Introduction
Bronchial hyperresponsiveness (BHR), defined as an
exaggerated sensitivity to a variety of physical, chemical or
pharmacologic stimuli, is characterized by episodes of intermittent
airflow obstruction caused by bronchospasm, which is also an
important clinical feature of asthma, and may be apparent in
certain other pulmonary diseases, such as chronic bronchitis and
emphysema (1). In general, BHR
occurs in response to a lower level of stimulus and is increased in
magnitude when compared with healthy individuals. BHR is the most
common clinical manifestation that is identified in asthmatic
patients, and intubation is the major factor that stimulates the
airway of the patients who receive general anesthesia (2). BHR is not uncommon and its prevalence
in the general population has been estimated to be 13% in the
general Chinese population (2).
Additionally, BHR is reported in asymptomatic individuals and those
individuals are considered to be susceptible to the development of
certain medical disorders that are characterized by bronchospasms,
such as in asthma (3). BHR in
response to intubation represents a major risk, and in certain
instances is life-threatening, to those patients who receive
general anesthesia (2). Therefore,
it is important to establish a biomarker to effectively predict the
occurrence of bronchospasm during general anesthesia, which may
facilitate the necessary actions to prevent BHR.
The neuronal nitric oxide synthase 1 gene,
NOS1 is responsible for the development of certain types of
bronchospasm-associated airway obstruction, and was demonstrated to
upregulate the expression level of NOS2, another regulatory factor
that is involved in regulating the balance between
bronchoconstriction and bronchodilation (4). The production of nitric oxide (NO)
catalyzed by NOS1, is physiologically associated with
bronchospasm as a neurotransmitter for non-adrenergic
non-cholinergic (NANC) nerves (5).
NANC mechanisms are believed to be essential in bronchoconstriction
and bronchodilation control, and a defect in NANC bronchodilation
underlies the molecular mechanism of BHR (6).
Micro (mi)RNAs are small non-coding RNA molecules,
~22 nucleotides long, and they function as inhibitors to suppress
the expression of ≤30% human protein-coding genes at the
post-transcriptional level by either translational repression or
messenger (m)RNA degradation (7).
Increasing evidence demonstrates that miRNAs are involved in
controlling the balance between bronchoconstriction and
bronchodilation (7,8).
MiR-146 is a family of miRNA precursors found in
mammals, including humans. MiR-146 predominantly participates in
the regulation of inflammation and other processes that are
associated with the innate immune system (9). A previous study revealed that the
minor C allele of the miR-146a rs2910164 polymorphism leads to a
reduction in the expression of its mature form by causing
mispairing within the miR-146a hairpin, resulting in a wide range
of functional alterations (10).
The majority of miR-146a rs2910164 studies were regarding the risk
of various types of cancer (11–13),
and only one described an association with a protection mechanism
against asthma in an Asian population (14).
As NOS1 functions as a regulator of
bronchospasms and NOS1 is a potential target of miR-146a,
the miRNA/mRNA association was verified. In addition, the
association between the miR-146a rs2910164 polymorphism and BHR in
response to intubation in patients exhibiting basic pulmonary
disease (such as asthma, emphysema and bronchitis) who received
general anesthesia was verified.
Materials and methods
Participants
As shown in Table
I, a total of 563 patients exhibiting basic pulmonary diseases,
such as asthma, emphysema and chronic bronchitis, who received a
general anesthetic (including intubation) prior to surgical
intervention at the First Hospital of Dalian Medical University
(Dalian, China) between September 2013 and September 2014 were
enrolled in the present study. Tissue samples were snap-frozen
immediately with liquid nitrogen, stored carefully, and
subsequently were used to extract DNA and RNA for molecular
analysis. Those patients who exhibited sinus bradycardia,
atrioventricular block (degree II or III), and/or sick sinus
syndrome were excluded from the present study. The study was
approved by the Research Ethics Committee in China Medical
University and informed consent was obtained from each patient.
 | Table IDemographic data and clinical
characteristics of study participants. |
Table I
Demographic data and clinical
characteristics of study participants.
| Characteristic | BHR+
(n=138) | BHR−
(n=425) | P-value |
|---|
| Age (years) | 56.3±9.7 | 58.4±10.1 | NS |
| Gender (M/F) | 72/66 | 276/149 | NS |
| Height (cm) | 163.3±7.5 | 164.1±10.8 | NS |
| Weight (kg) |
63.5±7.4 | 64.3±9.4 | NS |
| Smoking status |
| Current
smoker | 34 | 42 | |
| Ex-smoker | 52 | 89 | |
| Non-smoker | 52 | 294 | <0.05 |
| Basic pulmonary
disease |
| Emphysema | 36 | 146 | |
| Asthma | 63 | 187 | |
| Chronic
bronchitis | 39 | 92 | <0.05 |
| FVC (% of predicted
value) | 62.3±7.5 | 66.2±7.3 | <0.01 |
| FEV1 (%
of predicted value) | 43.6±5.3 | 53.5±5.9 | <0.01 |
| DLCO (% of
predicted value) | 66.4±5.5 | 69.4±6.2 | <0.01 |
Assessment of BHR
To assess BHR in response to intubation,
auscultation was performed on either side of the chest at the
second intercostal space in the parasternal line, the fifth
intercostal space in the midclavicular line, the fourth intercostal
space in the midaxillary line, prior to intubation and anesthesia
and 5 min after intubation. BHR was diagnosed by the presence of
wheezing, which was evaluated by an anesthesiologist blinded to the
protocol. Wheezing was defined as high-pitched expiratory rhonchi
that were audible in at least three of six auscultation sites.
Genotyping analysis
Genomic DNA was isolated from blood samples (5 ml)
obtained between September 2013 and September 2014 using a Qiagen
Genomic DNA extraction kit (Qiagen, Inc., Valencia, CA, USA). The
rs2910164 G/C polymorphism was genotyped in duplicate using the
TaqMan® Allelic Discrimination assay (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in an
ABI Prism® 7900 system (Applied Biosystems). In
addition, the genotyping results were confirmed by direct
sequencing in 10 random samples including five homozygote and five
heterozygote individuals, using the following primers: Forward,
5′-ATTTTACAGGGCTGGGACAG-3′ and reverse,
5′-TAGCAGCAGCAGCAAGAGAG-3′.
RNA isolation and reverse transcription
quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using miRNeasy (Qiagen,
Inc.). Trizol (Gibco; Thermo Fisher Scientific, Inc.) was used to
isolate the total cellular RNA from tissue samples and PTENCE
cells, according to the manufacturer's protocol, and subsequently
RNeasy Qiagen columns (Qiagen Inc.) were used to purify the RNA.
The RNA concentration was examined and the integrity was evaluated
by agarose gel electrophoresis (gels run at 100 V for 1 h; agarose
gel purchased from Invitrogen; Thermo Fisher Scientific, Inc.,
agarose gel electrophoresis equipment purchased from Sigma-Aldrich,
St. Louis, MO, USA). A TaqMan MicroRNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to
synthesize the copy (c)DNA (CANCAC1) from RNA with a mixture of 2
μg total RNA, 1 μl miRNA-specific primers (25
μM) and doubly distilled (dd)H2O (RNase-free) to
a final volume of 10 μl. Subsequently, the mixture was
denatured for 10 min at 70°C prior to the mixture being placed on
ice, followed by the addition of the reaction buffer, including 11
μl ddH2O (RNase-free), 4 μl deoxynucleotide
phosphates (dNTPs) mix, 4 μl 5xRT buffer and 1 μl
ReverTra Ace (100 units/μl; Toyobo Co., Ltd., Osaka, Japan).
The mixture was then maintained for 60 min at 42°C, followed by an
incubation for 10 min at 90°C. RT-qPCR was performed using an
Applied Biosystems 7500 Sequence Detection system (ABI7500 SDS;
Applied Biosystems, Thermo Fisher Scientific, Inc.). A TaqMan
MicroRNA Reverse Transcription kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used to synthesize the cDNA and RT was
performed using 1 μg total RNA. Quantitative amplification
was performed using a Roche LightCycler480-II real-time thermal
cycler (Roche Diagnostics) to determine the relative expression
level of miR-146a and NOS1 using RT2 SYBR
green/fluorescein qPCR Mastermix (Qiagen, Inc.). The experiment was
repeated at least three times. SnoNA U6 served as an internal
marker, and the 2−ΔΔCq method (15) was used to evaluate the results.
Cell culture, and mimic and inhibitor
miRNA transfection
Human pulmonary artery smooth muscle cells (PASMCs)
were purchased from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China) and added to Dulbecco's modified Eagle's
medium high glucose medium containing 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. The cells were passaged every 2 days. The
miR-146a-5p miRIDIAN duplex mimics, hairpin inhibitors and negative
control duplex mimics were purchased from Thermo Fisher Scientific,
Inc., and the oligonucleotides (Takara Biotechnology Co., Ltd.,
Dalian, China) were transfected into human PASMCs by reverse
transfection using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.).
Western blotting
Total protein was separated by 10% SDS-PAGE (Roche
Diagnostics, Basel, Switzerland) and the separated protein was
transferred to nitrocellulose membrane (Amersham Biosciences,
Piscataway, NJ, USA). The membrane was blocked using 5% non-fat
milk in phosphate-buffered saline (Invitrogen; Thermo Fisher
Scientific, Inc.). Anti-NOS1 antibody and anti-β-actin antibody
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA), and the membrane was incubated with the primary antibody for
2 h at room temperature (dilution, 1:1,000). A secondary antibody
(Santa Cruz Biotechnology, Inc.) was used at a dilution of 1:5,000,
and incubated for 2 h at room temperature. The fluorescent signal
was detected using an ECL detection kit (Applygen Technologies,
Inc., Beijing, China). The integrated intensities of the protein
bands were expressed relative to the protein levels of the
control.
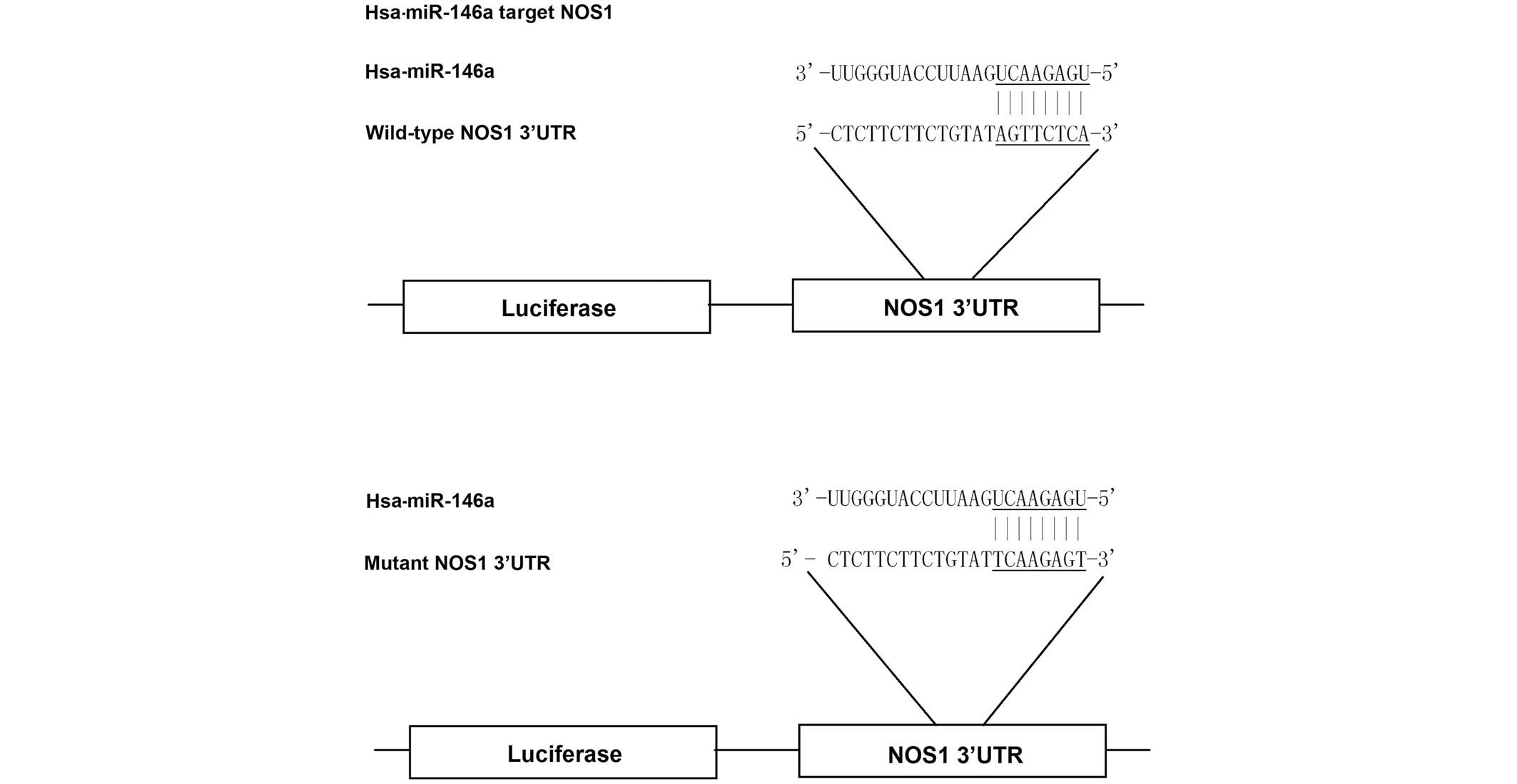
Luciferase reporter assay
A NOS1 wild-type 3′-untranslated region (UTR)
was amplified by PCR as described above and cloned into a pcDNA3
(Promega Corporation, Madison, WI, USA) that contained a firefly
luciferase reporter gene, at a position downstream of the
luciferase reporter. The vectors were designated as wild-type 3′UTR
of NOS1. Site-directed mutagenesis was used to introduce a
mutation to replace the wild-type seed sequence in the 3′UTR using
the Site-Directed Mutagenesis kit (Beijing SBS Genetech Co., Ltd.,
Beijing, China) and designated as mutant 3′UTR of NOS1.
Human PASMCs were seeded in a 48-well plate, and transfected with
miR-146a mimics and the wild-type or mutant 3′UTR of NOS1.
pRL-TK (5 ng), a plasmid expressing Renilla luciferase
(Promega Corporation), was also co-transfected and served as an
internal control. After 48 h, the cells were collected and the
luciferase reporter assay was performed in a TD-20/20 luminometer
(Promega Corporation).
Statistical analysis
Deviations from Hardy-Weinberg equilibrium (HWE)
were tested for using the χ2 test for observed and
expected values with the degrees of freedom adjusted by the number
of estimated independent frequencies. Characteristics of the case
and the control sample were compared using χ2 test
(binary variables), and Student's t-test or one-way analysis of
variance (binary variables). To adjust for additional covariates,
the data were reanalyzed by multiple logistic regression with BHR
as the outcome variable. P<0.05 was considered to indicate a
statistically significant difference. For an identified
association, the odds ratio (OR) and its 95% confidence interval
(CI) was computed as an approximation of the relative risk. The
statistical analyses were conducted using SPSS 21.0 software
package (IBM SPSS, Armonk, NY, USA).
Results
Participant characteristics
In the present study, a total of 563 patients with
basic pulmonary diseases, such as asthma, emphysema and chronic
bronchitis who received a general anesthetic (including intubation)
prior to surgical intervention at the First Hospital of China
Medical University were enrolled, and the polymorphisms in the
current study were in HWE (P>0.05). A total of 138 were positive
for BHR and 425 were negative. No difference was identified
regarding age, gender, height, weight and presence of emphysema,
asthma and chronic bronchitis between the BHR-positive and
-negative groups (Table I).
Additionally, these variables were included in the multivariate
logistic regression analysis to evaluate the potential effects on
the association between the rs2910164 G/C polymorphism and the risk
of BHR in response to intubation in patients who received a general
anesthetic.
Association between rs2910164 G/C
polymorphism and presence of BHR in response to intubation
The genotype frequency of the rs2910164 G/C
polymorphism among the BHR-positive and -negative groups, and the
association with the presence of BHR are presented in Table II. The frequencies of the GG, GC
and CC genotypes were 44, 50 and 6%, respectively, in the
BHR-positive group, and 39, 45 and 16%, respectively, in the
BHR-negative group. When the GG and GC genotype was used as the
reference, it was noted that the CC genotype was associated with a
significantly increased risk of BHR (OR, 0.38; 95% CI,
0.18–0.78).
 | Table IIAssociation between genotype
frequency and the presence of BHR. |
Table II
Association between genotype
frequency and the presence of BHR.
| Variable | BHR+
(n=138; %) | BHR−
(n=425; %) | P-value |
|---|
| Genotype |
| GG
(reference) | 60 (44) | 167 (39) | |
| GC | 69 (50) | 192 (45) | 0.999 |
| CC | 9 (6) | 66
(16) | 0.012 |
| Combined |
| GG + GC
(reference) | 129 (94) | 359 (84) | |
| CC | 9
(6) | 66
(16) | 0.009 |
Effect of the rs2910164 G/C polymorphism
on EGFR expression in lung cancer cells
Based on the computational analysis, NOS1 was
identified as a potential target gene of miR-146a (Fig. 1). To evaluate whether miR-146a
targets the NOS1 3′UTR in PASMCs, reporter vectors carrying
wild-type or mutant NOS1 3′UTR were constructed, as demonstrated in
Fig. 1. The reporter vectors were
subsequently used for transient transfection in PASMCs together
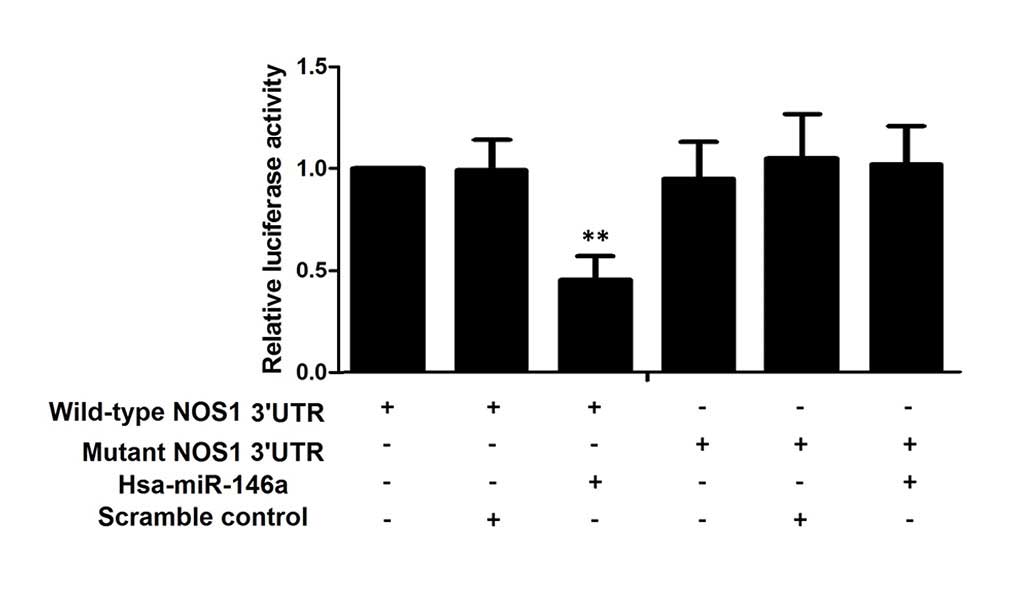
with miR-146a mimics or scramble controls. As shown in Fig. 2, only the luciferase activity from
the cells co-transfected with wild-type NOS1 3′UTR and
miR-146a mimics was identified to be significantly lower than the
control, whereas all other groups were comparable. The results
confirmed that NOS1 was a valid target of miR-146a in
PASMCs.
Determination of miR-146a and NOS1
expression patterns in lung tissue samples with different
genotypes
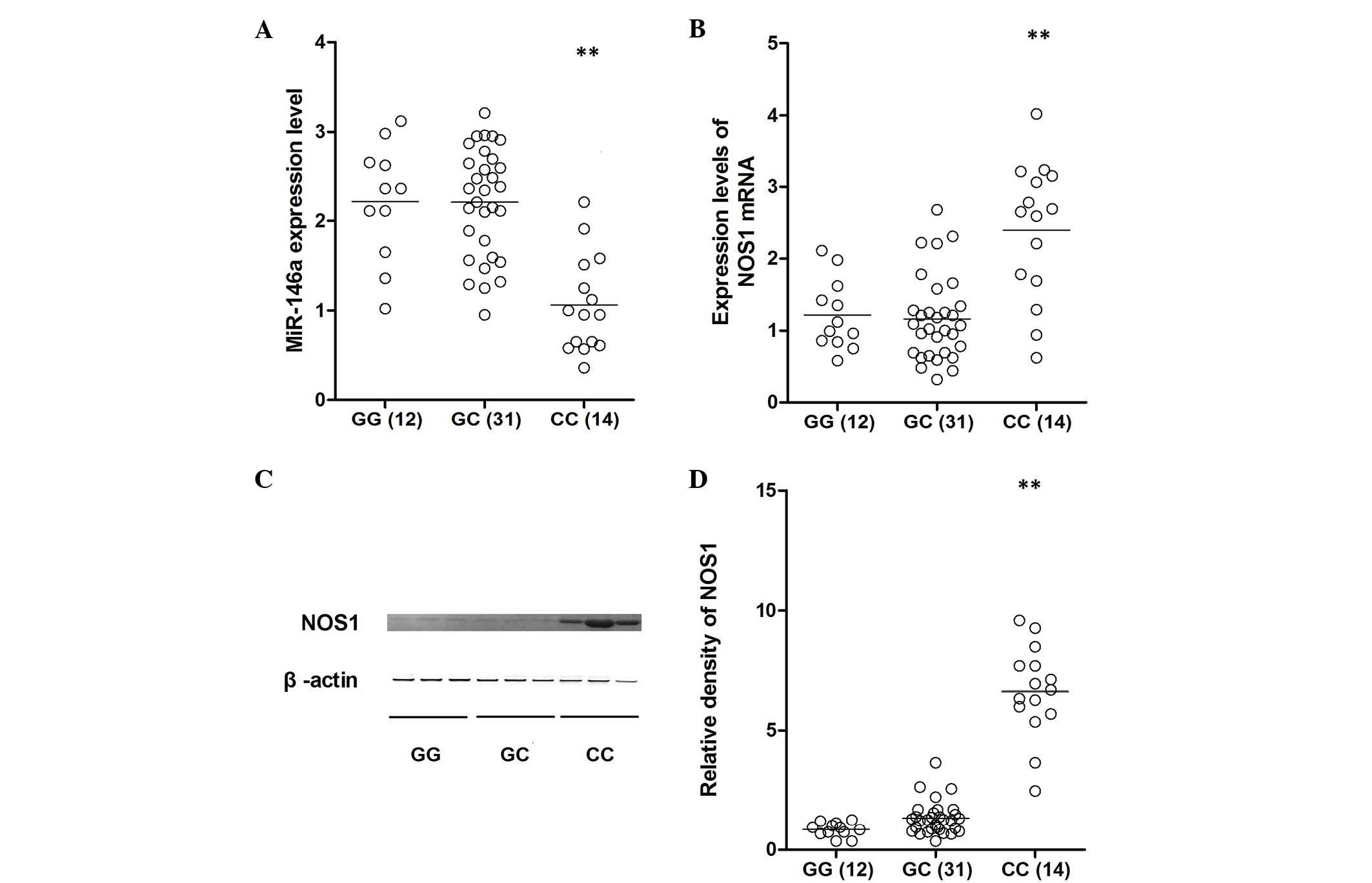
Lung tissue samples of three different genotypes
(GG, n=12; GC, n=31; CC, n=14) were used to further investigate the
impact of the polymorphism on NOS1 expression levels. Using
qPCR analysis, the expression of miR-146a was identified to be
comparable between the GG and GC groups, which were substantially
higher than that of the CC group (Fig.
3A). In addition, the mRNA and protein expression levels of
NOS1 were determined in all genotype groups using qPCR and
western blotting, which indicated that the level of NOS1 expression
was similar between the GG and GC groups, and the two were
significantly lower than that of the CC group (Fig. 3B–D). To further confirm the effect
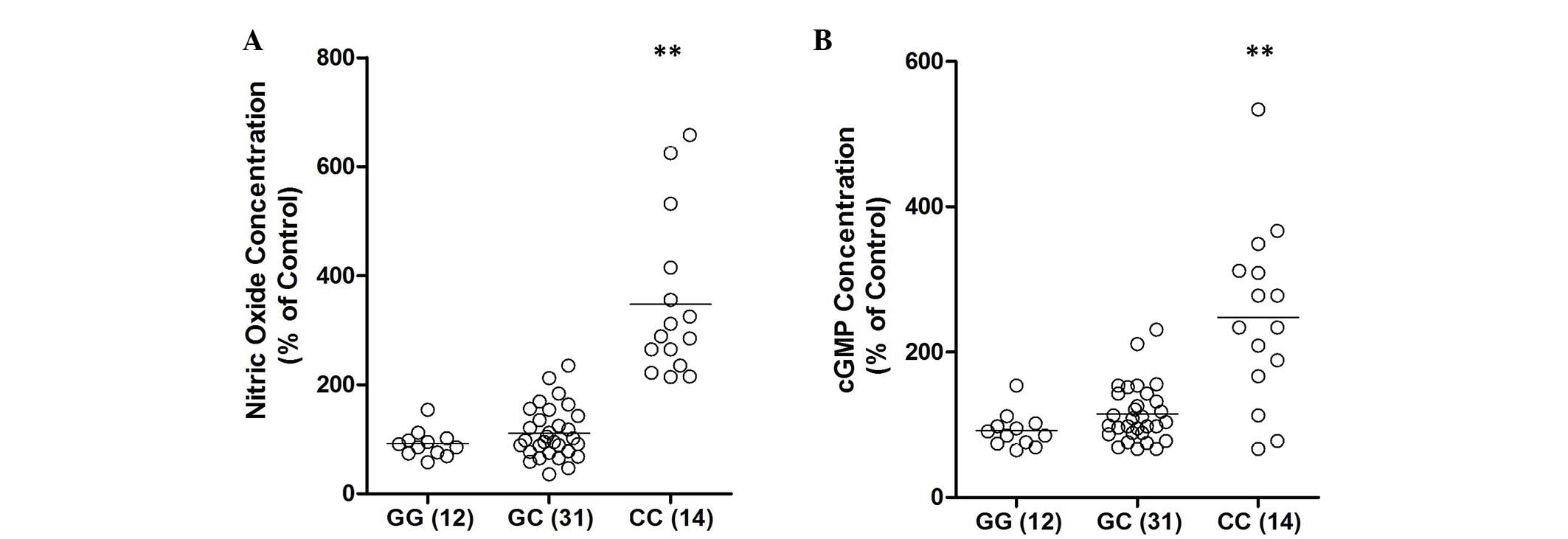
of rs2910164 G/C polymorphism on the signaling pathway, the
expression levels of NO and cyclic guanosine monophosphate (cGMP)
were examined. As shown in Fig. 4,
the concentration of NO and cGMP were consistently higher in the CC
group than in the GG or GC groups.
Alternation of miR-146a significantly
altered the expression of NOS1 and the production of its effectors,
NO and cGMP
The regulatory role of the control of NOS1
expression was further evaluated; the PASMCs were transfected with
miR-146a mimics and inhibitors, and upregulation of miR-146a was
identified to significantly decrease the expression level of
NOS1 (Fig. 5A and B) and
reduced the production of NO (Fig.
5C) and cGMP (Fig. 5D) in the
PASMCs in a dose dependent manner. While downregulation of miR-146a
significantly increased the expression level of NOS1 and the
production of NO and cGMP in PASMCs (Fig. 5).
Discussion
miRNAs are crucial regulators that control the
expression of thousands of human genes. In vivo and in
vitro studies have shown that variants interfering with miRNA
production processing or target recognition may be potentially
functional polymorphisms that lose their control over the
expression of various downstream target genes, which may be the
underlying molecular mechanism of the development of human
pathologies (8,16–20).
It has been previously reported that the miR-146a rs2910164
polymorphism affected the efficiency of premature miR-146a
processing, leading to a reduction of mature miRNAs (11). Furthermore, statistical analyses
indicated an association between the minor allele of the
polymorphism (the C allele) and either the risk for or protection
from various diseases (including, cancer, arthritis and asthma)
(11–14,19).
Jazdzewski et al (10)
demonstrated that this allele reduces the expression level of
mature miR-146a by ~2-fold and inhibits its target genes,
including Toll-like receptors, factor receptor-associated factor 6
and interleukin-1 receptor-associated kinase 1 (17,21,22).
In the present study, online in silico analysis was
conducted to identify the potential target of miR-146a, which is
functionally associated with BHR in response to physical stimuli,
and found that NOS1 is a candidate. To assess whether
miR-146a targets the NOS1 3′UTR in PASMCs, reporter vectors
carrying wild-type or mutant NOS1 3′UTR were constructed. As
shown in Fig. 2, only the
luciferase activity, from the cells co-transfected with wild-type
NOS1 3′UTR and miR-146a mimics, was significantly lower than
the control; all the other groups were comparable. The results
confirmed that NOS1 was a valid target of miR-146a in
PASMCs. Therefore, the present study hypothesized that C allele
carriers exhibit increased expression levels of NOS1, which
contributes to the molecular mechanism underlying the differential
acute response to intubation. In the current study, the carrier of
the CC genotype of the miR-146a gene was identified to be
associated with protection from BHR in response to intubation (OR,
0.38; 95% CI, 0.18–0.78).
The neuronal NOS gene, NOS1 has been reported
to be associated with airway obstruction and bronchial
inflammation, and it promotes NOS2 expression (4). NO is a potent vasodilator, and
neurally-derived NO, catalyzed by NOS1, is functionally
associated with bronchospasm, by modulating the balance between
bronchoconstriction and bronchodilation, as a neurotransmitter for
the NANC nervous system (5). NANC
mechanisms are important in maintaining the homeostasis of the
respiratory system, and a defect in NANC bronchodilation has been
indicated to be involved in the pathogenesis of certain
bronchospasm-based diseases, such as asthma (6). Additionally, NOS1 is
detectable in a wide spectrum of human tissues, particularly in
bronchial smooth muscle cells (23). In mice, NOS1 was detected in
epithelium of the trachea, the removal of which was found to
decrease the expression of NOS1, indicating that bronchial
epithelial cells are the major source of NOS1 production
(23). An in vivo study
showed that the inhibitory NANC relaxation of ovalbumin
(OVA)-exposed animals was not significantly affected by an NOS
inhibitor, indicating that the neural NO-induced relaxation was
markedly impaired by repeated antigen exposure (24). Markedly reduced NO production was
observed in NOS1 knock-out mice that were challenged with
OVA, when compared with that of the wild-type controls (25). In another study, Martinez et
al (26) identified that
stimulus exposure caused airway hyper-responsiveness via
compromised expression of bronchoprotective NOS1. These data
demonstrated a regulatory role of NOS1 in the mechanism
underlying the development of bronchospasms via promoting the
production of NO. In the current study, lung tissue samples were
collected from different genotypes, and the expression of miR-146a
was identified to be comparable between the GG and GC groups, which
were substantially greater than the CC group. Additionally, the
mRNA and protein expression levels of NOS1 were determined
in all of the genotype groups using qPCR and western blotting,
finding that NOS1 expression levels were similar between the
GG and GC groups, and the two were significantly lower than that of
the CC group (Fig. 3B–D). To
further demonstrate the effect of the rs2910164 G/C polymorphism on
the signaling pathway, the expression levels of NO and cGMP were
evaluated. As shown in Fig. 4, the
concentration of NO and cGMP were consistently higher in the CC
group when compared with the GG or GC group.
In the present study, multiple logistic regressions
analysis are included to reduce the false positive results from
controlling potential confounding factors. Spurious association as
a result of population stratification did not occur, as the
ethnicity of all subjects was Chinese Han, which is assumed to be a
homogenous population. Functional analysis was performed to support
the association study. However, the sample size was limited, as
only 563 patients were included in the statistical analysis, and
such a limited sample size may have compromised the statistical
power of the association study. In addition, as all of the
participants were patients who had received general anesthetic
prior to surgical intervention the study may contain selection
bias.
The association between the rs2910164 polymorphism
and BHR is notable, and further additional case-control studies in
other ethnic groups are warranted to confirm the finding of the
current study. Furthermore, investigations of other study cohorts
are required to gain insight into the molecular mechanisms
underlying this association and the role of mir-146a single
nucleotide polymorphisms in bronchospasm-based diseases.
In conclusion, the rs2910164 C allele is associated
with BHR in response to intubation in the patients who receive
general anesthetic. Thus, the rs2910164 C allele may serve as a
novel biomarker and as a potential therapeutic target to predict
and treat BHR.
References
|
1
|
Cockcroft DW and Hargreave FE: Airway
hyperresponsiveness. Relevance of random population data to
clinical usefulness. Am Rev Respir Dis. 142:497–500. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miura M, Yamauchi H, Ichinose M, Ohuchi Y,
Kageyama N, Tomaki M, Endoh N and Shirato K: Impairment of neural
nitric oxide-mediated relaxation after antigen exposure in guinea
pig airways in vitro. Am J Respir Crit Care Med. 156:217–222. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
A genome-wide search for asthma
susceptibility loci in ethnically diverse populations. The
collaborative study on the genetics of asthma (CSGA). Nat Genet.
15:389–392. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iijima H, Tulic MK, Duguet A, Shan J,
Carbonara P, Hamid Q and Eidelman DH: NOS 1 is required for
allergen-induced expression of NOS 2 in mice. Int Arch Allergy
Immunol. 138:40–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mattick JS: Non-coding RNAs: The
architects of eukaryotic complexity. EMBO Rep. 2:986–991. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Immervoll T, Loesgen S, Dütsch G, Gohlke
H, Herbon N, Klugbauer S, Dempfle A, Bickeböller H, Becker-Follmann
J, Rüschendorf F, et al: Fine mapping and single nucleotide
polymorphism association results of candidate genes for asthma and
related phenotypes. Hum Mutat. 18:327–336. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baltimore D, Boldin MP, O'Connell RM, Rao
DS and Taganov KD: MicroRNAs: New regulators of immune cell
development and function. Nat Immunol. 9:839–845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomankova T, Petrek M, Gallo J and
Kriegova E: MicroRNAs: Emerging regulators of immune-mediated
diseases. Scand J Immunol. 75:129–141. 2012. View Article : Google Scholar
|
|
9
|
Sonkoly E, Stahle M and Pivarcsi A:
MicroRNAs and immunity: Novel players in the regulation of normal
immune function and inflammation. Semin Cancer Biol. 2:131–140.
2008. View Article : Google Scholar
|
|
10
|
Jazdzewski K, Murray EL, Franssila K,
Jarzab B, Schoenberg DR and de la Chapelle A: Common SNP in
pre-miR-146a decreases mature miR expression and predisposes to
papillary thyroid carcinoma. Proc Natl Acad Sci USA. 105:7269–7274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao LB, Bai P, Pan XM, Jia J, Li LJ, Liang
WB, Tang M, Zhang LS, Wei YG and Zhang L: The association between
two polymorphisms in pre-miRNAs and breast cancer risk: A
meta-analysis. Breast Cancer Res Treat. 125:571–574. 2011.
View Article : Google Scholar
|
|
12
|
Tian T, Xu Y, Dai J, Wu J, Shen H and Hu
Z: Functional polymorphisms in two pre-microRNAs and cancer risk: A
meta-analysis. Int J Mol Epidemiol Genet. 1:358–366.
2010.PubMed/NCBI
|
|
13
|
Xu W, Xu J, Liu S, Chen B, Wang X, Li Y,
Qian Y, Zhao W and Wu J: Effects of common polymorphisms rs11614913
in miR-196a2 and rs2910164 in miR-146a on cancer susceptibility: A
meta-analysis. PLoS One. 6:e204712011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su XW, Yang Y, Lv ML, Li LJ, Dong W,
Miao-Liao, Gao LB, Luo HB, Yun-Liu, Cong RJ, et al: Association
between single-nucleotide polymorphisms in pre-miRNAs and the risk
of asthma in a Chinese population. DNA Cell Biol. 30:919–923. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oliveira SR, Vieira HL and Duarte CB:
Effect of carbon monoxide on gene expression in cerebrocortical
astrocytes: Validation of reference genes for quantitative
real-time PCR. Nitric Oxide. 49:80–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Chen XP and Li YJ: MicroRNA-146a and
human disease. Scand J Immunol. 71:227–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perry MM, Moschos SA, Williams AE,
Shepherd NJ, Larner-Svensson HM and Lindsay MA: Rapid changes in
microRNA-146a expression negatively regulate the IL-1beta-induced
inflammatory response in human lung alveolar epithelial cells. J
Immunol. 180:5689–5698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu B, Feng NH, Li PC, Tao J, Wu D, Zhang
ZD, Tong N, Wang JF, Song NH, Zhang W, et al: A functional
polymorphism in Pre-miR-146a gene is associated with prostate
cancer risk and mature miR-146a expression in vivo. Prostate.
70:467–472. 2010. View Article : Google Scholar
|
|
20
|
Vinci S, Gelmini S, Pratesi N, Conti S,
Malentacchi F, Simi L, Pazzagli M and Orlando C: Genetic variants
in miR-146a, miR-149, miR-196a2, miR-499 and their influence on
relative expression in lung cancers. Clin Chem Lab Med.
49:2073–2080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang Y, Luo X, Cui H, Ni X, Yuan M, Guo Y,
Huang X, Zhou H, de Vries N, Tak PP, et al: MicroRNA-146A
contributes to abnormal activation of the type I interferon pathway
in human lupus by targeting the key signaling proteins. Arthritis
Rheum. 60:1065–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chatzikyriakidou A, Voulgari PV, Georgiou
I and Drosos AA: A polymorphism in the 3′-UTR of interleukin-1
receptor-associated kinase (IRAK1), a target gene of miR-146a, is
associated with rheumatoid arthritis susceptibility. Joint Bone
Spine. 77:411–413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grasemann H, Yandava CN, Storm van's
Gravesande K, Deykin A, Pillari A, Ma J, Sonna LA, Lilly C,
Stampfer MJ, Israel E, et al: A neuronal NO synthase (NOS1) gene
polymorphism is associated with asthma. Biochem Biophys Res Commun.
272:391–394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miura M, Yamauchi H, Ichinose M, Ohuchi Y,
Kageyama N, Tomaki M, Endoh N and Shirato K: Impairment of neural
nitric oxide-mediated relaxation after antigen exposure in guinea
pig airways in vitro. Am J Respir Crit Care Med. 156:217–222. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Silverman EK and Palmer LJ: Case-control
association studies for the genetics of complex respiratory
diseases. Am J Respir Cell Mol Biol. 22:645–648. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martinez B, Barrios K, Vergara C, Mercado
D, Jiménez S, Gusmão L and Caraballo L: A NOS1 gene polymorphism
associated with asthma and specific immunoglobulin E response to
mite allergens in a Colombian population. Int Arch Allergy Immunol.
144:105–113. 2007. View Article : Google Scholar : PubMed/NCBI
|