Introduction
Rheumatoid arthritis (RA) is a chronic
immune-mediated disease that affects ~1% of the world's population.
It is characterized by synovial hyperplasia, activation of
RA-fibroblast-like synoviocytes (FLSs), articular inflammation and
invasion of the synovium into the adjacent bone and cartilage
(1–3). It has been reported that macrophages,
B-cells, T-cells, chondrocytes and osteoclasts are involved in the
pathogenesis of RA (4–8). A previous study determined that
activated RA-FLSs present in the rheumatoid synovium are crucial
for the progression of RA (9).
Subsequent to activation, RA-FLSs produce various cytokines,
chemokines and matrix-degrading enzymes that mediate the
interaction with neighboring inflammatory and endothelial cells and
lead to the progressive degradation of the articular cartilage and
bone (10). RA starts in a few
joints; however, it may spread to all of the joints during the
development of the disease, depending on the migration ability of
the RA-FLSs. They are able to migrate long distances through the
blood stream and move towards, attach to and invade distant exposed
cartilage matrix (11).
Additionally, RA-FLSs develop a unique aggressive phenotype that
leads to increased invasiveness into the extracellular matrix
(ECM), exacerbating joint damage (12,13).
Platelet-rich plasma (PRP) is autologous plasma that
has a platelet concentration that is elevated three to four times
above the baseline levels (13–15).
It has been established that the number of platelets is correlated
with rheumatoid activity. In addition, a patient with RA may have
an increasing number of platelets during the active stages of the
disease, which may be reduced with the decrease in inflammation
(16). Previous studies have
determined that the number of platelets and platelet
microparticles, derived from activated platelets, were abundant in
the RA synovial fluid (17,18).
Additionally, as a concentrated source of autologous platelets, PRP
contains various growth factors and cytokines, including
platelet-derived growth factor, transforming growth factor-β and
insulin-like growth factor-1. These have been used for the
treatment of bone defects, tendinopathies and intra-articular
pathology of the synovial joints (19–21).
Given the importance of the migration and invasion of RA-FLSs for
the pathogenesis of RA, the effect of PRP on these processes has
not been fully elucidated. Therefore, the aim of the present study
was to investigate the effect of PRP on the migration and invasion
of human RA-FLSs. It was determined that PRP may promote RA-FLS
cell-matrix adhesion, migration and invasion. In addition, PRP may
alter cell motility by cytoskeleton rearrangement and upregulation
of the expression levels of matrix metalloproteinase-1 (MMP-1).
Materials and methods
Cell culture
RA-FLSs, obtained from GuangZhou Jennio Biotech Co.,
Ltd. (Guangzhou, China) were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.) and antibiotics (100
mg/ml streptomycin and 100 U/ml penicillin) in a humidified
incubator at 37°C with 5% CO2. Cells used for
experiments were at passage 3–6.
PRP preparation
PRP was harvested from 10 ml cell culture (platelet
concentration, 1.2×1012/l; Red Cross Blood Station of
Yangzhou City, Yangzhou, China). The PRP samples were incubated
with 10% calcium chloride and 100 U/ml bovine thrombin
(Sigma-Aldrich, St. Louis, MO, USA), aggregated overnight at 4°C,
then centrifuged at 5,000 × g for 20 min at 4°C. The supernatant
was collected and stored at −20°C (16).
Scratch assay
Two different concentrations of PRP (2 and 5%) were
selected for the present study. RA-FLS were serum-starved for 24 h,
then plated in a 24-well plate. The cells were then incubated with
culture medium in the absence (normal control; NC) or presence of
PRP (2 and 5%) for 48 h. The cell monolayer was scraped in a
straight line to create a 'scratch' using a P200 pipet tip.
Subsequently, the cells were washed twice with phosphate-buffered
saline (PBS). Photographs of the treated cells migrating within the
scratch were captured at 0 and 24 h. Each experiment was performed
three times.
Transwell cell migration and invasion
assays
Cell migration in vitro was performed using
6.5 mm Transwell chambers with 8 µm pores (Corning, Corning,
NY, USA). RA-FLSs seeded at a density of 2.0×104 were
serum-starved for 24 h, then seeded in the upper chamber of DMEM
containing 2% FBS. The lower chamber contained DMEM and 10% FBS
with or without PRP (2 or 5%). The cells were incubated for 24 h at
37°C in a humidified atmosphere, and the non-migrating cells were
wiped with dry cotton swabs. Cells that migrated through the filter
were fixed with methanol for 15 min and stained with 0.1% crystal
violet for 20 min. For the cell invasion assay, RA-FLSs were seeded
at a density of 4.0×104 into Transwell inserts
pre-coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA;
100 µg/ml diluted with serum-free DMEM), and DMEM with or
without PRP (2 or 5%) was added to the lower chamber as mentioned
above. Cell invasion was allowed to occur for 24 h, and cells on
the top membrane surface were removed with cotton swabs. The
migrating or invading cells were counted from five randomly
selected microscopic fields at a magnification of ×200 per insert.
Three independent experiments were performed.
Cell-matrix adhesion assay
Cell-matrix adhesion assay was performed as
previously described with some modifications (13). Cells were seeded at a density of
1.0×105 cells/well in 24-well plates, which were coated
with 100 µg/ml type I collagen (BD Biosciences) at 4°C
overnight and blocked with 1% bovine serum albumin (BSA;
Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 1 h.
RA-FLSs were pretreated with or without PRP (2 or 5%) for 48 h, and
suspended in the adhesion buffer containing DMEM supplemented with
0.1% BSA. The cells were then plated onto the collagen I-coated
plates. They were then incubated for 20 min at 37°C, and unattached
cells were washed out three times with PBS. The number of the
attached cells was counted from five randomly selected microscopic
fields, at a magnification of ×200. The independent experiments
were performed three times.
Immunofluorescence analysis
RA-FLSs were plated onto coverslips in 24-well
plates and incubated for 48 h at 37°C with or without PRP (2 or 5%)
in DMEM supplemented with 10% FBS. Next, the cells were washed with
PBS and fixed with 4% paraformaldehyde diluted in PBS for 30 min.
The cells were permeabilized with 0.2% Triton X-100 in PBS for 10
min and blocked with 3% BSA in PBS for 1 h. The cells were
incubated with the rhodamine-conjugated phalloidin probe
(Sigma-Aldrich) for 1 h at 37°C in the dark, and then washed three
times with PBS. All cells were counterstained with
4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for the
nucleus. Following three additional washes with PBS, the cells were
examined under a fluorescence microscope, and images were captured
using Optronics digital camera (Optronics, Goleta, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol (Vazyme Biotech
Co., Ltd., Nanjing, China) according to the manufacturer's
protocol, and cDNA was synthesized from RNA using HiScript Q RT
SuperMix for qPCR (Vazyme Biotech Co., Ltd.). RT-qPCR was performed
using the Applied Biosystems 7500 Real-Time PCR System (Thermo
Fisher Scientific, Inc.) using the AceQ qPCR SYBR Green Master mix
(Vazyme Biotech Co., Ltd.) according to the manufacturer's
protocols. The thermocycling conditions were as follows: 95°C for 5
min; and 40 cycles of 95°C for 10 sec and 60°C for 34 sec.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the
internal control. The sequences of primers used were as follows:
MMP-1, forward (F) 5′-ACTCTGGAGTAATGTCACACCT-3′ and reverse (R)
5′-GTTGGTCCACCTTTCATCTTCA-3′; GAPDH, F 5′-GCACCGTCAAGGCTGAGAAC-3′
and R 5′-TGGTGAAGACGCCAGTGGA-3′. The relative levels of the MMP-1
mRNA normalized against GAPDH mRNA were evaluated using
2−ΔΔCq (22). All
reactions were performed three times.
Western blot analysis
RA-FLSs were lysed in radioimmunoprecipitation assay
(RIPA) buffer containing 50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1%
NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS),
1 µmol phenylmethylsulfonyl fluoride and a cocktail of
protease inhibitors (Roche Diagnostics, Basel, Switzerland). The
protein concentration was determined using a bicinchoninic acid
assay. Subsequently, the proteins were resolved on 10%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and
electro-transferred onto polyvinylidene fluoride membrane membranes
(Roche Diagnostics). The membranes were subsequently blocked with
PBS-Tween 20 containing 5% non-fat dried milk. Immunoblotting was
performed using primary antibodies against MMP-1 (1:500; R&D
Systems, Inc., Minneapolis, MN, USA; cat. no. MAB901) and GAPDH
(1:1,000; KangChen Bio-tech Co., Ltd., Shanghai, China; cat. no.
KC-5G4) overnight at 4°C, and the horseradish peroxidase-conjugated
goat anti-mouse IG secondary antibody (1:2,000; Santa Cruz
Biotechnology, Dallas, TX, USA; cat. no. sc-2301) incubated for 2 h
at room temperature. Protein bands were detected by enhanced
chemiluminescence (ECL) using the Pierce ECL Plus Western Blotting
Substrate kit (Thermo Fisher Scientific, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical comparison between groups was performed using one-way
analysis of variance followed by Dunnett's test using SPSS 19.0
software (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
PRP promotes migration and invasion of
RA-FLSs
RA-FLS migration and invasion are important to the
pathophysiology of RA (10,11).
In order to determine the importance of PRP in RA-FLS migration,
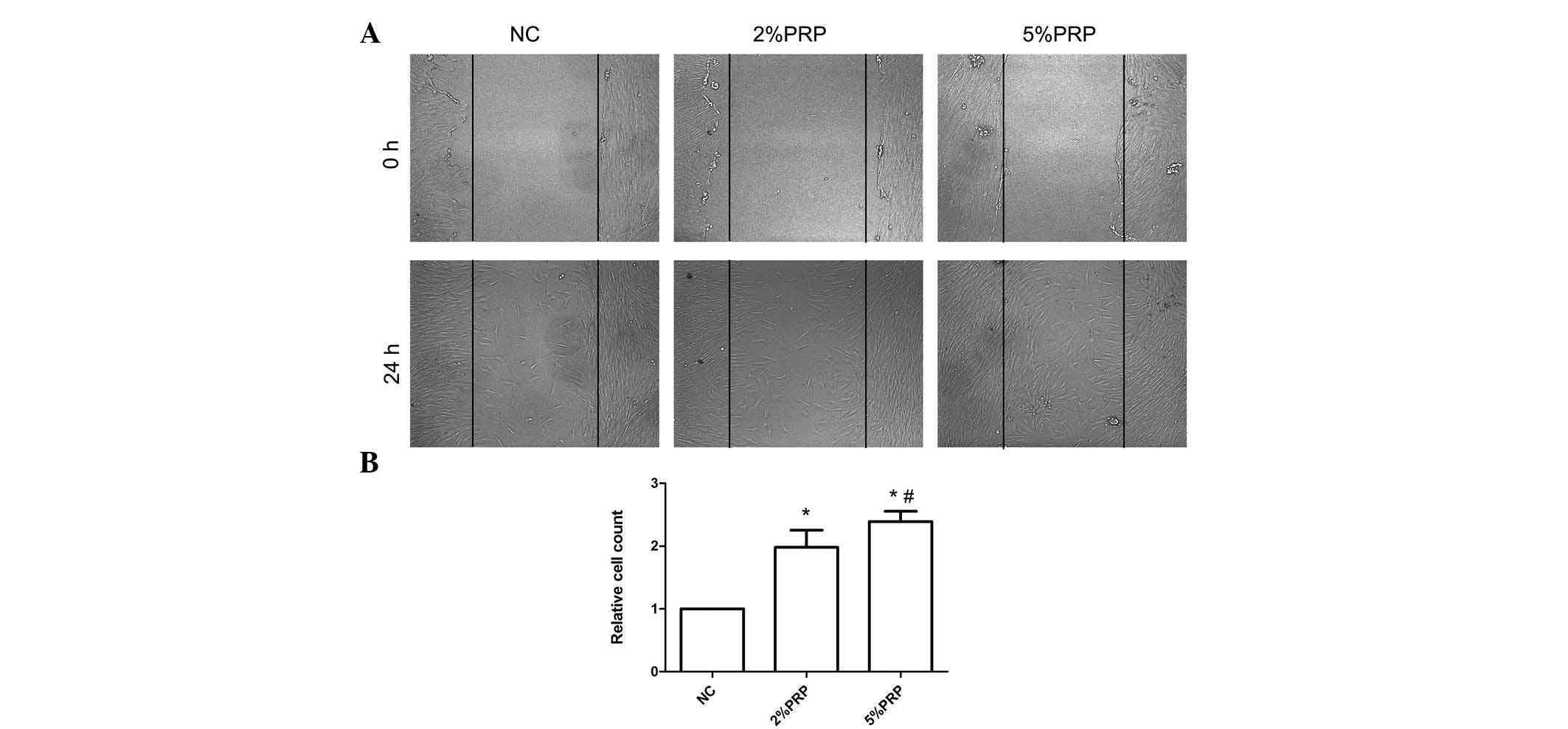
scratch and Transwell migration assays were performed. As presented
in Fig. 1, the scratch assay
highlighted a rapid movement of RA-FLSs. The migration front was
evident at 24 h, where a highly confluent monolayer region
gradually migrated into the cell-free 'scratch' region,
particularly in the 2 and 5% PRP groups (Fig. 1A). The scratch assay determined
that RA-FLSs incubated with 2 or 5% PRP have a significantly
greater migration ability compared with the NC group (P<0.05;
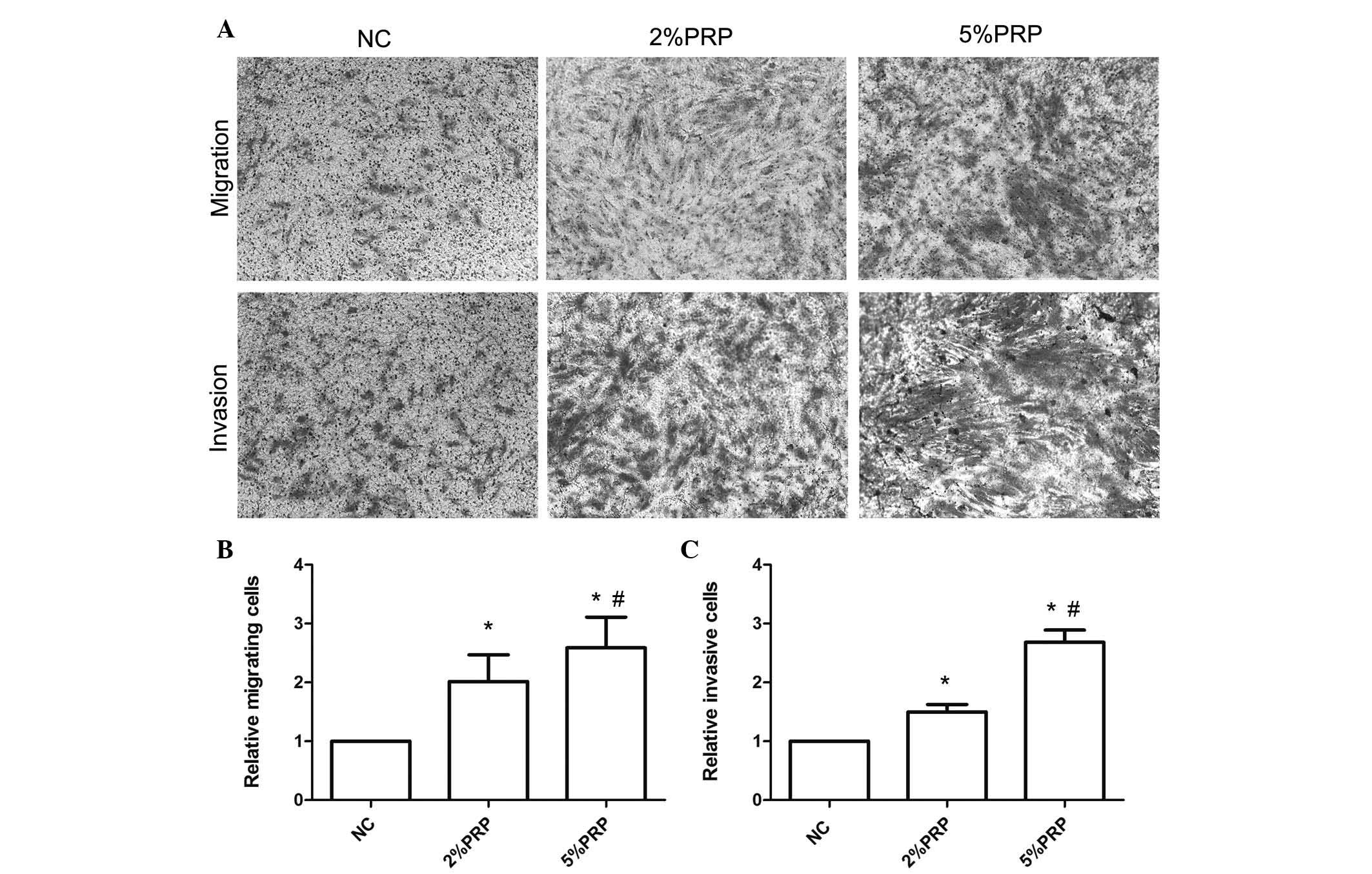
Fig. 1B). The Transwell migration
assay revealed that the number of RA-FLSs across the polycarbonate
membrane in the PRP group was significantly higher compared with
the NC group (P<0.05; Fig. 2).
The number of migrating cells in the 5% PRP group was greater
compared with the 2% PRP group (Fig.
2B), which was consistent with the results obtained with the
scratch assay (Fig. 1). The
aforementioned findings suggest that PRP enhanced the migration
ability of RA-FLSs. Additionally, to evaluate the effect of PRP on
the invasion of RA-FLSs, a Transwell invasion assay was performed.
RA-FLSs were seeded into Matrigel precoated Transwell inserts, and
allowed to invade for 24 h. As presented in Fig. 2, the number of invasive cells in
the chamber with PRP treatments was significantly higher compared
with the NC group (P<0.05; Fig.
2C). The promotion of cell migration did not appear to be due
to increased cell viability (data not shown). Considered together,
these findings demonstrated that PRP promoted migration and
invasion of human RA-FLS.
PRP promotes RA-FLS adhesion onto the
ECM
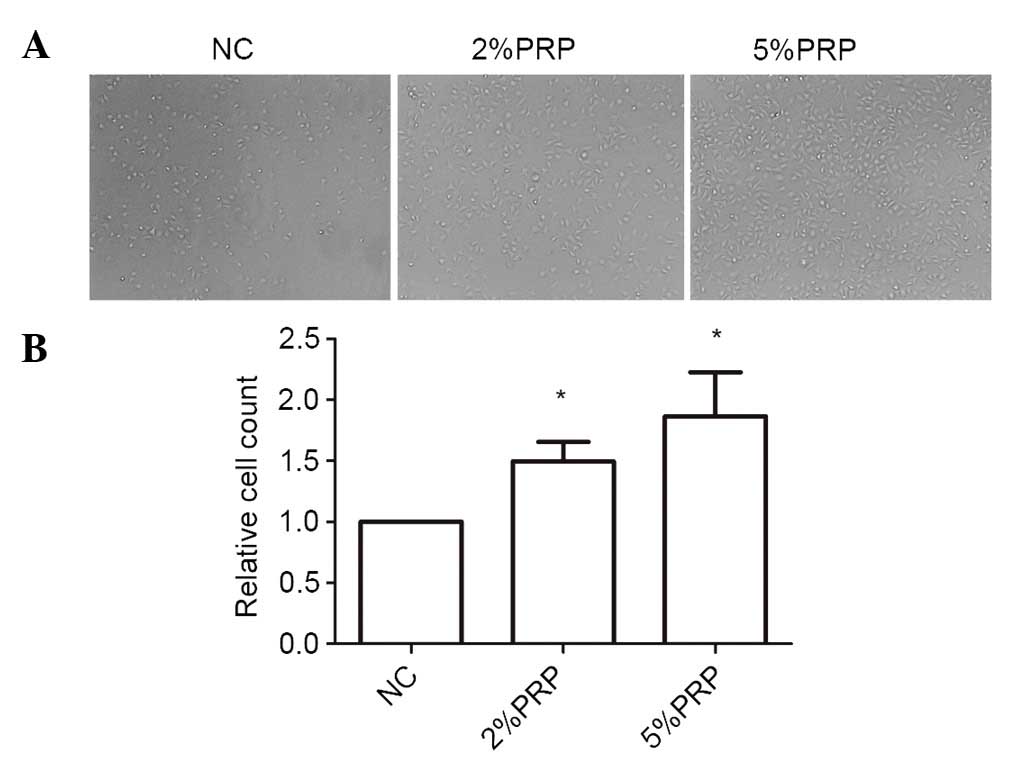
Cell adhesion and spreading to the ECM is a key step
in the migration and invasion process of RA-FLSs. As the cells
adhere and spread, they generate traction and thereby migrate on
the substrate. To investigate the importance of PRP to the
cell-matrix adhesion of RA-FLSs, they were seeded onto collagen I
matrix for 20 min. The cell adhesion ability on collagen I was
significantly higher in PRP-treated cells compared with the NC
group (P<0.05; Fig. 3). The
number of adherent cells in 5% PRP group was higher compared with
that in 2% PRP group (Fig. 3).
Therefore, it may be surmised that PRP enhanced cell-matrix
adhesion.
PRP induces the reorganization of the
actin cytoskeleton and formation of stress fibers and
lamellipodia
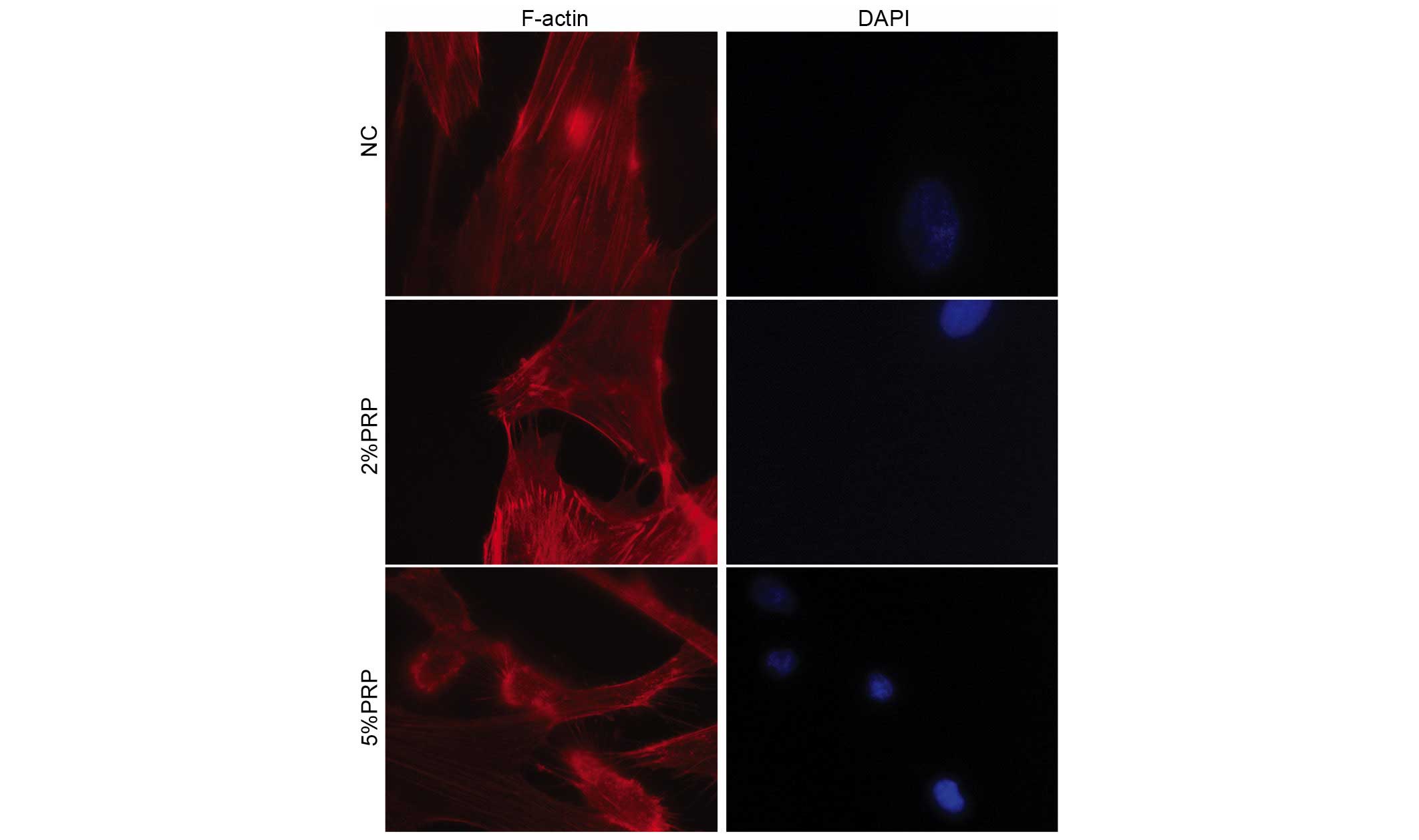
The actin cytoskeleton with its polymerization
dynamics is crucial for cell adhesion and migration (18). The present study investigated
whether PRP may interfere with the actin cytoskeleton rearrangement
and the production of filopodia and lamellipodia in RA-FLSs.
Therefore, rhodamine-conjugated phalloidin was used to stain the
filamentous actin (F-actin). PRP induced a significant decrease the
number of centrally located stress fibers, and led to an increase
in the formation of filopodia and lamellipodia in the detectable
leading edge protrusions of RA-FLSs, particularly in the 5% PRP
group (Fig. 4). These observations
revealed that PRP is important for the dynamic reorganization of
the actin cytoskeleton during adhesion and migration of
RA-FLSs.
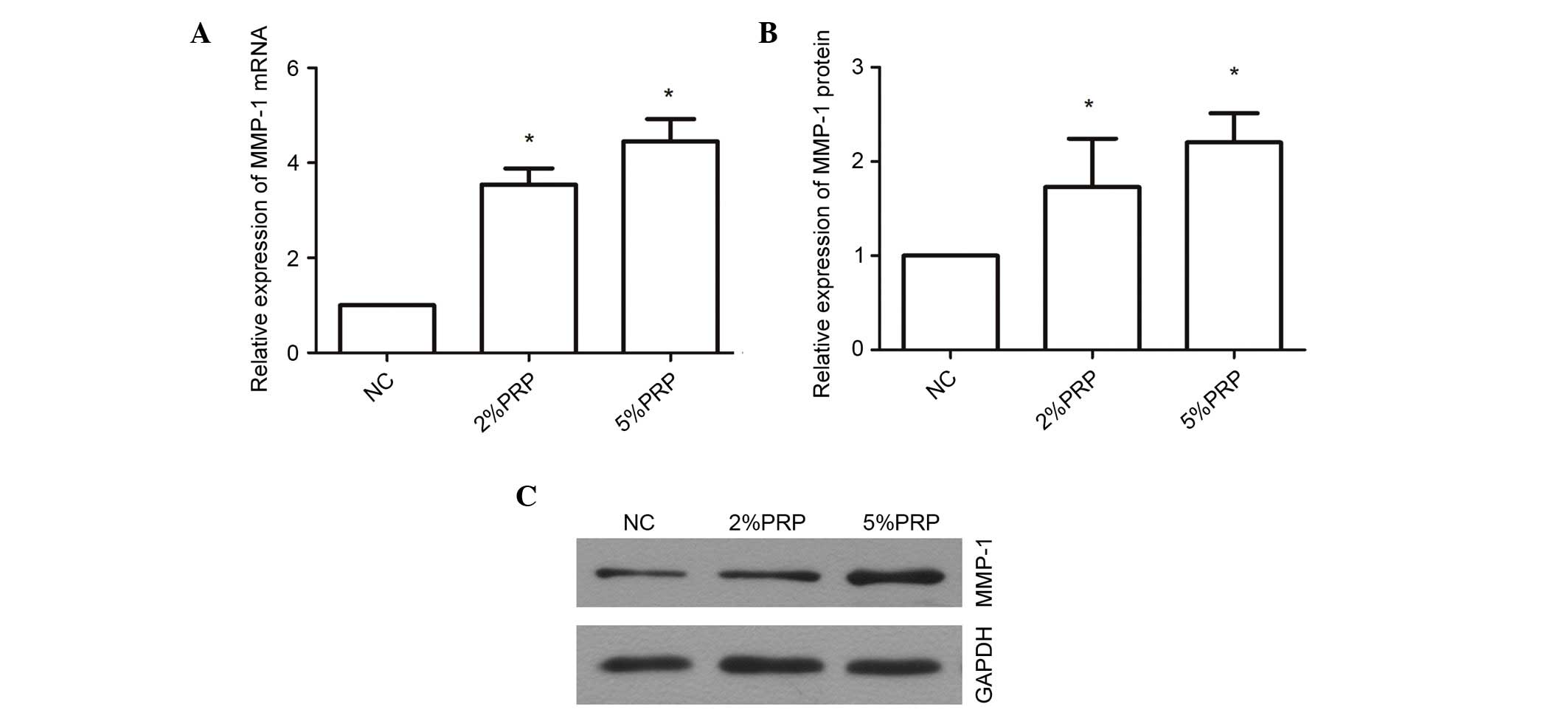
PRP upregulates the mRNA and protein
expression levels of MMP-1
MMP-1 is a proteolytic enzyme of collagen I, which
is important for collagen remodeling during wound healing (23). PRP evidently promoted the migration
and invasion of RA-FLSs; therefore, the underlying molecular
mechanism was examined through a determination of the expression
levels of MMP-1 (Fig. 5). The mRNA
expression level of MMP-1 was significantly upregulated
3.546±0.34-fold in RA-FLSs following treatment with 2% PRP compared
with the NC group (P<0.05; Fig.
5A), and 4.45±0.46-fold in the 5% PRP treatment group compared
with NC (P<0.05; Fig. 5A).
MMP-1 protein expression levels were also significantly greater in
the PRP-treated groups compared with the NC group (P<0.05;
Fig. 5B and C). These findings
indicate that MMP-1 may contribute to the migration and invasion of
RA-FLSs when induced by PRP. The upregulation of mRNA and protein
MMP-1 expression levels in RA-FLS exposed to PRP suggests that the
enrichment of PRP in the synovial joints may be associated with RA,
and the increased level of MMP-1 may be associated with catabolism
of cartilage.
Discussion
The biological effects of PRP on various types of
cell (chondrocytes, endothelial cells, osteoblasts, periodontal
ligament, and so forth) have previously been demonstrated (21,24).
A previous study has determined that the number of platelets is
associated with rheumatoid activity (16). Additionally, the platelets in PRP,
subsequent to incubation with CaCl2 and thrombin, were
demonstrated to secrete various growth factors and exhibit
biological activities, including promoting regeneration,
angiogenesis and wound healing (25,26).
To the best of our knowledge, the effects of PRP on RA-FLSs have
not been previously reported. The present study demonstrated that
PRP may promote migration and invasion of RA-FLSs. This conclusion
is supported by the observation that PRP also promoted cell
adhesion onto the ECM, interfered with the actin cytoskeleton
rearrangement and upregulated the mRNA and protein expression
levels of MMP-1.
RA usually arises in a few joints, and may spread to
all joints during the course of the disease. RA-FLSs undergo the
complex process of moving away from an affected joint, migrating
into a healthy joint and subsequently invading the articular
cartilage. During the pathogenesis of RA, FLSs may adhere onto the
ECM, invade into the cartilage and bone, and activate osteoclasts
to increase bone erosion and destruction, which are regarded as
important mechanisms that lead to the invasion of cartilage and
bone (27,28). In order to investigate whether PRP
affects RA-FLS migration and invasion, scratch, Transwell migration
and invasion assays were performed. It was determined that PRP
promoted RA-FLS migration, invasion and adhesion.
Cell adhesion and migration are complex and
interdependent cellular processes. The movement of cells requires
adhesion onto the ECM. As PRP is important for the positive
regulation of the migration and invasion of RA-FLSs, it was
determined that PRP may promote this migration through the
regulation of the cell-matrix adhesion. A cell-matrix adhesion
assay was performed, and it was revealed that PRP significantly
facilitated the adhesion of RA-FLSs to the cell matrix. The present
study determined that PRP may be a positive regulator in all three
processes of cell-matrix adhesion, cell migration and cell invasion
for RA-FLSs. The promotion of migration and invasion of RA-FLSs was
triggered by PRP, possibly due to the increase of their adhesion to
the cell-matrix.
The reorganization of cytoskeleton proteins,
including F-actin, has been associated with cell migration and
invasion. Migration of cells through the ECM is a multistep
process, which commences with the extension of lamellipodia. The
formation of actin stress fibers is frequently associated with cell
adhesion, whereas membrane ruffling and filopodia and lamellipodia
formation are associated with cell migration (29). Exposure of RA-FLSs to PRP resulted
in marked changes, characterized by alterations in the cell shape
and actin cytoskeleton. In order to confirm the effect of PRP on
the migration and invasion of RA-FLSs, F-actin reorganization was
assessed following treatment with PRP. It was determined that PRP
resulted in a decreased assembly of actin stress fibers and an
increased filopodia and lamellipodia formation in RA-FLSs. The
current study suggested that the facilitation of cell adhesion and
motility by PRP may be due to the regulation of actin
reorganization and the formation of filopodia and lamellipodia.
MMP-1 is a proteolytic enzyme of collagen I that is
important for collagen remodeling during wound healing (23). High expression levels of MMP-1 have
been associated with the increased invasive ability of several
types of cancer, including pancreatic, gastric, colorectal and
breast cancer (30,31). MMP-1 is vital for tissue
remodeling, tumor progression and metastasis due to its proteolytic
activities that aid in ECM degradation, invasion and cytokine
mobilization (32,33). The current study determined that
PRP upregulated the mRNA and protein expression levels of MMP-1.
Therefore, it is possible that PRP promoted the migration and
invasion of RA-FLSs due to its capacity to upregulate MMP-1
expression levels. The upregulation of MMP-1 in RA-FLSs that have
been exposed to PRP suggests that the application of PRP to
synovial joints may be associated with increased catabolism of
cartilage and extracellular matrix proteins.
In conclusion, the present study determined that PRP
promoted the migration and invasion of RA-FLSs, and it may
therefore be a novel target to limit the destruction of joints that
is associated with patients with RA. The regulation of cell
migration, invasion and adhesion on the ECM may be modulated
through the induction of actin cytoskeleton reorganization and
increased MMP-1 expression levels. Further studies are required to
clarify the complicated molecular mechanism of PRP-induced cell
migration and invasion in FLSs, and to elucidate the signaling
mechanism behind this. Migration of RA-FLSs to the cartilage and
bone has been considered to be the critical step in the aggravation
of RA, and regulating the migration and invasion of RA-FLSs may be
a novel therapeutic strategy to limit the destructive progress of
RA.
Acknowledgments
The present study was supported by the National
Nature Science Foundation of China (grant no. 81470070).
References
|
1
|
Karouzakis E, Neidhart M, Gay RE and Gay
S: Molecular and cellular basis of rheumatoid joint destruction.
Immunol Lett. 106:8–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Müller-Ladner U, Pap T, Gay RE, Neidhart M
and Gay S: Mechanisms of disease: The molecular and cellular basis
of joint destruction in rheumatoid arthritis. Nat Clin Pract
Rheumatol. 1:102–110. 2005. View Article : Google Scholar
|
|
3
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: Key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gravallese EM: Bone destruction in
arthritis. Ann Rheum Dis. 61(Suppl 2): ii84–ii86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Looney RJ: B cell-targeted therapy for
rheumatoid arthritis: An update on the evidence. Drugs. 66:625–639.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma Y and Pope RM: The role of macrophages
in rheumatoid arthritis. Curr Pharm Des. 11:569–580. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Skapenko A, Leipe J, Lipsky PE and
Schulze-Koops H: The role of the T cell in autoimmune inflammation.
Arthritis Res Ther. 7(Suppl 2): S4–S14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yasuda T: Cartilage destruction by matrix
degradation products. Mod Rheumatol. 16:197–205. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klareskog L, Catrina AI and Paget S:
Rheumatoid arthritis. Lancet. 373:659–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huber LC, Distler O, Tarner I, Gay RE, Gay
S and Pap T: Synovial fibroblasts: Key players in rheumatoid
arthritis. Rheumatology (Oxford). 45:669–675. 2006. View Article : Google Scholar
|
|
11
|
Lefèvre S, Knedla A, Tennie C, Kampmann A,
Wunrau C, Dinser R, Korb A, Schnäker EM, Tarner IH, Robbins PD, et
al: Synovial fibroblasts spread rheumatoid arthritis to unaffected
joints. Nat Med. 15:1414–1420. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tolboom TC, van der Helm-Van Mil AH,
Nelissen RG, Breedveld FC, Toes RE and Huizinga TW: Invasiveness of
fibroblast-like synoviocytes is an individual patient
characteristic associated with the rate of joint destruction in
patients with rheumatoid arthritis. Arthritis Rheum. 52:1999–2002.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karouzakis E, Gay RE, Gay S and Neidhart
M: Epigenetic control in rheumatoid arthritis synovial fibroblasts.
Nat Rev Rheumatol. 5:266–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tolboom TC, Pieterman E, van der Laan WH,
Toes RE, Huidekoper AL, Nelissen RG, Breedveld FC and Huizinga TW:
Invasive properties of fibroblast-like synoviocytes: Correlation
with growth characteristics and expression of MMP-1, MMP-3, and
MMP-10. Ann Rheum Dis. 61:975–980. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marx RE: Platelet-rich plasma (PRP): What
is PRP and what is not PRP? Implant Dent. 10:225–228. 2001.
View Article : Google Scholar
|
|
16
|
Wang F, Wang NS, Yan CG, Li JH and Tang
LQ: The significance of platelet activation in rheumatoid
arthritis. Clin Rheumatol. 26:768–771. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Endresen GK: Evidence for activation of
platelets in the synovial fluid from patients with rheumatoid
arthritis. Rheumatol Int. 9:19–24. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boilard E, Nigrovic PA, Larabee K, Watts
GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E,
Farndale RW, Ware J and Lee DM: Platelets amplify inflammation in
arthritis via collagen dependent microparticle production. Science.
327:580–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han J, Meng HX, Tang JM, Li SL, Tang Y and
Chen ZB: The effect of different platelet-rich plasma concentration
on proliferation and differentiation of human periodontal ligament
cells in vitro. Cell Prolif. 40:241–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anitua E, Andia I, Ardanza B, Nurden P and
Nurden AT: Autologous platelets as a source of proteins for healing
and tissue regeneration. Thromb Haemost. 91:4–15. 2004.
|
|
21
|
Kanno T, Takahashi T, Tsujisawa T,
Ariyoshi W and Nishihara T: Platelet-rich plasma enhances human
osteoblast-like cell proliferation and differentiation. J Oral
Maxillofac Surg. 63:362–369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Shin MK, Lee JW, Kim YI, Kim YO, Seok H
and Kim NI: The effects of platelet-rich clot releasate on the
expression of MMP-1 and type I collagen in human adult dermal
fibroblasts: PRP is a stronger MMP-1 stimulator. Mol Biol Rep.
41:3–8. 2014. View Article : Google Scholar
|
|
24
|
Kakudo N, Morimoto N, Kushida S, Ogawa T
and Kusumoto K: Platelet-rich releasate promotes angiogenesis in
vitro and in vivo. Med Mol Morphol. 47:83–89. 2014. View Article : Google Scholar
|
|
25
|
Kakudo N, Minakata T, Mitsui T, Kushida S,
Notodihardjo FZ and Kusumoto K: Proliferation-promoting effect of
platelet-rich plasma on human adipose-derived stem cells and human
dermal fibroblasts. Plast Reconstr Surg. 122:1352–1360. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kakudo N, Kushida S, Minakata T, Suzuki K
and Kusumoto K: Platelet-rich plasma promotes epithelialization and
angiogenesis in a split thickness skin graft donor site. Med Mol
Morphol. 44:233–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Volin MV, Huynh N, Klosowska K, Chong KK
and Woods JM: Fractalkine is a novel chemoattractant for rheumatoid
arthritis fibroblast-like synoviocyte signaling through MAP kinases
and Akt. Arthritis Rheum. 56:2512–2522. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gravallese EM, Manning C, Tsay A, Naito A,
Pan C, Amento E and Goldring SR: Synovial tissue in rheumatoid
arthritis is a source of osteoclast differentiation factor.
Arthritis Rheum. 43:250–258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akakura S and Gelman IH: Pivotal role of
AKAP12 in the regulation of cellular adhesion dynamics: Control of
cytoskeletal architecture, cell migration, and mitogenic signaling.
J Signal Transduct. 2012:5291792012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murray GI, Duncan ME, O'Neil P, Melvin WT
and Fothergill JE: Matrix metalloproteinase-1 is associated with
poor prognosis in colorectal cancer. Nat Med. 2:461–462. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McGowan PM and Duffy MJ: Matrix
metalloproteinase expression and outcome in patients with breast
cancer: Analysis of a published database. Ann Oncol. 19:1566–1572.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu X, Wang Q, Hu G, Van Poznak C, Fleisher
M, Reiss M, Massagué J and Kang Y: ADAMTS1 and MMP1 proteolytically
engage EGF-like ligands in an osteolytic signaling cascade for bone
metastasis. Genes Dev. 23:1882–1894. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pei D: Matrix metalloproteinases target
protease-activated receptors on the tumor cell surface. Cancer
Cell. 7:207–208. 2005. View Article : Google Scholar : PubMed/NCBI
|