinic ACh receptors, the co-transfected HEK 293
cells were treated with different concentrations of nicotine for 30
sec. Nicotine induced calcium influx in a concentration-dependent
manner (Fig. 5). The data obtained
were fitted to a logistic equation, and the nicotine concentration
required to elicit 50% of the maximal response was calculated to be
29.42 µM, with a 95% confidence interval of 13.32–65.42
µM).
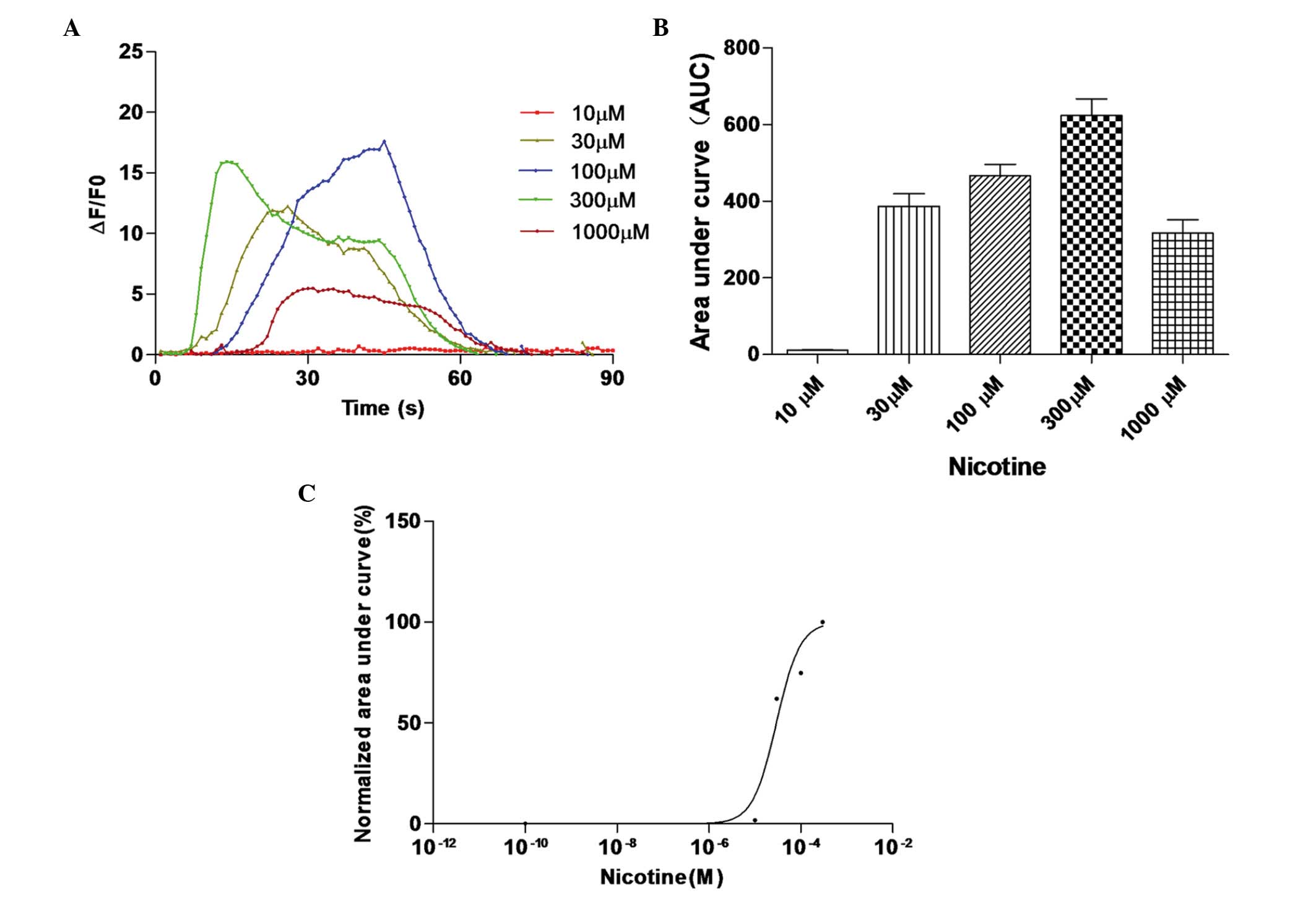 | Figure 5Nicotine induces calcium transients in
co-transfected HEK 293 cells. (A) Co-transfected HEK 293 cells were
treated with different concentrations of nicotine to induce calcium
influx. Fluorescence intensity changes in each transfected HEK 293
cell were recorded to generate concentration-response curves. (B)
Area under the curve for each transfected HEK 293 cell was
calculated, and the (C) response in each transfected HEK 293 cell
was normalized to the maximum area under the curve in each cell.
Data are presented as the mean ± standard error of the mean of
ΔF/F0 responses integrated for 60 sec following application of 10,
30, 100, 300 and 1,000 µM nicotine in 18, 26, 19, 29 and 15
co-transfected HEK 293 cells, respectively. **P<0.01.
HEK, human embryonic kidney. |
Discussion
Although the α7 subunit is able to
generate functional nicotinic ACh receptors when expressed in
Xenopus oocytes, considerable difficulty has been
encountered in the efficient expression of functional α7
nicotinic ACh receptors in cultured mammalian cell lines (9,11–13).
Previous studies (10,14) have demonstrated that α7
can efficiently generate functional nicotinic ACh receptors in
mammalian cell lines when co-expressed with either CeRIC-3 or its
human homolog, hric-3. RIC-3 is required for efficient receptor
folding, assembly and functional expression of homomeric
α7 nicotinic ACh receptors (15).
In the present study, the expression of functional
recombinant nicotinic ACh receptors in HEK 293 cells was induced by
co-expression with hRIC-3. In addition, immunohistochemistry and
western blot analysis were performed to confirm the protein
expression of the human α7 nicotinic ACh receptor, and
RT-PCR analysis was used to detect hric3 transcripts. Native HEK
293 cells did not express the human α7 nicotinic ACh
receptor pr hric-3 transcripts, whereas the human α7
nicotinic ACh receptor was detected following transfection. Even in
the absence of hric-3, α7 protein was detected in the
HEK 293 cells transiently expressing α7.
Using the calcium dye, Fluo-4, to record
intracellular calcium signals generated by the opening of
α7 nicotinic ACh receptors, images of calcium transients
were captured in the transfected HEK 293 cells expressing a high
level of α7 nicotinic ACh receptors. It was demonstrated
that these HEK 293 cells did not express detectable levels of
hric-3 transcripts, which was associated with a lack of functional
human α7 nicotinic ACh receptors. Subsequently, images
of calcium transients were captured in co-transfected HEK 293 cells
with high expression levels of α7 nicotinic ACh
receptors and hric-3 transcripts. The observed calcium transients
were predominantly derived from the opening of membrane
α7 nicotinic ACh receptors, as evidenced by the
following observations: (i) the signals were induced by treatment
with nicotine and (ii) human α7 nicotinic ACh receptors
were detectable in the co-transfected HEK 293 cells using
immunohistochemical and western blot analyses.
In conclusion, the findings of the present study
suggested that hRIC-3, when co-expressed with human α7
nicotinic ACh receptors in HEK 293 cells, supported the functional
expression of α7 nicotinic ACh receptors. These
observations may aid in the development of treatment strategies for
inflammation.
Acknowledgments
The authors would like to thank Editage for English
language editing. This study was supported by the National Natural
Science Foundation of China (grant no. 81171845) and the Songjiang
Foundation (grant no. 2011PD13).
References
|
1
|
Le Novere N, Corringer PJ and Changeux JP:
The diversity of subunit composition in nAChRs: Evolutionary
origins, physiologic and pharmacologic consequences. J Neurobiol.
53:447–456. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Millar NS and Gotti C: Diversity of
vertebrate nicotinic acetylcholine receptors. Neuropharmacology.
56:237–246. 2009. View Article : Google Scholar
|
|
3
|
Wang H, Yu M, Ochani M, Amella CA, Tanovic
M, Susaria S, Li JH, Wang H, Yang H, Ulloa L, et al: Nicotinic
acetylcholine receptor alpha7 subunit is an essential regulator of
inflammation. Nature. 421(6921): 384–388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Green WN and Millar NS: Ion-channel
assembly. Trends Neurosci. 18:280–287. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quik M, Choremis J, Komourian J, Lukas RJ
and Puchacz E: Similarity between rat brain nicotinic
alpha-bungarotoxin receptors and stably expressed
alpha-bungarotoxin binding sites. J Neurochem. 67:145–154. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng JH, Lucero L, Fryer J, Herl J,
Leonard SS and Lukas RJ: Inducible, heterologous expression of
human alpha7-nicotinic acetylcholine receptors in a native
nicotinic receptor-null human clonal line. Brain Res. 825:172–179.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Puchacz E, Buisson B, Bertrand D and Lukas
RJ: Functional expression of nicotinic acetylcholine receptors
containing rat alpha 7 subunits in human SH-SY5Y neuroblastoma
cells. FEBS Lett. 354:155–159. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao L, Kuo YP, George AA, Peng JH,
Purandare MS, Schroeder KM, Lukas RJ and Wu J: Functional
properties of homomeric, human alpha 7-nicotinic acetylcholine
receptors heterologously expressed in the SH-EP1 human epithelial
cell line. J Pharmacol Exp Ther. 305:1132–1141. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cooper ST and Millar NS: Host
cell-specific folding and assembly of the neuronal nicotinic
acetylcholine receptor alpha7 subunit. J Neurochem. 68:2140–2151.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Williams ME, Burton B, Urrutia A,
Shcherbatko A, Chavez-Noriega LE, Cohen CJ and Aiyar J: Ric-3
promotes functional expression of the nicotinic acetylcholine
receptor alpha7 subunit in mammalian cells. J Biol Chem.
280:1257–1263. 2005. View Article : Google Scholar
|
|
11
|
Chen D, Dang H and Patrick JW:
Contributions of N-linked glycosylation to the expression of a
functional alpha7-nicotinic receptor in Xenopus oocytes. J
Neurochem. 70:349–357. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kassner PD and Berg DK: Differences in the
fate of neuronal acetylcholine receptor protein expressed in
neurons and stably transfected cells. J Neurobiol. 33:968–982.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rangwala F, Drisdel RC, Rakhilin S, Ko E,
Atluri P, Harkins AB, Fox AP, Salman SS and Green WN: Neuronal
alpha-bungarotoxin receptors differ structurally from other
nicotinic acetylcholine receptors. J Neurosci. 17:8201–8212.
1997.PubMed/NCBI
|
|
14
|
Lansdell SJ, Gee VJ, Harkness PC, Doward
AI, Baker ER, Gibb AJ and Millar NS: RIC-3 enhances functional
expression of multiple nicotinic acetylcholine receptor subtypes in
mammalian cells. Mol Pharmacol. 68:1431–1438. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Millar NS: RIC-3: A nicotinic
acetylcholine receptor chaperone. Br J Pharmacol. 153(Suppl 1):
S177–S183. 2008. View Article : Google Scholar : PubMed/NCBI
|