Introduction
Colon (or colorectal) cancer is a common type of
malignant tumor, which is characterized by abnormal cell
proliferation without control in the lining of the colon and
rectum. It is the third most common type of cancer in Western
countries. Approximately 136,830 cases of colorectal cancer were
diagnosed, and ~50,310 patients succumbed to colorectal cancer in
the United States in 2014 (1). The
incidence, diagnostics and therapeutic options have also changed in
the last decades in China. In the past ten years, the incidence
rate has doubled and it reached ~13% in 2015. In addition, clinical
studies indicated that when screened for the disease, African
Americans tend to be diagnosed with colorectal cancer at a younger
age than Caucasians (2). When
colon cancer is diagnosed in the early stages, it is curable and
colon resection is an appropriate treatment for non-muscle invasive
colon cancer. However, surgery is not curative when cancer cells
have invaded into the muscle, and the prognosis for patients with
colon cancer at a more advanced stage remains poor. Therefore,
chemotherapy is an alternative treatment strategy. Exhibiting a
selective cytotoxicity to tumor cells, use of α-tocopherol
ether-linked acetic acid (α-TEA) has as a chemotherapeutic agent
has been a focus of in vivo and in vitro studies in
multiple types of cancer (3–8).
However, the exact impact and the mechanism underlying its effect
remains to be established.
Rho family members of GTPases have been reported to
be important in the regulation of certain biological functions
associated with cell movement and actin cytoskeleton rearrangement
(9). RhoA, as a member of GTPase
family, is involved in cell-cycle progression, gene transcription,
cell polarity and focal adhesion complex assembly (10). Similar to other GTPases, RhoA can
be changed from active to inactive states by the exchange between
GTP-bound and GDP-bound states. RhoA and its downstream effectors,
such as Rho-associated protein kinase (ROCK) and myosin light chain
(MLC), are closely associated with multiple cellular biological
functions such as cytoskeleton reorganization, smooth muscle
contraction, cell motility, proliferation and protein expression
(11–16). Rho-kinase modulates cell stress
fiber formation and intercellular connections to influence
metastasis, proliferation or anchorage-independent growth of tumor
cells (17–26). Considering that high level
expression of RhoA is detected in a number of malignant tumors, the
regulation of RhoA activity has been applied to cancer control due
to its participation in cancer-associated signaling pathways
(27–30). The protein expression of RhoA is
markedly higher in prostate cancer cells than in normal prostate
cells, as increased RhoA protein expression is associated with
abnormal cell growth (27). RhoA
silencing decreased androgen-regulated prostate cancer cell
survival and motility (27). RhoA
has also been shown to be activated in gastric cancer cells;
additionally, downregulation of RhoA activity prevented the
abnormal proliferation of gastric cancer cells by targeting
RhoA-mammalian Diaphanous 1 signaling (28). Furthermore, RhoA expression has
been found to be markedly increased in testicular tumor tissue
compared with that in normal tissue; protein expression of RhoA and
ROCK were also higher in advanced cancer stages compared with that
in early stage cancer (31,32).
The present study investigated the impact of α-TEA
on the proliferation and motility of colon cancer cells, and
determined whether the RhoA/ROCK signaling pathway is involved in
mechanism underlying the effect of α-TEA.
Materials and methods
Cell culture
HCT116 human colon carcinoma and SW480 human colon
adenocarcinoma cells (American Type Culture Collection; Manassas,
VA, USA) were grown in high glucose Dulbecco's modified Eagle's
medium (DMEM; Gibco, Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS; Lonza,
Levallois-Perret, France) and added to 100 µg/ml
penicillin/streptomycin. Cell lines were cultured at 37°C in a 5%
CO2 incubator.
Proliferation assay
Cell proliferation was assessed by MTT dye
conversion. Briefly, 104 cells were seeded in a 96-well
flat bottom plate after transfection. Cells were cultured in a
37°C, 5% CO2 incubator for 24 h, followed by another 4 h
after 20 µl MTT (5 mg/ml) was added to each well. Then, 200
µl dimethylsulfoxide (DMSO) was added to the washed well to
lyse cells. Absorbance was detected using an enzyme-linked
immunosorbent assay spectrophotometer at 490 nm.
Migration and invasion assay
Cell migration was assessed by a Transwell assay
using 6.5 mm chambers with 8-µm pore membranes. Then, 600
µl DMEM medium with or without α-TEA, which was synthesized
using a combination of previously described methods was added to
the lower chamber (33,34). The suspension of 5×104
cells in 100 µl DMEM medium with 1% fetal calf serum
(Sigma-Aldrich, St. Louis, MO, USA) was plated into the upper
chamber with or without α-TEA. After 20 h, cells on the
undersurface of the polycarbonate membranes were stained with
crystal violet (Amresco LLC, Cleveland, OH, USA) for 10 min at room
temperature and six randomly selected fields were observed with a
BX50 light microscope (Olympus, Tokyo, Japan) at ×100
magnification. The same procedure was conducted for the invasion
assay, except that 70 µl of 1 mg/ml Matrigel (BD
Biosciences) was added into the upper surface of the membrane and
the incubation time was prolonged to 24 h.
Transfection of HCT116 and SW480 cells by
anti-RhoA small interfering (si)RNA
A small RNA that does not match any known genes was
used as an siRNA control (Ambion, Austin, TX, USA). Cells
(2×106) were then transfected with RhoA or control
siRNAs (Ambion) using Lipofectamine-2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in 100-mm diameter cell culture dishes. At 24 h
after transfection, the cells were cultured in 100 mm dishes and
grown for 24 h prior to treatment with 10 µM α-TEA.
Reverse transcription-quantitative
polymerase chain reaction
RNA was extracted using the Total RNA Isolation kit
(A&A Biotechnology, Gdynia, Poland) according to the
manufacturer's instructions. Total RNA generated cDNA by reverse
transcription PCR using the RevertAid First Strand cDNA synthesis
kit (Fermentas International, Vilnius, Lithuania). The cDNA was
amplified using TaqMan Gene Expression Assay (Applied Biosystems,
Foster City, CA, USA) in the system containing specific primers for
RhoA and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and
FAM-labeled fluorescent probes. The following primers and probes
were used: Forward: 5′-AATGACGAGCACACGAGACGGGA-3′, reverse:
5′-ATGTACCCAAAAGCGCCAATCCT-3′. and TaqMan fluorogenic probe:
5′-CCCACCCTCTC-CGGTGTGTCTGTCGGTT-3′ for RhoA; and forward:
5′-CGACTTCAACAGCAACTCCCACTCTTCC-3′, reverse:
5′-TGGGTGGTCCAGGGTTTCTTACTCCTT-3′ and fluorogenic probe:
5′-ATGCCCTCCCCCATGCCATCCTGCGT-3′ for the GAPDH gene. The genes were
amplified by a first step of 120 sec at 95°C, followed by 45 cycles
of 30 sec at 95°C, 30 sec at 60°C, and 30 sec at 72°C. The
real-time fluorescence detection was performed with the ABI PRISM
7700 Sequence Detector (Applied Biosystem, Thermo Fisher
Scientific, Inc.). The quantity of mRNA expression for RhoA was
calculated using the formula 2−ΔΔCq and was normalized
to the level of GAPDH (35). The
relative quantity of mRNA in siRNA-treated cells was presented as
the relative value of mRNA in the untreated cells.
RhoA activity assays
The activity of RhoA was assessed in colon cancer
cells by a pull-down assay for GTP-bound RhoA (36). GTP-bound RhoA was precipitated from
cell lysates with Rhotekin RBD (Upstate Biotechnology, Lake Placid,
NY, USA). Active RhoA and total RhoA were detected by western
blotting using an anti-RhoA mAb.
MLC phosphorylation
Cells were starved for 24 h in serum-free DMEM
medium, and then were treated with or without α-TEA for 1 h in 5%
CO2 at 37°C. The cells were lysed with cell lysis buffer
B (1% Triton X-100, 30 mM HEPES NaOH, pH 7.4; 1 mM EGTA, 20 mM NaF,
1 mM Na3VO4, 40 mM
Na4P2O7, 100 mM NaCl, 10
µg/ml aprotinin, 10 µg/ml leupeptin, 10 µg/ml
pepstatin and 1 mM PMSF). The supernatants from the centrifuged
(10,000 × g for 10 min) cell lysates were collected and then were
assayed by western blotting using anti-MLC or anti-pMLC
antibodies.
Western blotting
Cells were washed with phosphate-buffered saline and
lysed in lysis buffer (50 mM HEPES pH 8.0; 1% Triton X-100, 1.5 mM
EDTA, 150 mM NaCl, 1 mM Na3VO4, 50 mM NaF, 1
mM MgCl2, 20 mM β-glycerophosphate, 10% glycerol, 1
µM pepstatin A, 1 mM phenylmethylsulphonyl fluoride and 10
µg/ml aprotonin). Cell lysate was centrifuged at 10,000 × g
for 10 min, and the supernatant was collected. Protein samples were
quantified using a bicinchoninic acid assay Protein Assay kit
(Beyotime, Beyotime Institute of Biotechnology, Jiangsu, China).
Total protein samples (50 µg) were separated by 12% sodium
dodecyl sulfate-polyacryl-amide gel electrophoresis and transferred
to polyvinylidene fluoride membranes (EMD Millipore, Beverly, MA,
USA). Rabbit anti-RhoA (1:1,000, cat. no. sc-179) and mouse
anti-GAPDH antibodies (1:5,000, cat. no. sc-365062) (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA), rabbit anti-MLC (1:1,000,
cat. no. #3672), rabbit anti-phosphorylated MLC (pMLC; 1:1,000,
cat. no. 3674S) antibodies, and horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG secondary antibody (1:2,000,
cat. no. #7074) (Cell Signaling Technology, Beverly, MA, USA) and
HRP conjugated horse anti-mouse IgG secondary antibody (1:2,000,
cat. no. #7076, Cell Signaling Technology) were used. Enhanced
chemiluminescence-detecting reagent (Amersham Biosciences,
Buckinghamshire, UK) was used for development. The protein blots
were quantified by densitometry using Quantity One software v 4.5.0
(Bio-Rad Laboratories Inc., Hercules, CA, USA), and the amounts
were expressed relative to the internal reference GAPDH.
Statistical analysis
SPSS version 11.0 (SPSS Inc., Chicago, IL, USA) was
used to analyze the experimental data. Data are presented as the
mean ± standard error of the mean. All of the experiments were
repeated in at least three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
α-TEA attenuates the migration and
invasion of colon cancer cells
To investigate whether α-TEA affects the motility of
colon cancer cells, migration and invasion assays were conducted
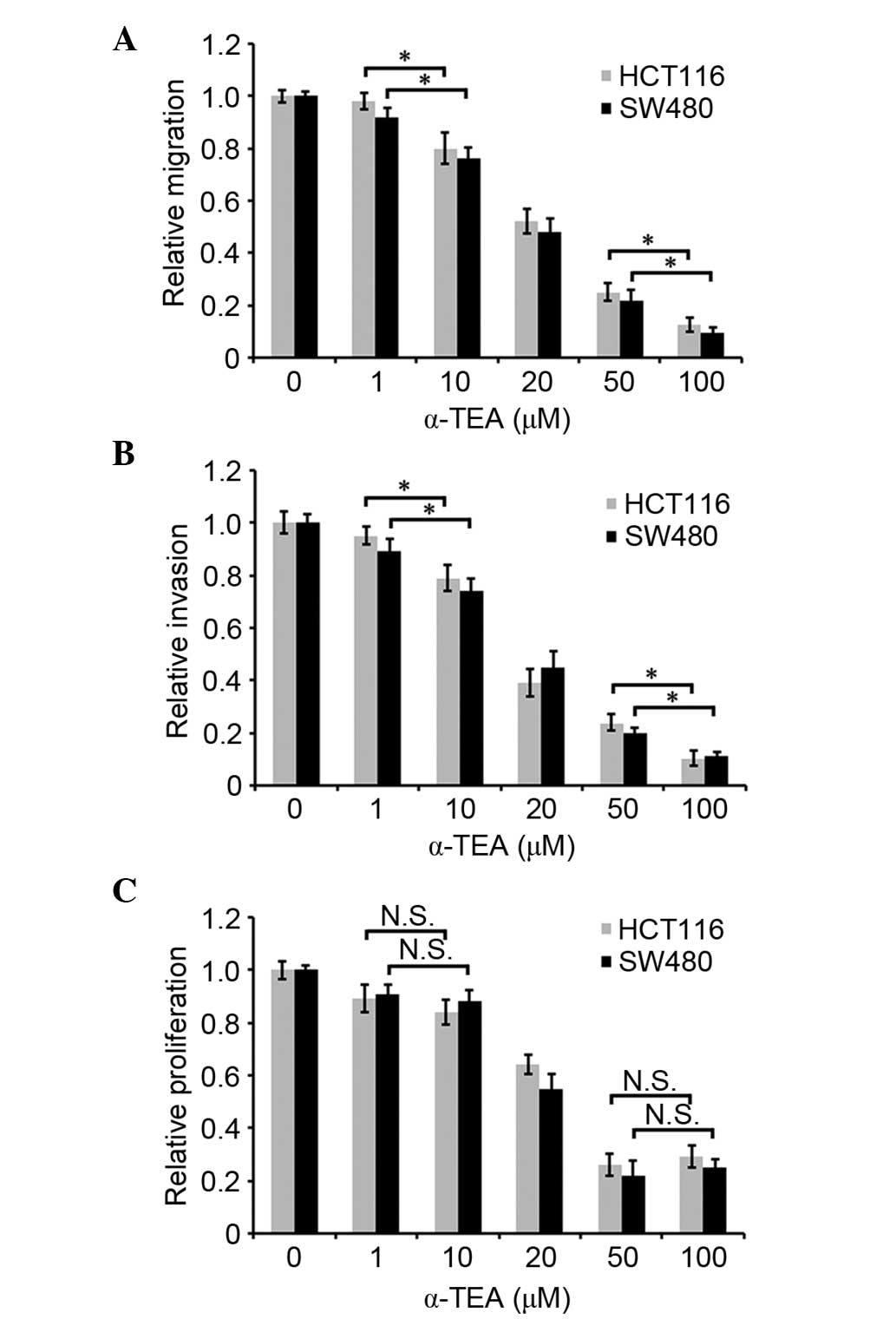
in vitro. As shown in Fig. 1A
and B, α-TEA attenuated cellular migration and invasion in
HCT116 and SW480 cells in a dose-dependent manner between 1
µM and 100 µM. Cell proliferation was assessed by an
MTT assay to determine whether it was regulated by α-TEA at various
concentrations for 24 h. α-TEA decreased the cell proliferation at
20, 50 or 100 µM for 24 h, and α-TEA mediated the most
significant decrease of cell proliferation at 50 µM
concentration compared with non-treated control group. (Fig. 1C). Cell proliferation was not
indicated to be significantly different between 1 and 10 µM
α-TEA treatment (Fig. 1C). These
data demonstrated that α-TEA inhibited cell migration and invasion
independently of its role in cell proliferation.
α-TEA decreases RhoA activity
It was then investigated how α-TEA attenuates cell
proliferation and motility. RhoA, as a Rho GTPase, participates in
the regulation of cell viability and cell-cycle progression. To
determine whether α-TEA affects the activity of RhoA in colon
cancer cells, GTP-bound RhoA was detected in HCT116 colon cancer
cells by a pull-down assay. The results demonstrated that the
activity of RhoA was decreased by 10 µM α-TEA treatment, and
the activity reached the trough at 30 min followed by a gradual
increase close to the initial level (Fig. 2). These results indicated that
α-TEA inhibited RhoA activity in HCT116 colon cancer cells.
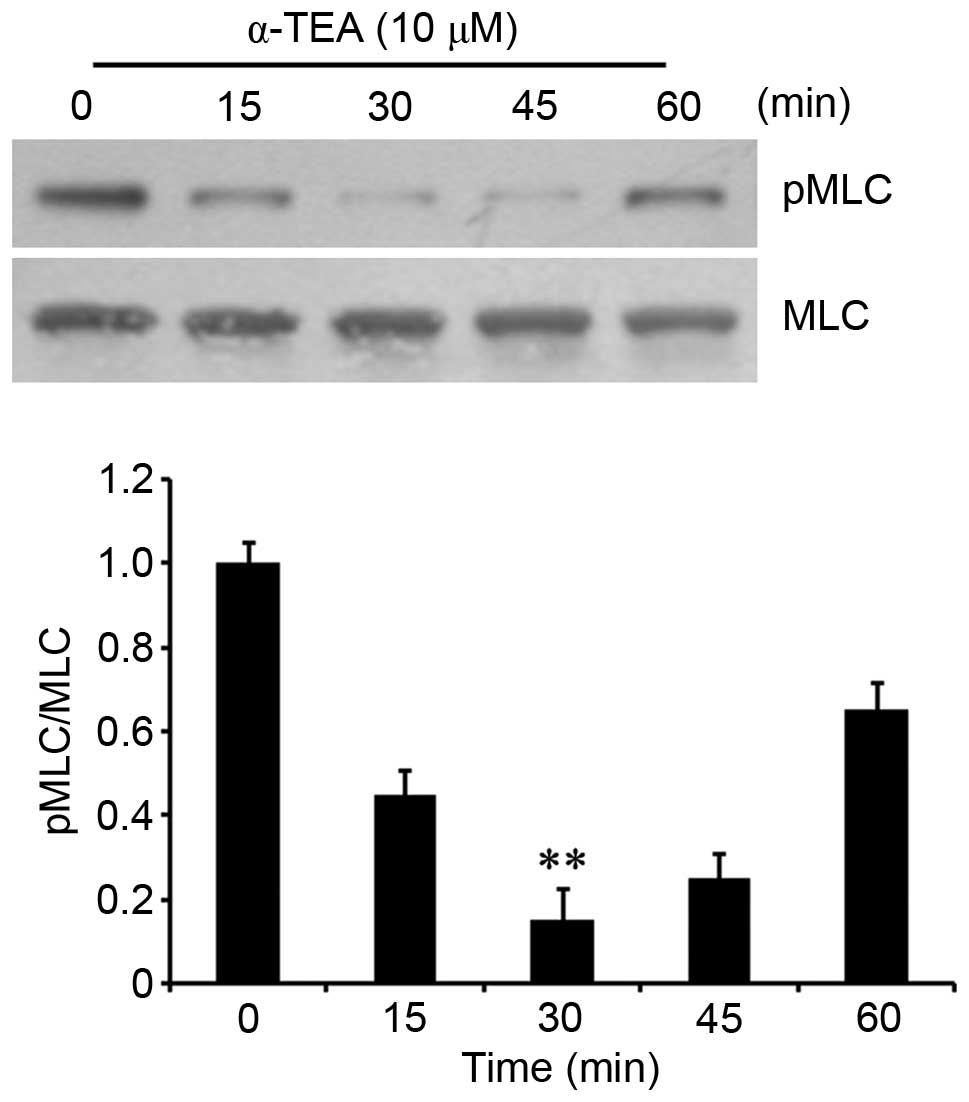
α-TEA downregulates MLC
phosphorylation
As RhoA can activate ROCK and then contribute to the
phosphorylation of MLC (14), its
phosphorylation in α-TEA-treated cells was determined. Western
blotting showed that the phosphorylation of MLC decreased
transiently in cells treated with 10 µM α-TEA, reaching a
trough at 30 min, and then followed by a gradual increase (Fig. 3). These results indicated that
α-TEA decreased MLC phosphorylation.
RhoA and ROCK inhibitors, and RhoA siRNA
augment α-TEA-induced inhibition of motility
To verify the participation of members of the
RhoA/ROCK signaling pathway in α-TEA-induced inhibition of
motility, HCT116 and SW480 cells were treated with 10 µM
α-TEA, plus 50 µg/ml RhoA inhibitor C3 exoenzyme and 50
µM ROCK inhibitor Y27632 for 24 h. Treatment with α-TEA or
inhibitors alone led to limited decrease in cell migration and
invasion, whereas α-TEA combined with each inhibitors markedly
augmented inhibition of migration and invasion relative to single
treatments (Fig. 4A and B). α-TEA
and inhibitors of RhoA and ROCK reduced the levels of MLC
phosphorylation. Combination treatment with RhoA or ROCK inhibitors
enhanced α-TEA inhibition of MLC phosphorylation (Fig. 4C), suggesting that RhoA and ROCK
mediated α-TEA-induced downregulation of MLC phosphorylation. In
addition, RhoA siRNA significantly decreased RhoA mRNA and protein
expression (Fig. 4D). Combination
of α-TEA and RhoA siRNA acted synergistically to inhibit cell
migration (Fig. 4E), and reduced
active RhoA and MLC phosphorylation in HCT116 cells (Fig. 4F). These data indicated that α-TEA
could downregulate active RhoA and MLC phosphorylation, and that
α-TEA acted synergistically with RhoA and ROCK chemical inhibitors
to inhibit colon cancer cell motility.
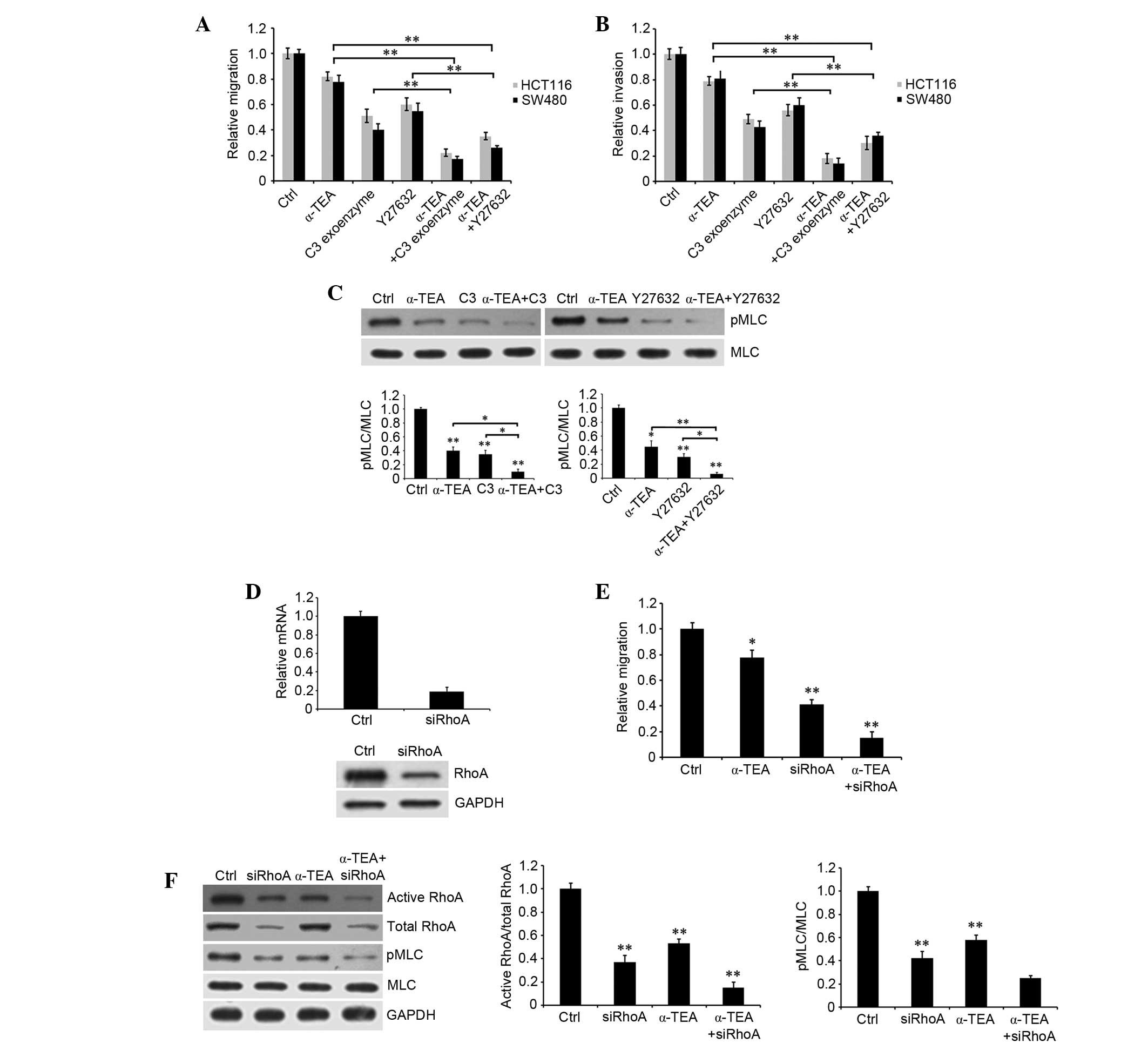 | Figure 4Effects of RhoA and ROCK inhibitors
and RhoA siRNA combined with α-TEA on the migration and invasion of
colon cancer cells. Untreated control and 10 µM
α-TEA-treated HCT116 and SW480 cells were induced with or without
50 µg/ml RhoA inhibitor C3 exoenzyme or 50 µM ROCK
inhibitor Y27632. (A) Migration and (B) invasion were detected
using a Transwell assay. (C) Impact of inhibitors of RhoA and ROCK
combined with α-TEA on MLC phosphorylation in HCT116 cells.
Untreated control and α-TEA-treated cells were induced with or
without inhibitors of RhoA and ROCK. MLC phosphorylation was
evaluated by western blotting using anti-MLC and anti-pMLC
antibodies. The blots were quantified by densitometry, and the
results are expressed as a ratio relative to the values obtained in
untreated control cells. Data are presented as the mean ± standard
error of the mean of three independent experiments.
*P<0.05 and **P<0.01. (D) Impact of
RhoA siRNA on RhoA mRNA and protein expression. HCT116 cells were
transfected with RhoA siRNA for 48 h. mRNA and protein were
extracted, and then reverse transcription-polymerase chain reaction
and western blotting were used to detect RhoA mRNA and protein
expression, respectively. (E) HCT116 cells were transfected with or
without RhoA siRNA for 48 h, and then treated with 10 µM
α-TEA and cell migration was evaluated by Transwell assay.
*P<0.05 and **P<0.01 vs. control. (F)
Activity of RhoA and MLC phosphorylation were assessed by western
blotting using anti-RhoA, anti-pMLC and anti-MLC antibodies. The
blots were quantified by densitometry. **P<0.01 vs.
control. MLC, myosin light chain; pMLC, phosphorylated MLC; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; siRNA, small interfering
RNA; α-TEA, α-tocopherol ether-linked acetic acid; ROCK,
Rho-associated protein kinase. |
RhoA and ROCK inhibitors enhance
α-TEA-induced proliferation inhibition
To investigate whether the RhoA/ROCK pathway is
associated with α-TEA-induced cell proliferation inhibition, HCT116
cells were treated with 20 µM α-TEA, plus 50 µg/ml C3
exoenzyme or 50 µM Y27632 for 24 h. Treatment with α-TEA or
an inhibitor alone resulted in a significant decrease in
proliferation. However, α-TEA in combination with each inhibitor
significantly enhanced inhibition of proliferation compared with
the single treatments (Fig. 5A and
B). Moreover, RhoA and ROCK inhibitors acted synergistically to
augment α-TEA inhibition of MLC phosphorylation, respectively
(Fig. 5A and B). These results
indicated that RhoA/ROCK signaling was involved in α-TEA-mediated
cell growth inhibition.
Discussion
Despite several treatment options, colon cancer
remains a leading cause cancer-related mortality. A major reason
for the poor prognosis of metastatic tumors is the development of
drug resistance. Thus, the development of novel antitumor agents to
prevent and treat colon cancer is required. α-TEA, a vitamin E
analogue, has chemopreventive and chemotherapeutic activities. In
recent years, it has been established that α-TEA has the ability to
inhibit tumor progression in vivo (37,38).
The antitumor activities of α-TEA have been extensively
characterized using in vitro systems. α-TEA has been
reported to be widely used in cancer treatment based on multiple
antitumor mechanisms in a variety of human cancers. α-TEA augments
the inhibition of trastuzumab on breast cancer with HER2/neu
expression (39). α-TEA inhibits
tumor growth by stimulating the anticancer immune response in
breast cancer (33). α-TEA induces
apoptosis via an increase in pro-death factors and decrease in
pro-survival factors in human prostate cancer cells (8), and via the JNK-p73-NOXA signaling
pathway in human breast cancer cells (40). α-TEA activates Fas signaling and
inhibits AKT and ERK activity, which induces the apoptosis of
cisplatin-sensitive and -resistant human ovarian cancer cells (76).
α-TEA has been reported to exhibit anti-tumor and antimetastatic
activities in cell culture and animal studies(6,41).
However, it is unclear whether α-TEA exhibits these effects on
colon cancer, and there are few studies regarding the mechanism
underlying the antimetastasis associated molecular mechanism of
α-TEA. In the present study, it was demonstrated that α-TEA
inhibited proliferation and motility of colon cancer cells and
researched the underlying mechanism of action.
RhoA expression is high in liver (42), skin (43) and colon (44) cancer. An increase in RhoA
expression is observed in conjunction with elevated RhoA activity,
poor prognosis and increased frequency of recurrence of cancer.
Furthermore, increased RhoA levels were reported in ovarian
(31), bladder (45), gastric (46), breast (47) and testicular (32) cancer. These data demonstrate that
RhoA is closely associated with cancer progression. Metastasis is a
key reason for cancer-related mortality, and is the final step in
the progression of a number of solid tumors. Migration and invasion
properties of tumor cells show cellular metastatic ability. In
order to improve the status of cancer patients, consideration of
malignant properties is required. MLC phosphorylation induces
actomyosin contraction, which is closely associated with cellular
migration and invasion (41,48,49).
In addition, RhoA can activate ROCK and stimulate the
phosphorylation of MLC (14).
Therefore, it is assumed that the inhibition of cellular migration
and invasion mediated by α-TEA may result from abnormal
phosphorylation of MLC via RhoA/ROCK signaling. As expected, α-TEA
reduced RhoA activity and downregulated MLC phosphorylation.
Moreover, the effect of α-TEA was enhanced by co-treatment with
RhoA and MLC inhibitors. However, RhoA regulates cellular
biological functions in cancer through several signaling
mechanisms. p27 is a RhoA binding protein, which is critical for
modulating the growth and proliferation of cells. p27 regulates the
cell cycle and is crucial in cell migration and motility. Binding
of p27 and RhoA is involved in the regulation of the activation of
the RhoA/ROCK pathway (50). In
this study, p27 may participate in α-TEA-induced inhibition of
proliferation and motility of colon cancer cells via the RhoA/ROCK
pathway. Additionally, p27RF-RhoA and membrane type-1 matrix
metalloproteinase (MT1-MMP) are critical in tumor cell invasion.
p27RF-Rho stimulates RhoA activation and promotes the formation of
punctate actin structures termed invadopodia, which are important
for regulating tumor cell invasion. RhoA induces invadopodia with
localized concentrations of matrix protease activity that
colocalize with MT1-MMP, actin and cortactin in invasive tumor
cells (51). p90 ribosomal S6
kinase is an effector of the Ras-MAPK cascade and it inhibits
RhoA-induced cell motility by disturbing actomyosin stability.
Therefore, whether other signaling pathways or proteins are
involved in the activity of α-TEA on colon cancer cell malignance
remains to be established.
In conclusion, α-TEA downregulates RhoA/ROCK
signaling and inhibits cancer progression. Thus, α-TEA combined
with RhoA/ROCK/MLC signaling pathway inhibitors may be a beneficial
therapeutic strategy for preventing the development of colon
cancer.
Acknowledgments
The authors would like to thank Summus Biological
Technology Co., Ltd. (Harbin, China) for their technical
support.
References
|
1
|
American Cancer Society: Cancer Facts
& Figures 2014. American Cancer Society; Atlanta: 2014
|
|
2
|
Colon cancer: Healthy women. http://www.healthywomen.org/condition/colon-cancer.
Accessed July 8, 2016.
|
|
3
|
Anderson K, Lawson KA, Simmons-Menchaca M,
Sun L, Sanders BG and Kline K: Alpha-TEA plus cisplatin reduces
human cisplatin-resistant ovarian cancer cell tumor burden and
metastasis. Exp Biol Med (Maywood). 229. pp. 1169–1176. 2004
|
|
4
|
Lawson KA, Anderson K, Simmons-Menchaca M,
Atkinson J, Sun L, Sanders BG and Kline K: Comparison of vitamin E
derivatives α-TEA and VES in reduction of mouse mammary tumor
burden and metastasis. Exp Biol Med (Maywood). 229:954–963.
2004.
|
|
5
|
Shun MC, Yu W, Gapor A, Parsons R,
Atkinson J, Sanders BG and Kline K: Pro-apoptotic mechanisms of
action of a novel vitamin E analog (alpha-TEA) and a naturally
occurring form of vitamin E (delta-tocotrienol) in MDA-MB-435 human
breast cancer cells. Nutr Cancer. 48:95–105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hahn T, Szabo L, Gold M, Ramanathapuram L,
Hurley LH and Akporiaye ET: Dietary administration of the
proapoptotic vitamin E analogue alpha-tocopheryloxyacetic acid
inhibits metastatic murine breast cancer. Cancer Res. 66:9374–9378.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu W, Shun MC, Anderson K, Chen H, Sanders
BG and Kline K: alpha-TEA inhibits survival and enhances death
pathways in cisplatin sensitive and resistant human ovarian cancer
cells. Apoptosis. 11:1813–1823. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia L, Yu W, Wang P, Sanders BG and Kline
K: In vivo and in vitro studies of anticancer actions of alpha-TEA
for human prostate cancer cells. Prostate. 68:849–860. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sanz-Moreno V, Gaggioli C, Yeo M,
Albrengues J, Wallberg F, Viros A, Hooper S, Mitter R, Féral CC,
Cook M, et al: ROCK and JAK1 signaling cooperate to control
actomyosin contractility in tumor cells and stroma. Cancer Cell.
20:229–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Basile JR, Gavard J and Gutkind JS:
Plexin-B1 utilizes RhoA and Rho kinase to promote the
integrin-dependent activation of Akt and ERK and endothelial cell
motility. J Biol Chem. 282:34888–34895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Samuel MS, Lopez JI, McGhee EJ, Croft DR,
Strachan D, Timpson P, Munro J, Schröder E, Zhou J, Brunton VG, et
al: Actomyosin-mediated cellular tension drives increased tissue
stiffness and β-catenin activation to induce epidermal hyperplasia
and tumor growth. Cancer Cell. 19:776–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rösel D, Brábek J, Tolde O, Mierke CT,
Zitterbart DP, Raupach C, Bicanová K, Kollmannsberger P, Panková D,
Vesely P, et al: Up-regulation of Rho/ROCK signaling in sarcoma
cells drives invasion and increased generation of protrusive
forces. Mol Cancer Res. 6:1410–1420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gadea G, de Toledo M, Anguille C and Roux
P: Loss of p53 promotes RhoA-ROCK-dependent cell migration and
invasion in 3D matrices. J Cell Biol. 178:23–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amano M, Ito M, Kimura K, Fukata Y,
Chihara K, Nakano T, Matsuura Y and Kaibuchi K: Phosphorylation and
activation of myosin by Rho-associated kinase (Rho-kinase). J Biol
Chem. 271:20246–20249. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riento K and Ridley AJ: Rocks:
Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kolodney MS and Elson EL: Contraction due
to microtubule disruption is associated with increased
phosphorylation of myosin regulatory light chain. Proc Natl Acad
Sci USA. 92:10252–10256. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Somlyo AV, Bradshaw D, Ramos S, Murphy C,
Myers CE and Somlyo AP: Rho-kinase inhibitor retards migration and
in vivo dissemination of human prostate cancer cells. Biochem
Biophys Res Commun. 269:652–659. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–2641. 2003.PubMed/NCBI
|
|
19
|
Nakajima M, Katayama K, Tamechika I,
Hayashi K, Amano Y, Uehata M, Goto N and Kondo T: WF-536 inhibits
metastatic invasion by enhancing the host cell barrier and
inhibiting tumour cell motility. Clin Exp Pharmacol Physiol.
30:457–463. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakajima M, Hayashi K, Egi Y, Katayama K,
Amano Y, Uehata M, Ohtsuki M, Fujii A, Oshita K, Kataoka H, et al:
Effect of Wf-536, a novel ROCK inhibitor, against metastasis of B16
melanoma. Cancer Chemother Pharmacol. 52:319–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue F, Takahara T, Yata Y, Xia Q, Nonome
K, Shinno E, Kanayama M, Takahara S and Sugiyama T: Blockade of
Rho/Rho-associated coiled coil-forming kinase signaling can prevent
progression of hepatocellular carcinoma in matrix
metalloproteinase-dependent manner. Hepatol Res. 38:810–817. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong CC, Wong CM, Tung EK, Man K and Ng
IO: Rho-kinase 2 is frequently overexpressed in hepatocellular
carcinoma and involved in tumor invasion. Hepatology. 49:1583–1594.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sahai E, Ishizaki T, Narumiya S and
Treisman R: Transformation mediated by RhoA requires activity of
ROCK kinases. Curr Biol. 9:136–145. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ying H, Biroc SL, Li WW, Alicke B, Xuan
JA, Pagila R, Ohashi Y, Okada T, Kamata Y and Dinter H: The Rho
kinase inhibitor fasudil inhibits tumor progression in human and
rat tumor models. Mol Cancer Ther. 5:2158–2164. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng
Y, Liu M, Chen J, Liu S, Qiu M, et al: RhoA regulates G1-S
progression of gastric cancer cells by modulation of multiple INK4
family tumor suppressors. Mol Cancer Res. 7:570–580. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zohrabian VM, Forzani B, Chau Z, Murali R
and Jhanwar-Uniyal M: Rho/ROCK and MAPK signaling pathways are
involved in glioblastoma cell migration and proliferation.
Anticancer Res. 29:119–123. 2009.PubMed/NCBI
|
|
27
|
Schmidt LJ, Duncan K, Yadav N, Regan KM,
Verone AR, Lohse CM, Pop EA, Attwood K, Wilding G, Mohler JL, et
al: RhoA as a mediator of clinically relevant androgen action in
prostate cancer cells. Mol Endocrinol. 26:716–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng
Y, Liu M, Chen J, Liu S, Qiu M, et al: RhoA regulates G1-S
progression of gastric cancer cells by modulation of multiple INK4
family tumor suppressors. Mol Cancer Res. 7:570–580. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Doublier S, Riganti C, Voena C, Costamagna
C, Aldieri E, Pescarmona G, Ghigo D and Bosia A: RhoA silencing
reverts the resistance to doxorubicin in human colon cancer cells.
Molecular Cancer Research. 6:1607–1620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Molli PR, Pradhan MB, Advani SH and Naik
NR: RhoA: A therapeutic target for chronic myeloid leukemia.
Molecular cancer. 11:162012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horiuchi A, Imai T, Wang C, Ohira S, Feng
Y, Nikaido T and Konishi I: Up-regulation of small GTPases, RhoA
and RhoC, is associated with tumor progression in ovarian
carcinoma. Lab Invest. 83:861–870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kamai T, Yamanishi T, Shirataki H, Takagi
K, Asami H, Ito Y and Yoshida K: Overexpression of RhoA, Rac1 and
CDC42 GTPases is associated with progression in testicular cancer.
Clin Cancer Res. 10:4799–4805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hahn T, Jagadish B, Mash EA, Garrison K
and Akporiaye ET: α-Tocopheryloxyacetic acid: A novel
chemotherapeutic that stimulates the antitumor immune response.
Breast Cancer Res. 13:R42011. View
Article : Google Scholar
|
|
34
|
Lawson KA1, Anderson K, Menchaca M,
Atkinson J, Sun L, Knight V, Gilbert BE, Conti C, Sanders BG and
Kline K: Novel vitamin E analogue decreases syngeneic mouse mammary
tumor burden and reduces lung metastasis. Mol Cancer Ther.
2:437–444. 2003.PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Yanagisawa M and Anastasiadis PZ: p120
catenin is essential for mesenchymal cadherin-mediated regulation
of cell motility and invasiveness. J Cell Biol. 174:1087–1096.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fariss MW, Fortuna MB, Everett CK, Smith
JD, Trent DF and Djuric Z: The selective antiproliferative effects
of alpha-tocopheryl hemisuccinate and cholesteryl hemisuccinate on
murine leukemia cells result from the action of the intact
compounds. Cancer Res. 54:3346–3351. 1994.PubMed/NCBI
|
|
38
|
Malafa MP, Fokum FD, Mowlavi A, Abusief M
and King M: Vitamin E inhibits melanoma growth in mice. Surgery.
131:85–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hahn T, Bradley-Dunlop DJ, Hurley LH,
Von-Hoff D, Gately S, Mary DL, Lu H, Penichet ML, Besselsen DG,
Cole BB, et al: The vitamin E analog, alpha-tocopheryloxyacetic
acid enhances the anti-tumor activity of trastuzumab against
HER2/neu-expressing breast cancer. BMC Cancer. 11:4712011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang P, Yu W, Hu Z, Jia L, Iyer VR,
Sanders BG and Kline K: Involvement of JNK/p73/NOXA in vitamin E
analog-induced apoptosis of human breast cancer cells. Mol
Carcinog. 47:436–445. 2008. View Article : Google Scholar
|
|
41
|
Schlienger S, Campbell S and Claing A:
ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast
cancer cell invasion. Mol Biol Cell. 25:17–29. 2014. View Article : Google Scholar :
|
|
42
|
Li XR, Ji F, Ouyang J, Wu W, Qian LY and
Yang KY: Overexpression of RhoA is associated with poor prognosis
in hepatocellular carcinoma. Eur J Surg Oncol. 32:1130–1134. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Collisson EA, Carranza DC, Chen IY and
Kolodney MS: Isoprenylation is necessary for the full invasive
potential of RhoA overexpression in human melanoma cells. J Invest
Dermatol. 119:1172–1176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fritz G, Just I and Kaina B: Rho GTPases
are over-expressed in human tumors. Int J Cancer. 81:682–687. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kamai T, Tsujii T, Arai K, Takagi K, Asami
H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway
with invasion and metastasis of bladder cancer. Clin Cancer Res.
9:2632–2641. 2003.PubMed/NCBI
|
|
46
|
Pan Y, Bi F, Liu N, Xue Y, Yao X, Zheng Y
and Fan D: Expression of seven main Rho family members in gastric
carcinoma. Biochem Biophys Res Commun. 315:686–691. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jiang WG, Watkins G, Lane J, Cunnick GH,
Douglas-Jones A, Mokbel K and Mansel RE: Prognostic value of rho
GTPases and rho guanine nucleotide dissociation inhibitors in human
breast cancers. Clin Cancer Res. 9:6432–6440. 2003.PubMed/NCBI
|
|
48
|
Shin DH, Chun YS, Lee KH, Shin HW and Park
JW: Arrest defective-1 controls tumor cell behavior by acetylating
myosin light chain kinase. PLoS One. 4:e74512009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kidera Y, Tsubaki M, Yamazoe Y, Shoji K,
Nakamura H, Ogaki M, Satou T, Itoh T, Isozaki M, Kaneko J, et al:
Reduction of lung metastasis, cell invasion, and adhesion in mouse
melanoma by statin-induced blockade of the Rho/Rho-associated
coiled-coil-containing protein kinase pathway. J Exp Clin Cancer
Res. 29:1272010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Larrea MD, Wander SA and Slingerland JM:
p27 as Jekyll and Hyde: Regulation of cell cycle and cell motility.
Cell Cycle. 8:3455–3461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hoshino D, Tomari T, Nagano M, Koshikawa N
and Seiki M: A novel protein associated with membrane-type 1 matrix
metalloproteinase binds p27 (kip1) and regulates RhoA activation,
actin remodeling, and matrigel invasion. J Biol Chem.
284:27315–27326. 2009. View Article : Google Scholar : PubMed/NCBI
|