Introduction
Infiltrating ductal carcinoma (IDC) is one of the
most common malignant tumors in female breast cancer (1). Tumor-specific immune promoting and
inhibiting responses are important for the pathogenesis of IDC
(2). CD4+ regulatory T
cells at tumor sites may significantly suppress immune responses,
leading to immune tolerance of breast cancer cells (3). Conversely, CD8+ T cells
may lead to an antitumor response against neoplastic cells
(4). A recent study suggested that
the plasticity of tumor-infiltrated T-cell subsets was influenced
by several factors, including the tumor microenvironment and
antigen-presenting cells (APCs) (5). APCs, such as macrophages, may
regulate the differentiation of T cells via
co-stimulatory/inhibitory molecules expressed on the surface of
cells and soluble products, including various cytokines, such as
interleukin (IL)-10 and -6. Based on the polarization of type 1 and
type 2 T helper cells (Th1 and Th2), macrophages may be divided
into the classically activated (M1) macrophage phenotype and the
alternatively activated (M2) macrophage phenotype (6,7). M1
macrophages promote the antitumor response of T cells, and M2
macrophages promote regulatory immune responses and enhance tumor
growth. However, the exact function of tumor-infiltrated
macrophages in the progression of IDC remains to be elucidated
(8–10).
B7 co-stimulatory/inhibitory family molecules are
expressed by macrophages and tumor cells, and are important
regulators of the balance between antitumor and tumor-promoting
immune responses. CD80/86 promotes interferon-γ (IFN-γ) expression,
which in turn mediates the Th1 response, and are frequently
expressed by M1 macrophages. Programmed death-ligand 1 (PD-L1)/L2
and B7-H4 reduce T-cell responses and are expressed by M2
macrophages. B7-H4, also termed B7x and B7S1, is a co-inhibitory
molecule for T-cell activation signaling. Due to this function,
B7-H4 may limit the proliferation, cytokine secretion and the
development of cytotoxicity of T cells, including CD4+
and CD8+ T cells (11,12).
The expression of the co-inhibitory molecule B7-H4 in cancer cells
may be associated with tumor progression, due to its importance in
the tumor microenvironment and its significance in the activation
of T cells. The present study demonstrated that B7-H4 may be
overexpressed in the breast IDC microenvironment, and
tumor-infiltrated macrophages may also express B7-H4. However, the
factor that induces B7-H4 expression in macrophages remains to be
elucidated. It is possible that specific cytokines in the breast
IDC micro environment may be associated with B7-H4 expression in
macrophages.
The present study characterized B7-H4 expression in
tumor-infiltrated macrophages in situ. In addition, in
vitro experiments revealed that macrophages of different
polarizations may express various levels of B7-H4. In congruence
with this, the M1 distinctive cytokine IL-6 and the M2 distinctive
cytokine IL-10 (13) were
increased in the IDC microenvironment when compared with
pericarcinomatous (PC) tissue. Furthermore, different expression
levels of IL-6 and -10 were detected in M1 and M2 phenotype cell
cultures. The present study revealed the association between B7-H4
and specialized subpopulations of macrophages, along with the
potential influence B7-H4 expression may have in the IDC
microenvironment.
Materials and methods
Human tissue biopsies
Paired IDC and PC tissues were collected from 61
patients with IDC were obtained from Outdo BioTech Co., Ltd.
(Shanghai, China). In addition, six frozen IDC samples from breast
cancer surgery between 2008 and 2012 were obtained from the tissue
bank in the Third Affiliated Hospital of Harbin Medical University
(Harbin, China) for immunofluorescence. The patients had a clear
pathological diagnosis according to the American Joint Committee on
Cancer (AJCC) staging system. The present study was approved by the
ethics committee of the Third Affiliated Hospital of Harbin Medical
University and all patients provided written informed consent. In
addition, the expression levels of estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor 2 (HER2) were detected in 31 patients. For
paraffin-embedded sections of IDC and PC samples, the reagents for
detection of ER, PR and HER2 were obtained from Maixin Biotech Co.,
Ltd (Fuzhou, China).
Cell culture, and M1 and M2 phenotype
macrophage polarization
Human blood monocytes were obtained from peripheral
blood mononuclear cells (PBMCs), which were obtained from healthy
female volunteer blood samples (mean age, 34.5 years), using
Miltenyi Biotec MACS Separator Starting kits and Human CD14
MicroBeads (Miltenyi Biotec Ltd., Surrey, UK).
M0 cells are human blood monocytes without cytokine
stimulus, they were used as control cells in the present study. The
M1 phenotype of cells may be induced by granulocyte-macrophage
colony-stimulating factor (GM-CSF), IFN-γ and lipopolysaccharides
(LPS) treatments. The M2 phenotype of cells may be induced by
M-CSF, IL-4 and IL-13. All of the cytokines used were obtained from
PeproTech, Inc. (Rocky Hill, NJ, USA). GM-CSF and M-CSF were added
to the blood monocytes in RPMI 1640 (GE Healthcare Life Sciences,
Little Chalfont, UK) supplemented with 10% fetal bovine serum
(Biological Industries, Beit Haemek, Israel). After a 48 h
incubation at 37°C, IFN-γ and LPS were added to the M1 phenotype
culture media, and IL-4 and IL-13 were added to the M2 culture
media (13). Following a further
48 h incubation, the M1 and M2 cells underwent the following
experiments. The concentrations of GM-CSF and M-CSF used were 30
ng/ml, the remaining cytokines were used at 10 ng/ml. Similar
manipulations to obtain the M1 and M2 phenotypes were performed on
the Thp1 human monocyte cell line (Bioleaf Biotech, Co., Ltd.,
Shanghai, China).
Enzyme-linked immunosorbent assay
(ELISA)
The supernatant of the tissue samples was acquired
following incubation with a lysis buffer (from ELISA kits) and
ultrasonic processing. The lysates were analyzed using RayBio Human
IL-6 and IL-10 ELISA kits (RayBio, Inc., Norcross, GA, USA)
according to the manufacturer's protocol. In addition, the
supernatant of the cultured monocytes, M1 and M2 phenotypes, was
collected and were analyzed using RayBio Human IL-6 and IL-10 ELISA
kits.
Immunohistochemistry (IHC) and
scoring
The paraffin-embedded breast IDC and PC tissue
samples were prepared by Outdo BioTech Co., Ltd., they were
sectioned (4 µm) using a Leica RM2245 microtome (Leica
Microsystems, Wetzlar, Germany). The samples were stained with
rabbit anti-B7-H4 monoclonal primary immunoglobulin (Ig)G antibody
(1:300, GeneTex, Inc., Irvine, CA, USA; cat. no. GTX42699)
overnight at 4°C with agitation, followed by ImmunoCruz rabbit LSAB
Staining system (Santa Cruz Biotechnology, Inc., Dallas, TX, USA;
cat. no. sc-2051). Subsequently, images were obtained using Nikon
Eclipse 80i microscope (Nikon Corporation, Tokyo, Japan).
IHC scoring was performed on the 61 paired IDC and
PC samples from patients with IDC (14). Proportion and intensity scores were
calculated for each sample. The intensity of immunostaining was
scored by visual assessment of the intensity (brown color) in
positively stained cells: 0, none; 1, weak; 2, intermediate and 3,
strong. The proportion score represented the percentage of
positively stained cells in the entire tissue section: 0, none; 1,
<5%; 2, 5–25%; 3, 26–50%; 4, 51–75% and 5, >75%. Overall
B7-H4 expression in the IDC and PC samples was expressed as a
histoscore, which was the sum of the proportion (0-5) and the
intensity scores (0–3), producing a range between 0–8, with a
maximum possible score of 8.
Immunofluorescence
Frozen breast IDC and PC tissue samples were
sectioned using a Microm HM525 freezing microtome (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The specimens were stained
with primary antibodies overnight at 4°C, as follows: Rabbit
anti-B7-H4 polyclonal primary IgG antibody (1:200, Santa Cruz
Biotechnology, Inc.; cat. no. 68872), mouse anti-CD68 monoclonal
IgG antibody (1:200, Santa Cruz Biotechnology, Inc.; cat. no.
20060), rabbit anti-CD163 polyclonal primary IgG antibody (1:200;
Abcam, Cambridge, UK; cat. no. ab87099), followed by Alexa Fluor
488-conjugated donkey anti-mouse IgG and Alexa Fluor 555-conjugated
donkey anti-rabbit IgG (1:200; Abcam; cat. no. ab150105) for 1 h at
37°C in the dark. The DAPI solution (1 µg/ml; Solarbio
Science & Technology Co., Ltd., Beijing, China) was used for
nuclear staining. The images were obtained using a Nikon Eclipse
80i fluorescence microscope.
Western blot analysis
The macrophages obtained from PBMCs and Thp1 cells
were resuspended in lysis buffer and 2 mM phenylmethane sulfonyl
fluoride (Solarbio Science & Technology Co., Ltd.). Lysates
were centrifuged at 12,000 × g for 5 min at 4°C. The protein
concentration was quantified by BCA Protein Assay kit (Solarbio
Science & Technology Co., Ltd.) and 40 µg was loaded
into 12% SDS-PAGE gels. The proteins were transferred to
nitrocellulose membranes. Subsequently, the expression levels of
B7-H4 were detected by immunoblotting using β-actin as a
housekeeping protein. The membranes were blocked with 5% skim milk
at room temperature for 1 h. The primary antibodies used were
polyclonal rabbit anti-B7-H4 IgG antibody (1:300; Santa Cruz
Biotechnology, Inc.; cat. no. sc-68872) and mouse anti-β-actin
monoclonal antibody (1:4,000; ProteinTech Group, Inc., Chicago, IL,
USA; cat. no. 66009-1) at 4°C overnight. The secondary antibodies
used were horseradish peroxidase (HRP)-conjugated affinipure goat
anti-rabbit (1:2,000; cat. no. SA00001-1) and anti-mouse IgG
(1:2,000; ProteinTech Group, Inc.; cat. no. SA00001-2) at 37°C for
1 h. The Luminata Forte Western HRP substrate (EMD Millipore,
Billerica, MA, USA) was used for enhanced chemiluminescence
visualization. Finally, the rabbit anti-human B7-H4 monoclonal
primary IgG antibody (1:300, GeneTex, Inc.) was used as a repeated
verification test. The western blots were analyzed sing ImageJ 1.48
software (imagej.nih.gov).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from macrophages obtained from
PBMCs and Thp1 cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific., Inc.). High-Capacity cDNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc.) was used for
mRNA RT. The RT was conducted as follows: 25°C for 10 min; 37°C for
2 h; 85°C for 5 min; and then paused at 12°C. The primer sequences
used for RT-qPCR were as follows: B7-H4, forward (F)
5′-TCTGGGCATCCCAAGTTGAC-3′, and reverse (R)
5′-TCCGCCTTTTGATCTCCGATT-3′; IL-6, F 5′-ACTCACCTCTTCAGAACGAATTG-3′
and R 5′-CCATCTTTGGAAGGTTCAGGTTG-3′; and IL-10, F
5′-GACTTTAAGGGTTACCTGGGTTG-3′ and R 5′-TCACATGCGCCTTGATGTCTG-3′.
The primer sequences for the control, glyceraldehyde 3-phosphate
dehydrogenase, were as follows: F 5′-CAAGTTCAACGGCACAGTCAA-3′ and R
5′-GTGGTCATGAGCCCTTCCA-3′. The primers were obtained from
Invitrogen (Thermo Fisher Scientific, Inc.). The reaction was
performed using ABI Power SYBR Green PCR Master mix on a ABI
StepOne Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows:
95°C for 10 min; 40 cycles of 95°C for 20 sec and 60°C for 30 sec.
The results were quantified and analyzed using a Cq method
(15,16) and melting curve analysis.
Flow cytometry
The present study determined the purity of monocytes
from PBMCs using mouse fluorescein isothiocyanate-conjugated
anti-IgG CD14 (1 µg/test; cat. no. 11-0141-81). The M1
phenotype was verified using mouse phycoerythrin-conjugated
anti-CD86 IgG (0.5 µg/test; cat. no. 12-0861-81) and M2
cells were verified by mouse APC-conjugated anti-CD163 (0.5
µg/test; cat. no. 17-1639-41). All of the
fluorescent-labeled antibodies were obtained from eBioscience, Inc.
(San Diego, CA, USA) and incubated with cells for 20 min at 4°C and
an Accuri C6 flow cytometer was used (BD Biosciences, Franklin
Lakes, NJ, USA).
Statistical analysis
IHC scores were reported as the mean ± standard
error of the mean. Mann-Whitney U test was used to analyze the
differences in the expression of B7-H4 between IDC and PC tissues,
χ2 test was used to estimate the frequency of B7-H4
expression. The results of ELISA, western blotting and RT-qPCR were
analyzed using Student's t-test. All statistical analyses were
performed using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Breast IDC tissues and tumor-associated
macrophages overexpress B7-H4
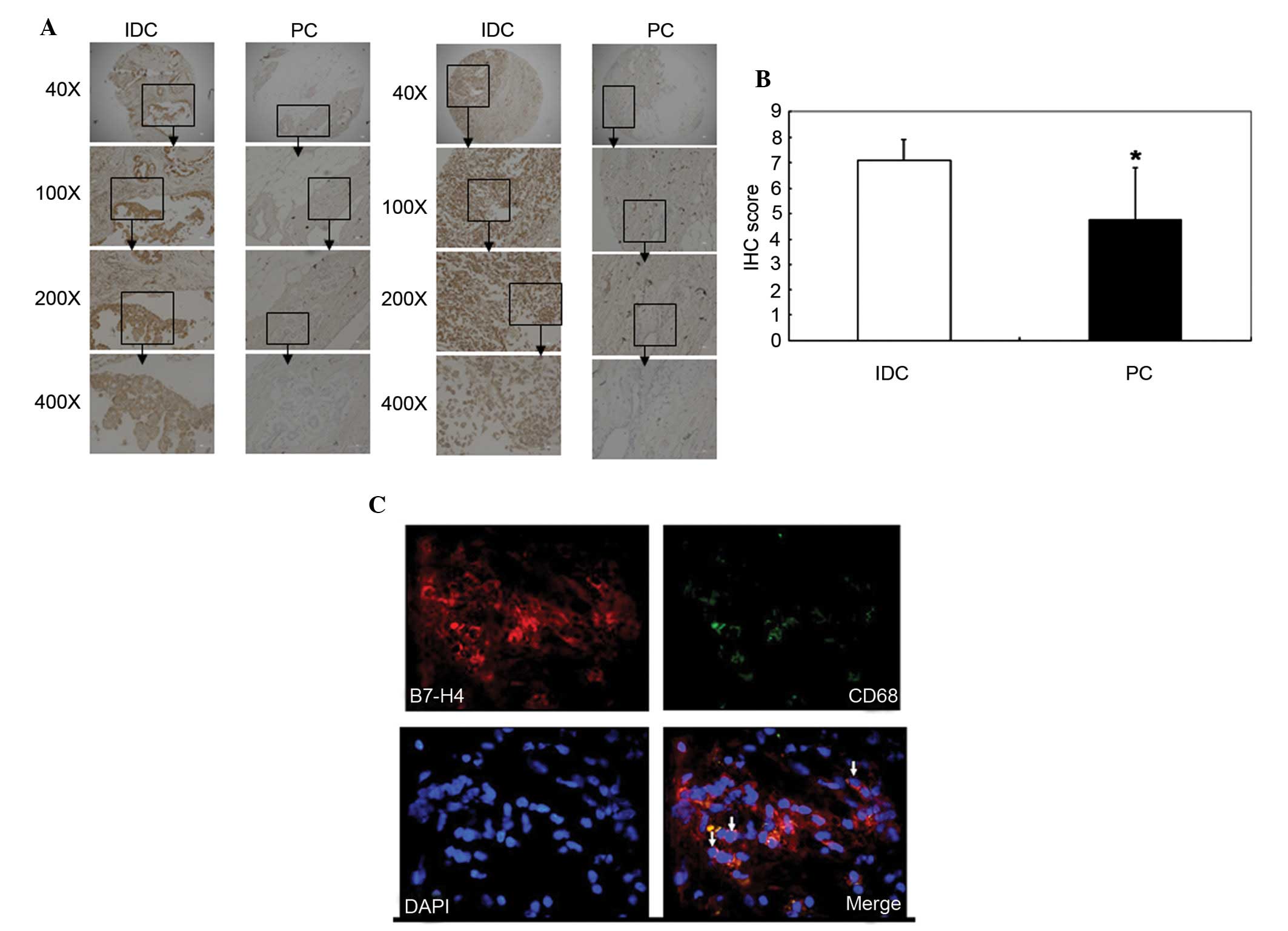
The expression levels of B7-H4 in breast IDC were
characterized in situ. A cohort of 61 patients with IDC was
selected (mean age, 53.28±7.79 years). Table I summarizes the clinical
characteristics of the cohort. Paraffin-embedded sections of IDC
and PC samples were stained with a B7-H4 antibody. IHC scores were
calculated and used to determine B7-H4 expression. B7-H4 had a
higher expression level in the IDC tissues compared with the PC
tissues (P=0.011; Fig. 1A and B
and Table I). The positive rate of
B7-H4 expression was also significantly higher in the tumor
environment compared with the pericarcinomatous tissue as
determined by χ2 analysis (P<0.01; data not shown).
No significant difference was identified between B7-H4 expression
levels and AJCC staging in patients with IDC (P>0.05). In
addition, the expression levels of estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor-2 (HER2) were detected in 31 patients. There was a
significant difference in B7-H4 expression between IDC and PC
tissues in ER-, PR- and HER2-positive and negative patients
(P<0.05; Table II). However,
when examining IDC tissues alone, no significant difference in
B7-H4 expression was identified between ER-, PR- and HER2-positive
and negative patients (P>0.05; Table II).
 | Table IClinical characteristics of patients
with IDC and the association between B7-H4 expression in IDC and PC
tissues. |
Table I
Clinical characteristics of patients
with IDC and the association between B7-H4 expression in IDC and PC
tissues.
| Variable | IDC | PC | P-value |
|---|
| Clinical
staginga | | | |
| I | 5 | N/A | N/A |
| II | 31 | N/A | N/A |
| III | 14 | N/A | N/A |
| ER | | | |
| + | 19 | N/A | N/A |
| − | 12 | N/A | N/A |
| PR | | | |
| + | 15 | N/A | N/A |
| − | 16 | N/A | N/A |
| HER2 | | | |
| + | 12 | N/A | N/A |
| − | 19 | N/A | N/A |
| Age (years) | 53.28±7.79 | N/A | N/A |
| B7-H4
expressionb | 7.10±0.78 | 4.75±2.08 | 0.011c |
 | Table IIAssociation between clinical
characteristics of breast IDC and B7-H4 expression (n=31). |
Table II
Association between clinical
characteristics of breast IDC and B7-H4 expression (n=31).
| Variable | Expression | IDC | PC | P-value |
|---|
| ER | + | 7.05±0.90 | 5.00±1.79 | 0.002a |
| − | 7.08±0.92 | 5.00±1.83 | 0.020a |
| | | | 0.952b |
| PR | + | 6.93±0.87 | 4.93±1.70 | 0.005a |
| − | 7.19±0.91 | 5.06±1.94 | 0.006a |
| | | | 0.446b |
| HER2 | + | 6.92±0.94 | 4.75±2.38 | 0.045a |
| − | 7.16±0.89 | 5.16±1.55 | 0.001a |
| | | | 0.589b |
Immunofluorescence analysis detected B7-H4
expression in IDC tissue. B7-H4 was shown to be expressed on the
surface of macrophages, which were CD68+; therefore, it
is possible that B7-H4 was not solely expressed on the surface of
breast cancer cells but also on macrophages in the breast IDC
microenvironment (Fig. 1C).
M0, M1 and M2 cells express various
levels of B7-H4
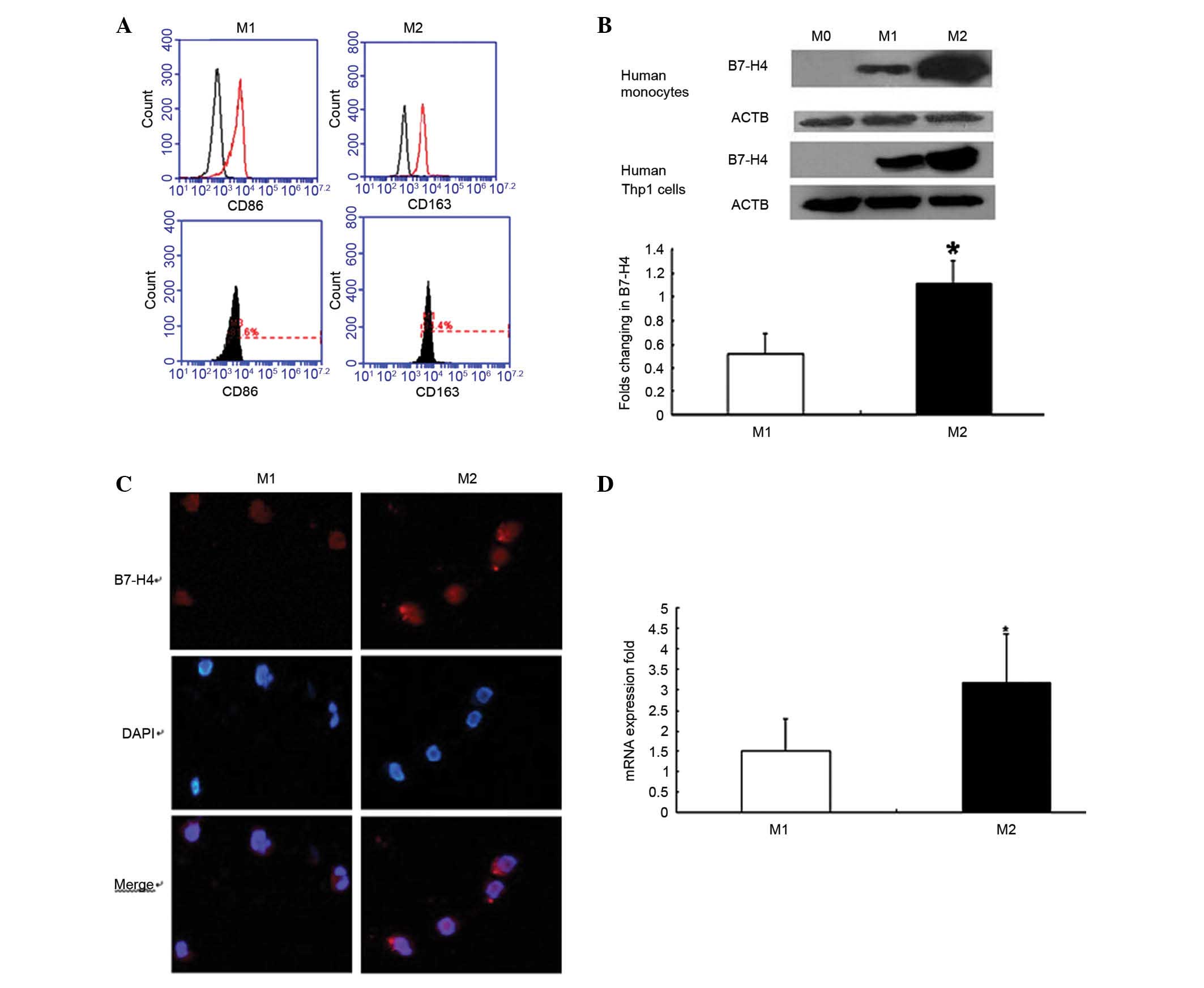
The CD14+ monocytes from PBMC were
assessed for purity in the present study, and the proportion of
CD14+ cells was >97% (data not shown). Distinctive
surface markers were also highly expressed on macrophages; CD86 was
highly expressed on M1 cells and CD163 was highly expressed on M2
cells (Fig. 2A).
Western blot analysis demonstrated that the
expression levels of B7-H4 were significantly higher in M2 cells
compared with in M1 cells, in human monocytes and Thp1 cells
(P<0.05; Fig. 2B). The
verification of the western blot analysis, using a B7-H4 antibody
from a different supplier, confirmed this result (data not
shown).
Immunofluorescence analysis confirmed that M1 and M2
macrophages expressed B7-H4 (Fig.
2C). B7-H4 expression differed on the surface of M0, M1 and M2
cells, as detected by flow cytometry. RT-qPCR analysis revealed
that B7-H4 expression was higher in M2 cells compared with in M1
cells (P<0.05; Fig. 2D).
IL-6 and -10 are highly expressed in IDC
tissues
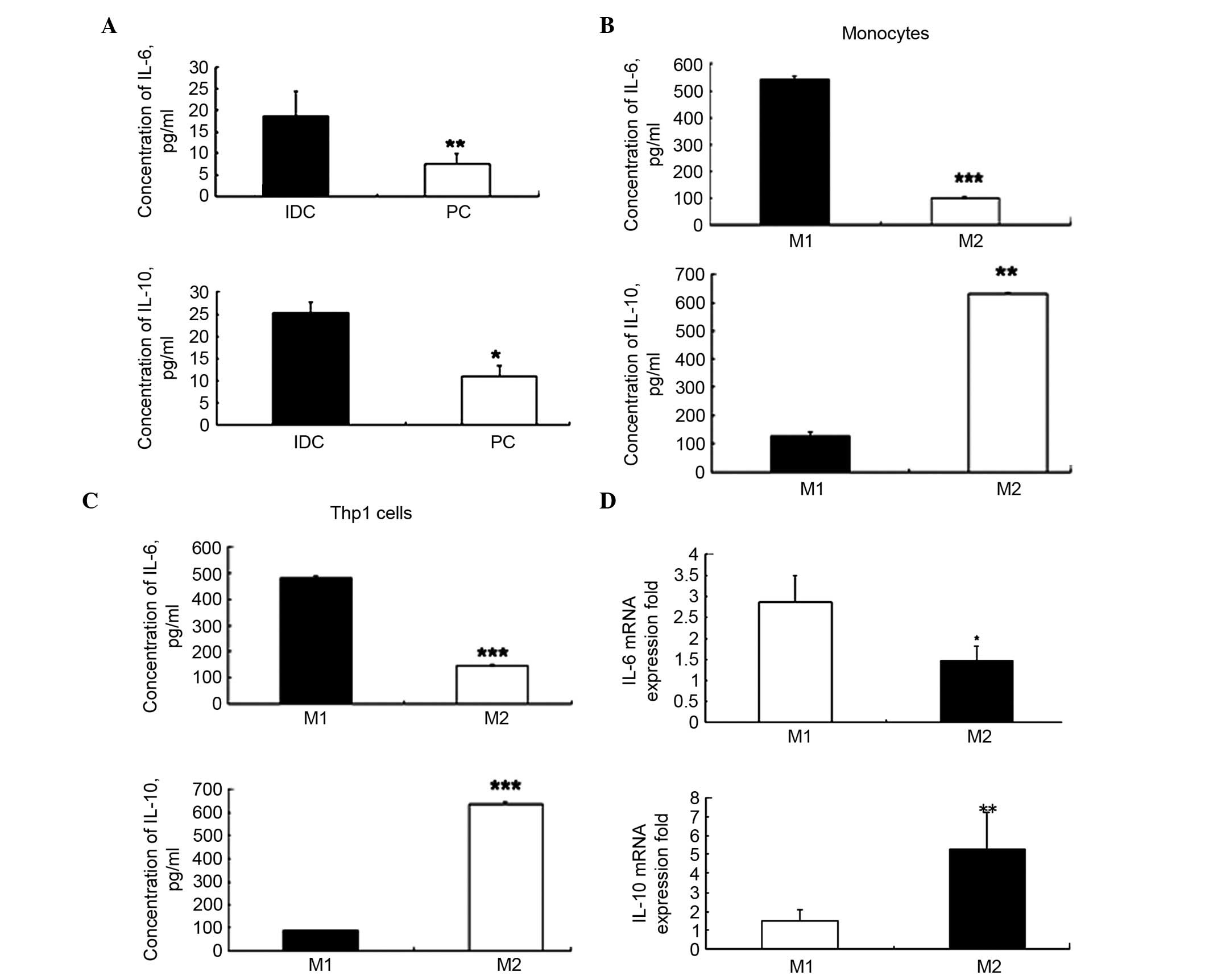
The present study demonstrated that IL-6 and -10
levels were significantly higher in the IDC tissues when compared
with the PC tissues (P=0.006 and P=0.031; Fig. 3A). There was a significant
difference in the average concentrations of IL-6 (18.72±5.69 vs.
5.80±2.96 pg/ml; P=0.006) and IL-10 (27.39±3.87 vs. 11.64±1.77
pg/ml; P=0.031) between IDC and PC tissues. These results suggest
that the levels of IL-6 and -10 were three-fold higher in IDC
tissues compared with in PC tissues.
High expression levels of IL-6 in the M1
phenotype and IL-10 in the M2 phenotype
The levels of IL-6 and -10 in M1 and M2 macrophages
obtained from human Thp1 cells and PBMC CD14+ monocytes
were detected. The ELISA results demonstrated that in M1 cells IL-6
was significantly upregulated compared with in M2 cells
(540.54±13.50 vs. 144.63±3.24 pg/ml; P=0.00018; Fig. 3B). In addition, IL-10 was
significantly decreased in M1 cells compared with in M2 cells
(124.61±14.21 vs. 628.48±4.98 pg/ml, P=0.0012; Fig. 3B). Similar levels were observed in
the Thp1 cell line (IL-6, P=0.00017; IL-10, P=0.00012; Fig. 3C). As IL-6 is termed a
representative cytokine for the M1 phenotype in cells and IL-10 as
one for M2 cells (13), the
obtained results fulfilled the expectation of polarization in
macrophages.
The RT-qPCR analysis also revealed that the mRNA
expression levels of IL-6 were significantly higher in the M1
phenotype when compared with the M2 phenotype (P<0.05; Fig. 3D). The mRNA expression levels of
IL-10 were significantly higher in the M2 phenotype compared with
the M1 phenotype (P<0.01, Fig.
3D). These results were similar to the results of the ELISA
analysis.
Discussion
Macrophages are important for tumorigenesis due to
their secretion of specific cytokines and proteases (17,18).
The polarization of macrophages may also affect the progression and
metastasis of cancer, including breast cancer (19–22).
A previous study functionally classified macrophages into M1 and M2
phenotypes (23). M1 cells, which
express CD86 and major histocompatibility complex II, are capable
of secreting IL-6 and nitric oxide synthases. However, M2 cells,
which express CD163, secrete IL-10 and transforming growth factor-β
(TGF-β). According to a previous study, macrophages may be
stimulated by IL-10, IL-4 and TGF-β in the tumor microenvironment
in order to induce the M2 phenotype (13). Due to the characteristics of
macrophages in the tumor microenvironment, overexpression of the
suppressive co-stimulatory molecule B7-H4 is a possibility.
As a member of the B7 family, B7-H4 has an
inhibitory effect on cellular immune responses. Therefore, B7-H4
should be highly expressed in the tumor microenvironment, which is
under a state of immune suppression. Previous studies have detected
high B7-H4 expression levels in various tumors, including ovarian,
lung and breast cancer (24–28).
Furthermore, a previous study demonstrated that B7-H4-expressing
macrophages were significantly higher in peripheral blood from
patients with cancer compared with those from healthy donors
(25). Cytokines in peripheral
blood may be the induction factors for B7-H4-expressing macrophages
in patients with cancer. Therefore, the present study selected
human monocytes from peripheral blood and the Thp1 monocyte cell
line as targets. The macrophages were stimulated into two different
polarization types in vitro. As determined by the detection
of surface markers and cytokine secretion, the polarization of
macrophages was successful in the present study.
In the tumor microenvironment, M1 cells may limit
the development and progression of tumors. Conversely, M2 cells are
able to induce tumor promotion (29,30).
Due to the inhibitory function of B7-H4, it was hypothesized that
M2 macrophages would express higher levels of B7-H4 compared with
M1 cells. In the present study, both M1 and M2 human macrophages
expressed B7-H4; however, compared with M1 cells, M2 cells
exhibited significantly higher levels of B7-H4.
The high expression of B7-H4 in IDC tissues may be
due to specific cytokines. Therefore, the present study
investigated the levels of IL-6 and -10, and demonstrated that they
were higher in IDC tissues compared with PC tissues. This has also
been observed in ovarian cancer research, which has revealed that
IL-6 and -10 induced B7-H4 expression in macrophages (23). Different concentrations and mRNA
expression levels of IL-6 and -10 were observed in the M1 and M2
cells in the present study. Future research will aim to determine
the source of IL-6 and -10 in the breast cancer microenvironment,
and the association between the expression levels of IL-6 and -10
and B7-H4 expression in macrophages of different polarization
states. IL-6 and -10 may stimulate signal transducer and activator
of transcription 3 (Stat3) via the janus kinase/Stat signaling
pathway (31,32). Therefore, IL-6 and -10 may be
regulated with B7-H4, and the association between B7-H4 and IL-6 or
IL-10 signaling regulation should be investigated in future
studies.
As a co-inhibitory molecule, B7-H4 has the ability
to regulate the immune response in the tumor microenvironment. In
addition, B7-H4, as a negative stimulatory molecule, has an
inhibitory capacity for activation of the immune response, contrary
to the combination of B7-CD28 (33). Therefore, B7-H4 may be a potential
target for immunotherapy in various tumors, including breast cancer
(34).
In conclusion, the present study indicated that
B7-H4 may be overexpressed on the majority of cells in the IDC
microenvironment, including macrophages. In vitro
experiments revealed that M1 and M2 cells expressed B7-H4. Compared
with M1 cells, M2 cells exhibited significantly higher expression
levels of B7-H4. In addition, the expression levels of IL-6 and -10
were higher in human breast IDC tissues compared with breast distal
PC tissues, and various levels of IL-6 and -10 were observed in the
M1 and M2 macrophages.
Abbreviations:
|
IDC
|
infiltrating ductal carcinoma
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor
receptor-2
|
|
AJCC
|
American Joint Committee on Cancer
|
|
IL
|
interleukin
|
|
IFN γ
|
interferon γ
|
|
GM-CSF
|
granulocyte-macrophage
colony-stimulating factor
|
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
PBMC
|
peripheral blood monouclear cells
|
|
LPS
|
lipopolysaccharides
|
|
IHC
|
immunohistochemistry
|
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81201594), the China
Postdoctoral Science Foundation funded project (grant no.
2012M520036) and the Postdoctoral Science Foundation of
Heilongjiang Province China (grant no. LRB 2011300).
References
|
1
|
Evans AJ, Pinder SE, Snead DR, Wilson AR,
Ellis IO and Elston CW: The detection of ductal carcinoma in situ
at mammographic screening enables the diagnosis of small, grade 3
invasive tumours. Br J Cancer. 75:542–544. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Standish LJ, Sweet ES, Novack J, Wenner
CA, Bridge C, Nelson A, Martzen M and Torkelson C: Breast cancer
and the immune system. J Soc Integr Oncol. 6:158–168. 2008.
|
|
3
|
Watanabe MA, Oda JM, Amarante MK and Cesar
Voltarelli J: Regulatory T cells and breast cancer: Implications
for immunopathogenesis. Cancer Metastasis Rev. 29:569–579. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grieco V, Rondena M, Romussi S, Stefanello
D and Finazzi M: Immunohistochemical characterization of the
leucocytic infiltrate associated with canine seminomas. J Comp
Pathol. 130:278–284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carvalho MI, Pires I, Prada J and Queiroga
FL: A role for T-lymphocytes in human breast cancer and in canine
mammary tumors. Biomed Res Int. 2014:1308942014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gordon S and Taylor PR: Monocyte and
macrophage heterogeneity. Nat Rev Immunol. 5:953–964. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sica A and Bronte V: Altered macrophage
differentiation and immune dysfunction in tumor development. J Clin
Invest. 117:1155–1166. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Biswas SK, Sica A and Lewis CE: Plasticity
of macrophage function during tumor progression: Regulation by
distinct molecular mechanisms. J Immunol. 180:2011–2017. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian ZB and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sica GL, Choi IH, Zhu G, Tamada K, Wang
SD, Tamura H, Chapoval AI, Flies DB, Bajorath J and Chen L: B7-H4,
a molecule of the B7 family, negatively regulates T cell immunity.
Immunity. 18:849–861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zang X, Loke P, Kim J, Murphy K, Waitz R
and Allison JP: B7x: A widely expressed B7 family member that
inhibits T cell activation. Proc Natl Acad Sci USA.
100:10388–10392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Biswas SK and Mantovani A: Macrophage
plasticity and interaction with lymphocyte subsets: Cancer as a
paradigm. Nat Immunol. 11:889–896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maitra A, Ashfaq R, Gunn CR, Rahman A, Yeo
CJ, Sohn TA, Cameron JL, Hruban RH and Wilentz RE: Cyclooxygenase 2
expression in pancreatic adenocarcinoma and pancreatic
intraepithelial neoplasia: An immunohistochemical analysis with
automated cellular imaging. Am J Clin Pathol. 118:194–201. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar M and Nandi S: Development of a SYBR
Green based real-time PCR assay for detection and quantitation of
canine parvovirus in faecal samples. J Virol Methods. 169:198–201.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kabayiza JC, Andersson ME, Welinder-Olsson
C, Bergström T, Muhirwa G and Lindh M: Comparison of rectal swabs
and faeces for real-time PCR detection of enteric agents in Rwandan
children with gastroenteritis. BMC Infect Dis. 13:4472013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng J, Huo DH, Kuang DM, Yang J, Zheng L
and Zhuang SM: Human macrophages promote the motility and
invasiveness of osteopontin-knockdown tumor cells. Cancer Res.
67:5141–5147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gocheva V, Wang HW, Gadea BB, Shree T,
Hunter KE, Garfall AL, Berman T and Joyce JA: IL-4 induces
cathepsin protease activity in tumor-associated macrophages to
promote cancer growth and invasion. Genes Dev. 24:241–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Zhang Z, Chen C, Liu Y, Si Q,
Chuang TH, Li N, Gomez-Cabrero A, Reisfeld RA, Xiang R and Luo Y:
MicroRNA-19a-3p inhibits breast cancer progression and metastasis
by inducing macrophage polarization through downregulated
expression of Fra-1 proto-oncogene. Oncogene. 33:3014–3023. 2014.
View Article : Google Scholar
|
|
20
|
Jang JY, Lee JK, Jeon YK and Kim CW:
Exosome derived from epigallocatechin gallate treated breast cancer
cells suppresses tumor growth by inhibiting tumor-associated
macrophage infiltration and M2 polarization. BMC Cancer.
13:4212013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oghumu S, Varikuti S, Terrazas C, Kotov D,
Nasser MW, Powell CA, Ganju RK and Satoskar AR: CXCR3 deficiency
enhances tumor progression by promoting macrophage M2 polarization
in a murine breast cancer model. Immunology. 143:109–119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia X, Yu F, Wang J, Iwanowycz S, Saaoud
F, Wang Y, Hu J, Wang Q and Fan D: Emodin suppresses pulmonary
metastasis of breast cancer accompanied with decreasedmacrophage
recruitment and M2 polarization in the lungs. Breast Cancer Res
Treat. 148:291–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mills CD, Kincaid K, Alt JM, Heilman MJ
and Hill AM: M-1/M-2 macrophages and the Th1/Th2 paradigm. J
Immunol. 164:6166–6173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kryczek I, Zou L, Rodriguez P, Zhu G, Wei
S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al: B7-H4
expression identifies a novel suppressive macrophage population in
human ovarian carcinoma. J Exp Med. 203:871–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen C, Zhu YB, Shen Y, Zhu YH, Zhang XG
and Huang JA: Increase of circulating B7-H4-expressing CD68+
macrophage correlated with clinical stage of lung carcinomas. J
Immunother. 35:354–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salceda S, Tang T, Kmet M, Munteanu A,
Ghosh M, Macina R, Liu W, Pilkington G and Papkoff J: The
immunomodulatory protein B7-H4 is overexpressed in breast and
ovarian cancers and promotes epithelial cell transformation. Exp
Cell Res. 306:128–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tringler B, Zhuo S, Pilkington G, Torkko
KC, Singh M, Lucia MS, Heinz DE, Papkoff J and Shroyer KR: B7-h4 is
highly expressed in ductal and lobular breast cancer. Clin Cancer
Res. 11:1842–1848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mugler KC, Singh M, Tringler B, Torkko KC,
Liu W, Papkoff J and Shroyer KR: B7-h4 expression in a range of
breast pathology: Correlation with tumor T-cell infiltration. Appl
Immunohistochem Mol Morphol. 15:363–370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raes G, Brys L, Dahal BK, Brandt J,
Grooten J, Brombacher F, Vanham G, Noël W, Bogaert P, Boonefaes T,
et al: Macrophage galactose-type C-type lectins as novel markers
for alternatively activated macrophages elicited by parasitic
infections and allergic airway inflammation. J Leukoc Biol.
77:321–327. 2005. View Article : Google Scholar
|
|
30
|
Loke P, Nair MG, Parkinson J, Guiliano D,
Blaxter M and Allen JE: IL-4 dependent alternatively-activated
macrophages have a distinctive in vivo gene expression phenotype.
BMC Immunol. 3:72002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang L, Yi T, Kortylewski M, Pardoll DM,
Zeng D and Yu H: IL-17 can promote tumor growth through an
IL-6-Stat3 signaling pathway. J Exp Med. 206:1457–1464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kortylewski M, Kujawski M, Wang T, Wei S,
Zhang S, Pilon-Thomas S, Niu G, Kay H, Mulé J, Kerr WG, et al:
Inhibiting Stat3 signaling in the hematopoietic system elicits
multicomponent antitumor immunity. Nat Med. 11:1314–1321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen L: Co-inhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smith JB, Stashwick C and Powell DJ Jr:
B7-H4 as a potential target for immunotherapy for gynecologic
cancers: A closer look. Gynecol Oncol. 134:181–189. 2014.
View Article : Google Scholar : PubMed/NCBI
|