Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer and the second leading cause of cancer-associated
mortality in the United States (1). In China, colorectal cancer has become
the second and fourth leading cause of cancer-associated mortality
in women and men, respectively (2). Despite recent progress, novel
therapeutic agents are required in intestinal oncology (3). Previously, gene therapy has been
proposed as a potential novel treatment strategy for colorectal
cancer, preclinical data using adenovirus vectors has been
promising and a number of clinical trials utilizing this vector are
underway (4,5). The safety of adenovirus vectors have
been established by various phase I trials, however, the factors
determining the efficiency of gene delivery remain to be
elucidated.
The coxsackie and adenovirus receptor (CAR), a
trans-membrane component of the tight junction complex, was
initially identified as a viral attachment site on the surface of
epithelial cells, which was required for subsequent virus uptake
(6,7). Walters et al (8) demonstrated that CAR is key in gene
transfer efficacy and functions as a primary receptor for the
coxsackie B virus and adenovirus. Pandha et al (9) determined that CAR levels are closely
associated with adenovirus attachment, infection and transgene
expression. Attenuated adenoviruses, which may be
replication-incompetent to deliver therapeutic genes or viruses
replicating only in certain cell types, may be used as a cancer
therapy (10). Thus, the presence
of CAR is considered an important determinant for the efficacy of
therapeutic strategies using adenoviruses.
Analysis of CAR expression in different types of
tumor demonstrated varied results. Accumulating evidence indicates
that CAR expression levels are low in a number of types of tumor,
including ovarian, lung, breast and bladder (11–14),
particularly in those tumors exhibiting poor differentiation and
advanced disease stage (12,15,16).
In addition, downregulated expression of CAR predicted a poor
clinical outcome for gastric and bladder cancer patients (12,17).
By contrast, CAR upregulation was also observed in cancer of the
endometrium, ovary, cervix, breast and lung, as well as
neuroblastomas and medulloblastomas (18–24).
Furthermore, high CAR expression has been associated with poor
prognosis in breast and lung cancer (15,20).
It remains to be elucidated whether these results reflect
differences in CAR expression levels or are a result of racial and
methodological differences.
In CRC, Zhang et al (25) observed a high variability in CAR
expression levels with ~75% of the cases demonstrating CAR
downregulation. Reeh et al (26) also demonstrated that CAR expression
levels were decreased in CRC. However, Stecker et al
(27) indicated that CAR
facilitates complex effects during colorectal carcinogenesis,
potentially mediated by its stage-dependent subcellular
distribution, and loss of CAR expression promotes growth and
metastasis of primary CRC (27).
These results suggested CAR has a complex role in carcinogenesis.
However, to the best of our knowledge, no research has focused on
the association between CAR expression levels and
clinicopathological features of CRC. In our previous study, an
oncolytic adenovirus was developed by inserting a CRC-specific
suppressor gene, ST13, into a CRC-specific oncolytic virus. This
virus exhibited marked antitumor effects, which inhibited tumor
growth in CRC xenografts (28).
However, as a key determinant of the efficacy of gene transfer, the
clinical relevance of CAR expression in CRC requires further
determination.
In the present study, immunohistochemistry was
conducted to assess CAR expression in CRC and adjacent normal
tissue samples in a tissue microarrays (TMA). Large sample sizes
were selected to generate data allowing increased understanding the
role of CAR in the pathological progress of CRC. In addition,
potential targets for adenovirus-mediated therapies based on CAR
expression may also be identified.
Materials and methods
CRC patients in tissue microarray
The CAR protein expression levels were assessed with
immunohistochemical staining of tissue microarrays, which were
purchased from Shanghai Biochip Co., Ltd. (Shanghai, China). The
TMAs containing a total of 502 formalin-fixed, paraffin-embedded
archival samples from a total of 251 CRC patients from the Chinese
Han population, in addition to 251 corresponding controls derived
from adjacent normal tissue samples.
The patient cohort consisted of 139 males and 112
females, with a median age of 66 years (range, 27–91 years) at the
time of surgery. All patients had follow-up records for >5
years. The survival time was calculated from the date of surgery to
the follow-up deadline or mortality.
Immunohistochemistry analysis
TMA sections were used for subsequent
immunohistochemical analysis. Briefly, TMA sections were
deparaffinized and dehydrated employing standard procedures using
xylene and graded alcohol. Antigen retrieval was conducted by
autoclaving in 0.01 M citrate buffer (pH 6.0) for 3 min. The
sections were then treated with 3% hydrogen peroxide to quench
endogenous peroxidase activity, and incubated with 10% normal goat
serum to reduce background non-specific binding. TMA sections were
incubated with a rabbit anti-human primary polyclonal antibody
against CAR (dilution, 1:50; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA; cat. no. sc-15405) overnight at 4°C. PBS used in
place of the primary antibody served as a negative control.
Subsequently, a biotinylated goat anti-rabbit immunoglobulin
(Histostain-Plus IHC kit; Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) was used as a secondary antibody, followed
by treatment with streptavidin-biotinylated horseradish peroxidase
complex (Invitrogen; Thermo Fisher Scientific, Inc.). The slides
were stained with 3,3-diaminobenzidine and counterstained with
hematoxylin, dehydrated in a graded alcohol series and mounted.
Evaluation of immunohistochemical
staining
Immunohisto-chemical staining for CAR was observed
predominantly in the membrane and cytoplasm of tumor cells. The
degree of immunostaining was reviewed under light microscopy by two
expert pathologists who were blinded to the clinical data and
scored independently. Immunopositivity at the plasma membrane was
also assessed separately. The CAR expression level was determined
using the intensity of staining and percentage of immunoreactive
cancer cells. Staining intensity was graded as follows: 0, no
staining; 1, weak staining; 2, moderate staining; and 3, strong
staining. Staining percentage was graded according to the
proportion of positively stained tumor cells as follows: 0, <5%
positive tumor cells; 1, 6–25% positive tumor cells; 2, 26–50%
positive tumor cells; and 3, >51% positive tumor cells. The
immunoreactive score was calculated by percentage of positive cells
multiplied by staining intensity score. For further evaluation, a
staining index score of ≤3 was used to define tumors with
CAR-negative, and a tumor with a staining index score of ≥4 was
regarded as CAR-positive.
Genomic analysis
Gene-therapeutic agent interaction data was
downloaded from the Comparative Toxicogenomics Database (CTD;
ctdbase.org). R 3.2.1 (R Foundation for Statistical
Computing; www.r-project.org) was utilized to
perform the analyses.
Statistical analysis
All statistical analyses were performed using SPSS
13.0 (SPSS Inc., Chicago, IL, USA). Categorical data were analyzed
using the χ2 or Fisher's exact test to assess the
association between the expression of CAR and the
clinicopathological parameters of patients with colon cancer. The
Kaplan-Meier method was performed to estimate survival curves,
accompanying the log-rank test to calculate differences between the
curves. Cox proportional hazards regression model was used to
perform multivariate survival analysis to assess predictors
associated with prognosis. In addition, correlation between CAR
protein expression levels and clinicopathological features were
estimated using the Spearman correlation method. All P-values were
two-sided and P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of CAR in CRC samples was
lower than in corresponding normal tissue samples
Immunohistochemistry was used to analyze the
presence and distribution of CAR. It was observed that
immunostaining of CAR was predominantly located in the membrane and
cytoplasm of the cells (Fig. 1).
Positive expression of CAR protein was detected in 240 of 251
(95.6%) noncancerous colorectal mucosa samples, which was
significantly higher than in CRC (40.6%, 102/251,
χ2=174.7, P<0.001; detailed data not shown).
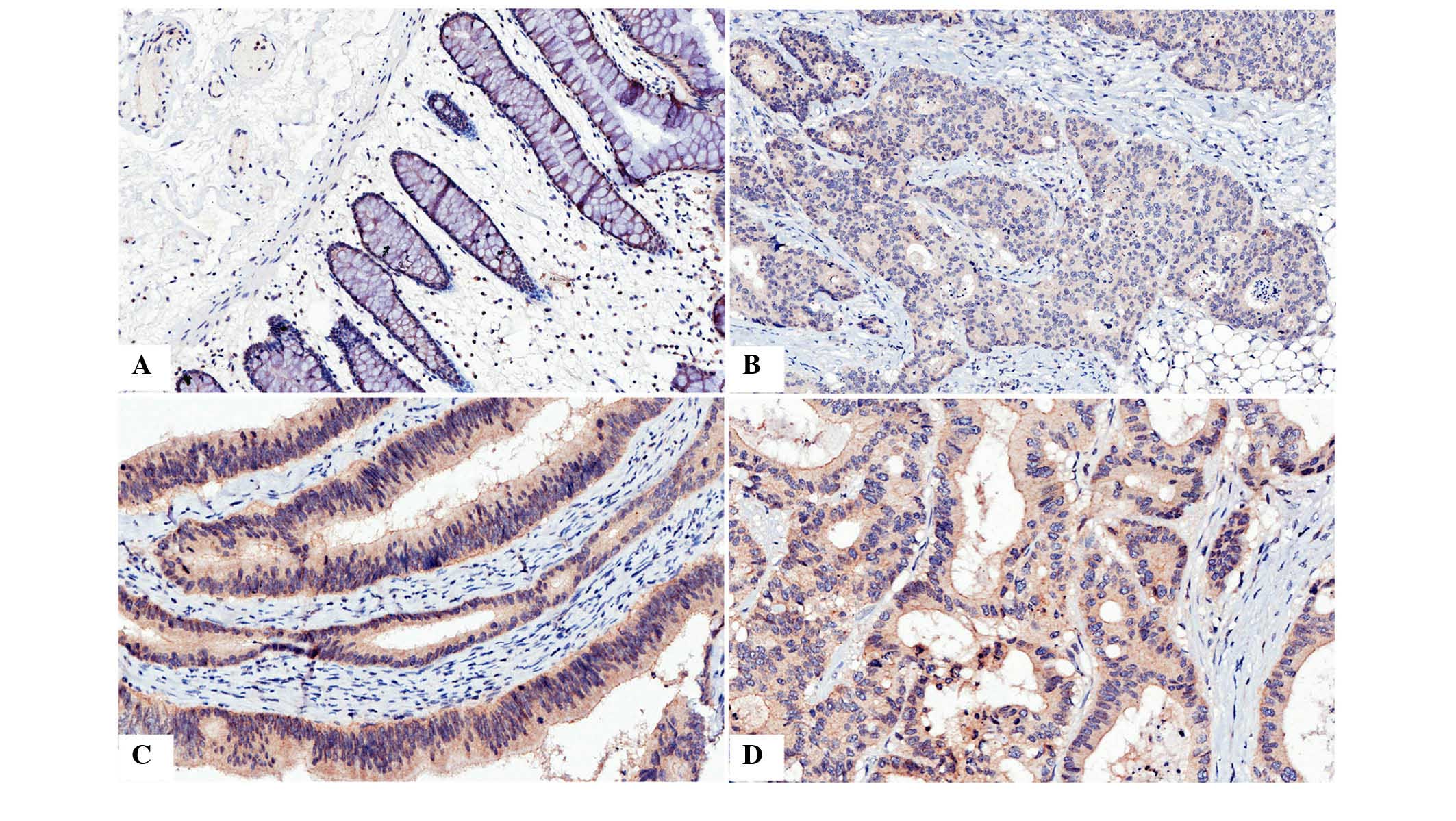 | Figure 1Immunohistochemical analysis of CAR
in colon cancer and normal tissue. (A) Immunostaining of CAR in
normal colon tissue, positive staining was observed in the
cytoplasm and, predominantly, the membrane. (B) Immunostaining of
CAR in poorly differentiated colon cancer, positive staining was
predominantly observed in the cytoplasm. (C) Immunostaining of CAR
in moderately differentiated colon cancer, positive staining was
observed in the membrane and, predominantly, the cytoplasm. (D)
Immunostaining of CAR in colon cancer with liver matastasis,
positive staining was observed in the cytoplasm and membrane.
Magnification, ×400. CAR, coxsackie and adenovirus receptor. |
CAR immunopositivity is associated with
metastasis in CRC
In order to investigate whether the presence of CAR
is associated with the development of CRC, the CAR expression was
compared with clinicopathological parameters of CRC. The results
demonstrated that the prevalence of cytoplasmic CAR
immunopositivity was not significantly associated with gender, age,
tumor size, differentiation, TNM stage, lymph node metastasis or
distant metastasis (Table I).
However, CAR immunopositivity was detected in 83.3% (5/6) of
patients with colorectal liver metastasis, which was significantly
higher than those without liver metastasis (39.6%, 97/245,
P=0.042).
 | Table IAssociation between CAR expression
and clinicopathological features of colon cancer. |
Table I
Association between CAR expression
and clinicopathological features of colon cancer.
| Clinical
parameters | CAR expression
|
|---|
| Negative (%) | Positive (%) | χ2 | P-value |
|---|
| Gender | | | 0.148 | 0.701 |
| Male | 84 (60.4) | 55 (39.6) | | |
| Female | 65 (58.0) | 47 (42.0) | | |
| Age (yrs) | | | 0.4595 | 0.441 |
| <60 | 40 (63.5) | 23 (36.5) | | |
| ≥60 | 109 (58.0) | 79 (42.0) | | |
| Tumor diameter | | | 1.151 | 0.283 |
| <20 cm | 76 (62.8) | 45 (37.2) | | |
| ≥20 cm | 73 (56.2) | 57 (43.8) | | |
|
Differentiation | | | 2.943 | 0.230 |
| High | 28 (56.0) | 22 (44.0) | | |
| Moderate | 86 (57.0) | 65 (43.0) | | |
| Poor | 35 (70.0) | 15 (30.0) | | |
| TNM stage | | | 0.053 | 0.818 |
| TNM I+II | 84 (58.7) | 59 (41.3) | | |
| TNM III+IV | 65 (60.2) | 43 (39.8) | | |
| Lymph node
metastasis | | | 0.636 | 0.425 |
| No | 86 (57.3) | 64 (42.7) | | |
| Yes | 63 (62.4) | 38 (37.6) | | |
| Distant
metastasis | | | 2.675 | 0.164 |
| No | 146 (60.6) | 96 (39.7) | | |
| Yes | 3 (33.3) | 6 (66.7) | | |
| Liver
metastasis | | | 4.645 | 0.042 |
| Negative | 148 (60.4) | 97 (39.6) | | |
| Positive | 1 (16.7) | 5 (83.3) | | |
Survival analysis indicates CAR
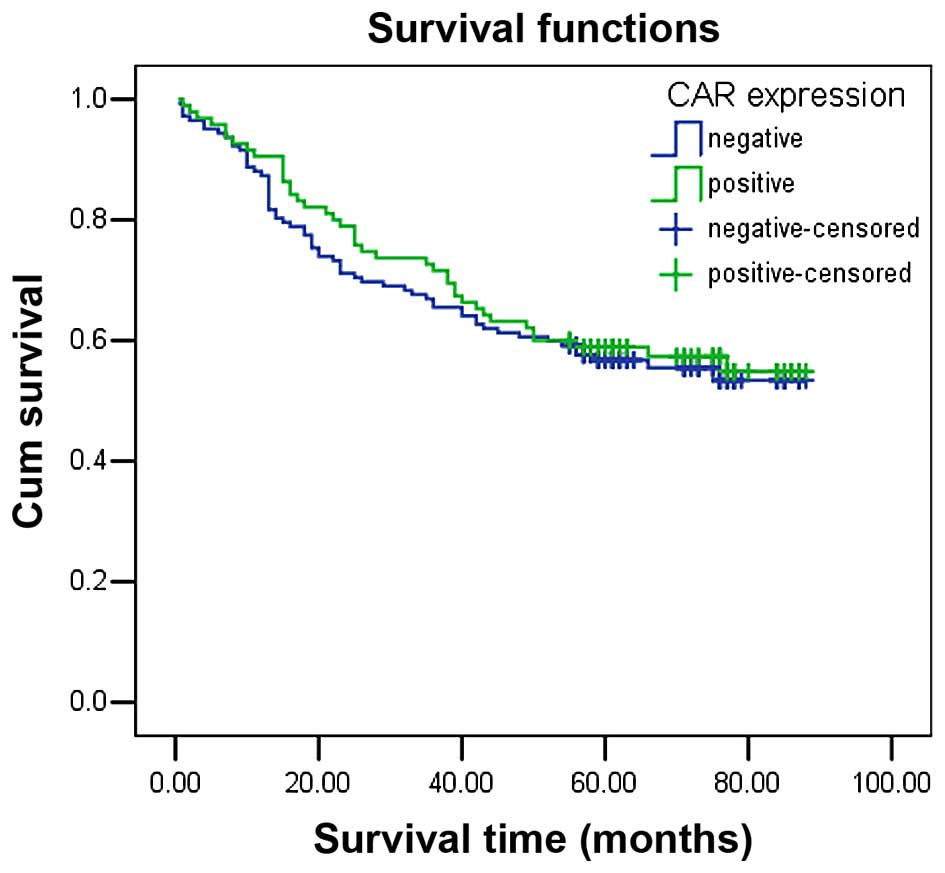
immunopositivity does not significantly decrease survival time
The mean survival time in colon cancer patients with
CAR immunopositivity was 61.50±3.31 months, which demonstrated no
significant difference between survival time compared with
CAR-negative patients (58.96±2.87 months, P=0.654). The
Kaplan-Meier survival curve also demonstrated that CAR expression
had no significant association with overall survival (Fig. 2). In addition, factors with
possible prognostic effects in colon cancer were analyzed by Cox
regression analysis, and the results indicated that distant
metastasis was an independent prognostic factors in patients with
CRC (P=0.001), while CAR expression was not (P=0.355; Table II).
 | Table IICox-regression analysis of the
clinicopathological parameters in colon cancer patients. |
Table II
Cox-regression analysis of the
clinicopathological parameters in colon cancer patients.
| Parameters | Coefficient | HR | 95.0% CI for
HR | P |
|---|
| Gender | 0.321 | 1.116 | 0.748–1.667 | 0.590 |
| Age | 0.110 | 1.379 | 0.848–2.243 | 0.195 |
| Distant
metastasis | 0.328 | 5.474 | 1.947–15.386 | 0.001 |
| Tumor diameter | 0.299 | 1.388 | 0.929–2.076 | 0.110 |
| Lymph node
metastasis | 0.424 | 1.528 | 0.497–4.701 | 0.460 |
| TNM stage | 0.258 | 1.295 | 0.397–4.223 | 0.668 |
|
Differentiation | 1.700 | 1.348 | 0.986–1.843 | 0.061 |
| CAR expression | −0.192 | 0.826 | 0.550–1.239 | 0.355 |
Plasma membrane expression of CAR in CRC
was decreased compared with normal mucosal samples
CAR is a trans-membrane glycoprotein, which is a
viral attachment site on the surface of epithelial cells. CAR
expression in the plasma membrane is important role in virus
uptake. Thus, the present study analyzed the presence of CAR in the
plasma membrane. CAR was observed at the plasma membrane of normal
mucosa samples (29.5%, 74/251), which was indicated to be
significantly higher than in CRC samples (4.0%, 10/251,
P<0.001). However, further analysis demonstrated that none of
the clinicopathological parameters were significantly associated
with the plasma membrane expression of CAR in primary CRC (data not
shown).
Certain therapeutic agents may upregulate
CAR expression levels
The CTD database was searched for therapeutic agents
and chemicals that may upregulate the mRNA or protein expression
levels of CAR, based on their applications in cancer management.
The results indicated that a number of therapeutic agents,
including PJ-34, dietary fats, calcitriol, testosterone,
gentamicins and torcetrapib may upregulate the expression of
CAR.
Discussion
In the present study of the Chinese Han population,
high CAR expression levels was observed in normal colorectal
tissues, which is consistent with previous studies (12,27,29).
A decrease in CAR expression in primary CRC samples was also
observed, with 40.6% positive detection rate. These data suggest
that reduced CAR expression contributes to carcinogenesis and
progression of primary CRC. Furthermore, low CAR protein expression
in CRC may result in poor adenoviral transduction efficiency. In
order to assess the profile of CAR in the development of CRC, the
association between CAR expression and clinicopathological features
of CRC was analyzed. CAR immunopositivity was not significantly
associated with age, gender or any other clinicopathological
feathers, which was consistent with previous observations by
Stecker et al (27).
However, CAR immunopositivity was significantly associated with
liver metastasis. Korn et al (29) reported that 60% of cases of CRC
with metastases in the liver exhibited CAR expression. Rauen et
al (30) also demonstrated
that CAR immunopositivity was significantly higher in prostate
cancer with metastasis than in primary cancer. However, as the
number of examined patients with liver metastases in the present
study is small, the current study hypothesizes that CAR may exert a
complex effect on the process of colon cancer development,
potentially depending on its stage in Chinese Han population, and
further studies are required to confirm the result.
CRC may be surgically treated with chemoradiotherapy
as a adjuvant therapy. However, the outcome is not ideal, and liver
metastasis may result in post-operative relapse (31). Cady and Stone (32) indicated that ~20–40% of patients
had liver metastasis when first diagnosed, however, the incidence
of liver metastasis following radical resection of CRC was 40–50%.
Furthermore, liver metastasis occurred in >50% of CRC-associated
mortality. Tomlinson et al (33) reported that Liver-directed therapy
in colorectal liver metastasis in addition to rational
implementation of chemotherapeutic regimens have resulted in median
survival time of >30 months and the potential for cure with
resection. The present study demonstrated that CAR immunopositivity
was significantly upregulated in patients with liver metastasis,
which suggest that high CAR expression may support the
establishment of metastasis, particularly in liver metastasis.
Thus, the present study hypothesizes that CAR may be used for
monitoring and/or predicting the outcome of adenovirus-mediated
gene therapy, particularly to improve the efficacy of therapeutic
strategies using adenoviruses in CRC with liver metastasis.
There are few studies that aimed to determine the
value of CAR expression in the prognosis of cancer. Thus, the
present study aimed to investigate the clinical importance of CAR
expression in the prognosis of patients with CRC. It was observed
that survival time in patients with moderate or strong tumor CAR
expression was longer than those with low expression, however, this
was not a statistically significant difference when analyzed using
univariate analysis. Martin et al (20) indicated that elevated levels of CAR
expression were markedly associated with poor overall survival in
patients with breast cancer. It had also been previously
demonstrated that the soluble splice variants CAR 3/7 and CAR 4/7,
but not the full-length human CAR were independent prognostic
factors in progression-free and overall survival of patients with
ovarian cancer (23). These
results suggest that in addition to its important role in coxsackie
and adenovirus cell-entry, CAR is also involved in cell-cell
adhesions, exerting effects of a cell surface receptor and
resulting in different characteristics depending on the tumor type,
and the prognostic value of CAR in CRC remains to be determined by
investigating larger sample sizes and adjusting for other relative
factors.
Considering the reduced expression of CAR, a number
of adenovirus targeting strategies that aim to improve the efficacy
of adenovirus-mediated gene therapy in CRC. Structural alteration
of adenovirus vectors or drug-induced CAR expression has become a
focus of research in the field of adenovirus-mediated therapy. For
example, chemical modification of the adenovirus capsid or fiber
alteration (swapping and replacement) may promote CAR-independent
gene transfer efficiency (34–36).
The fiber-swapping of adenovirus type 5 (Ad5) fibers with subgroup
B adenovirus fibers, such as Ad3, Ad35, or Ad11, results in vectors
with CAR-independent transduction, mediated via the group B Ad
receptors, including cluster of differentiation (CD)46, CD80/CD86,
or 'receptor X' (37–39). In addition, it has been
demonstrated that the trimerization function of the fiber knob may
be replaced by the fold on domain from the bacteriophage T4
fibritin protein (40) or
extrinsic trimerization motifs, such as the MoMuLV envelope
glycoprotein trimerization domain (41).
Previous studies (as presented in Table III) have demonstrated that a
variety of therapeutic agents enhance the efficacy of
adenovirus-mediated gene transduction via elevating CAR expression
in target cells. Trichostatin A induced the expression of CAR in
esophageal squamous cell carcinoma cell lines via the
mitogen-activated protein kinase/extracellular-regulated kinase 1/2
signaling pathway (42). Treatment
of cells with the histone deacetylase inhibitor, FR901228 (a
depsipeptide) increased CAR RNA levels in cancer cell lines,
including carcinoma of the thyroid, colon, renal cell, breast and
hepatic cell, and it is associated with enhanced adenoviral
transgene expression following infection (43). Yoo et al (44) also demonstrated that docetaxel
enhanced p53 transduction using an adenovirus by increasing CAR
expression. In addition, interaction network analysis was performed
using the CTD, and CAR was searched in the CTD database for
therapeutic agents and chemicals that may upregulate the mRNA or
protein expression levels of CAR, and these were selected based on
their applications in cancer management. The result demonstrated
that a number of therapeutic agents, such as PJ-34 may upregulate
the expression of CAR, which is crucial for the efficacy of the
treatment of adenovirus-mediated gene therapy in CRC.
 | Table IIITherapeutic agents previously
described to upregulate the expression of CAR. |
Table III
Therapeutic agents previously
described to upregulate the expression of CAR.
| Agent | Function | Source | Cancer type | Description | Ref. |
|---|
| Kitazono, 2001 | FR901228 | Histone deacetylase
inhibitor | Depsipeptide
fermentation product from Chromobacterium violaceum, first
isolated by the Fujisawa Pharmaceutical Company, Ltd. (Osaka,
Japan) | Carcinoma of
thyroid, colon, renal cell, breast and hepatic cell | Increased CAR mRNA
levels observed in all cell lines following 1 ng/ml for 72 h | (43) |
| Ma, 2012 | TSA | Histone deacetylase
inhibitor | Sigma-Aldrich (St.
Louis, MO, USA) | Esophageal squamous
cell carcinoma | CAR protein
expression levels increased in a dose-dependent manner in EC1 cells
following TSA treatment (0.3, 0.5 and 1.0 µmol/l) | (42) |
| Yoo, 2004 | Docetaxel |
G2M-arresting agent | Sanofi S.A. (Paris,
France | Head and neck
cancer | Docetaxel treatment
(25 ng/ml for 24 h) increased the expression of CAR, analyzed by
fluorescence-activated cell sorting, and resulted in increased
adenoviral transduction rates | (44) |
The results of the present study suggest that CAR
facilitates complex effects during CRC carcinogenesis in the
Chinese Han population, potentially depending on the stage of the
cancer development and progression. In addition, the current study
indicated various therapeutic agents that may increase the
expression of CAR in order to improve adenovirus-mediated gene
therapy efficacy in CRC.
In conclusion, CAR expression has potential as a
marker for monitoring and/or predicting the outcome of gene
therapy, and increasing its expression levels may contribute to the
upregulation of cellular sensitivity towards adenovirus
infection.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81201783, 81372463
and 81472210), the Zhejiang Provincial Natural Science Foundation
of China (grant nos. LY15H160051, LY14H160041 and LY13H080005), the
Funds of Science Technology Department of Zhejiang Province (grant
no. 2014C37101), the Zhejiang Province Bureau of Health (grant no.
2015ZA009), and the Open Fund of Zhejiang Provincial Top Key
Discipline of Biology.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu
L and He J: Report of incidence and mortality in China cancer
registries, 2009. Chin J Cancer Res. 25:10–21. 2013.PubMed/NCBI
|
|
3
|
Chang GJ, Kaiser AM, Mills S, Rafferty JF
and Buie WD; Standards Practice Task Force of the American Society
of Colon and Rectal Surgeons: Practice parameters for the
management of colon cancer. Dis Colon Rectum. 55:831–843. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rayburn ER, Wang W, Zhang R and Wang H:
Experimental therapy for colon cancer: Anti-cancer effects of TLR9
agonism, combination with other therapeutic modalities, and
dependence upon p53. Int J Oncol. 30:1511–1519. 2007.PubMed/NCBI
|
|
5
|
Zuckerman DS and Clark JW: Systemic
therapy for metastatic colorectal cancer: Current questions.
Cancer. 112:1879–1891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bergelson JM, Cunningham JA, Droguett G,
Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL and
Finberg RW: Isolation of a common receptor for Coxsackie B viruses
and adenoviruses 2–5. Science. 275:1320–1333. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cohen CJ, Shieh JT, Pickles RJ, Okegawa T,
Hsieh JT and Bergelson JM: The coxsackievirus and adenovirus
receptor is a transmembrane component of the tight junction. Proc
Natl Acad Sci USA. 98:15191–15196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walters RW, Freimuth P, Moninger TO,
Ganske I, Zabner J and Welsh MJ: Adenovirus fiber disrupts
CAR-mediated intercellular adhesion allowing virus escape. Cell.
110:789–799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pandha HS, Stockwin LH, Eaton J, Clarke
IA, Dalgleish AG, Todryk SM and Blair GE: Coxsackie B and
adenovirus receptor, integrin and major histocompatibility complex
class I expression in human prostate cancer cell lines:
Implications for gene therapy strategies. Prostate Cancer Prostatic
Dis. 6:6–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasuya H, Takeda S, Shimoyama S, Shikano
T, Nomura N, Kanazumi N, Nomoto S, Sugimoto H and Nakao A:
Oncolytic virus therapy-foreword. Curr Cancer Drug Targets.
7:123–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abdolazimi Y, Mojarrad M, Pedram M and
Modarressi MH: Analysis of the expression of coxsackievirus and
adenovirus receptor in five colon cancer cell lines. World J
Gastroenterol. 13:6365–6369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anders M, Vieth M, Röcken C, Ebert M,
Pross M, Gretschel S, Schlag PM, Wiedenmann B, Kemmner W and Höcker
M: Loss of the coxsackie and adenovirus receptor contributes to
gastric cancer progression. Br J Cancer. 100:352–359. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Thorne S, Hannock J, Francis J, Au
T, Reid T, Lemoine N, Kirn D and Halldén G: A novel assay to assess
primary human cancer infectibility by replication-selective
oncolytic adenoviruses. Clin Cancer Res. 11:351–360.
2005.PubMed/NCBI
|
|
14
|
Yamashita M, Ino A, Kawabata K, Sakurai F
and Mizuguchi H: Expression of coxsackie and adenovirus receptor
reduces the lung metastatic potential of murine tumor cells. Int J
Cancer. 121:1690–1696. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wunder T, Schmid K, Wicklein D, Groitl P,
Dobner T, Lange T, Anders M and Schumacher U: Expression of the
coxsackie adenovirus receptor in neuroendocrine lung cancers and
its implications for oncolytic adenoviral infection. Cancer Gene
Ther. 20:25–32. 2013. View Article : Google Scholar
|
|
16
|
Wunder T, Schumacher U and Friedrich RE:
Coxsackie adenovirus receptor expression in carcinomas of the head
and neck. Anticancer Res. 32:1057–1062. 2012.PubMed/NCBI
|
|
17
|
Matsumoto K, Shariat SF, Ayala GE, Rauen
KA and Lerner SP: Loss of coxsackie and adenovirus receptor
expression is associated with features of aggressive bladder
cancer. Urology. 66:441–446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dietel M, Häfner N, Jansen L, Durst M and
Runnebaum IB: Novel splice variant CAR 4/6 of the coxsackie
adenovirus receptor is differentially expressed in cervical
carcinogenesis. J Mol Med (Berl). 89:621–630. 2011. View Article : Google Scholar
|
|
19
|
Giaginis CT, Zarros AC, Papaefthymiou MA,
Papadopouli AE, Sfiniadakis IK and Theocharis SE: Coxsackievirus
and adenovirus receptor expression in human endometrial
adenocarcinoma: Possible clinical implications. World J Surg Oncol.
6:592008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin TA, Watkins G and Jiang WG: The
Coxsackie-adenovirus receptor has elevated expression in human
breast cancer. Clin Exp Med. 5:122–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martino TA, Petric M, Weingartl H,
Bergelson JM, Opavsky MA, Richardson CD, Modlin JF, Finberg RW,
Kain KC, Willis N, et al: The coxsackie-adenovirus receptor (CAR)
is used by reference strains and clinical isolates representing all
six serotypes of coxsackievirus group B and by swine vesicular
disease virus. Virology. 271:99–108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Persson A, Fan X, Widegren B and Englund
E: Cell type- and region-dependent coxsackie adenovirus receptor
expression in the central nervous system. J Neurooncol. 78:1–6.
2006. View Article : Google Scholar
|
|
23
|
Reimer D, Steppan I, Wiedemair A, Concin
N, Hofstetter G, Marth C, Müller-Holzner E and Zeimet AG: Soluble
isoforms but not the transmembrane form of coxsackie-adenovirus
receptor are of clinical relevance in epithelial ovarian cancer.
Int J Cancer. 120:2568–2575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Wang S, Bao Y, Ni C, Guan N, Zhao
J, Salford LG, Widegren B and Fan X: Coxsackievirus and adenovirus
receptor expression in non-malignant lung tissues and clinical lung
cancers. J Mol Histol. 37:153–160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang NH, Song LB, Wu XJ, Li RP, Zeng MS,
Zhu XF, Wan DS, Liu Q, Zeng YX and Zhang XS: Proteasome inhibitor
MG-132 modifies coxsackie and adenovirus receptor expression in
colon cancer cell line lovo. Cell Cycle. 7:925–933. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reeh M, Bockhorn M, Görgens D, Vieth M,
Hoffmann T, Simon R, Izbicki JR, Sauter G, Schumacher U and Anders
M: Presence of the coxsackievirus and adenovirus receptor (CAR) in
human neoplasms: A multitumour array analysis. Br J Cancer.
109:1848–1858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stecker K, Vieth M, Koschel A, Wiedenmann
B, Röcken C and Anders M: Impact of the coxsackievirus and
adenovirus receptor on the adenoma-carcinoma sequence of colon
cancer. Br J Cancer. 104:1426–1433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou X, Xie G, Wang S, Wang Y, Zhang K,
Zheng S, Chu L, Xiao L, Yu Y, Zhang Y and Liu X: Potent and
specific antitumor effect for colorectal cancer by CEA and Rb
double regulated oncolytic adenovirus harboring ST13 gene. PLoS
One. 7:e475662012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Korn WM, Macal M, Christian C, Lacher MD,
McMillan A, Rauen KA, Warren RS and Ferrell L: Expression of the
coxsackievirus- and adenovirus receptor in gastrointestinal cancer
correlates with tumor differentiation. Cancer Gene Ther.
13:792–797. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rauen KA, Sudilovsky D, Le JL, Chew KL,
Hann B, Weinberg V, Schmitt LD and McCormick F: Expression of the
coxsackie adenovirus receptor in normal prostate and in primary and
metastatic prostate carcinoma: Potential relevance to gene therapy.
Cancer Res. 62:3812–3818. 2002.PubMed/NCBI
|
|
31
|
van Gijn W, Krijnen P, Lemmens VE, den
Dulk M, Putter H and van de Velde CJ: Quality assurance in rectal
cancer treatment in the Netherlands: A catch up compared to colon
cancer treatment. Eur J Surg Oncol. 36:340–344. 2010. View Article : Google Scholar
|
|
32
|
Cady B and Stone MD: The role of surgical
resection of liver metastases in colorectal carcinoma. Semin Oncol.
18:399–406. 1991.PubMed/NCBI
|
|
33
|
Tomlinson JS, Jarnagin WR, DeMatteo RP,
Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH and
D'Angelica M: Actual 10-year survival after resection of colorectal
liver metastases defines cure. J Clin Oncol. 25:4575–4580. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fisher KD, Stallwood Y, Green NK, Ulbrich
K, Mautner V and Seymour LW: Polymer-coated adenovirus permits
efficient retargeting and evades neutralising antibodies. Gene
Ther. 8:341–348. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lanciotti J, Song A, Doukas J, Sosnowski
B, Pierce G, Gregory R, Wadsworth S and O'Riordan C: Targeting
adenoviral vectors using heterofunctional polyethylene glycol FGF2
conjugates. Mol Ther. 8:99–107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Menezes KM, Mok HS and Barry MA: Increased
transduction of skeletal muscle cells by fibroblast growth
factor-modified adenoviral vectors. Hum Gene Ther. 17:314–320.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Segerman A, Atkinson JP, Marttila M,
Dennerquist V, Wadell G and Arnberg N: Adenovirus type 11 uses CD46
as a cellular receptor. J Virol. 77:9183–9191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Short JJ, Pereboev AV, Kawakami Y, Vasu C,
Holterman MJ and Curiel DT: Adenovirus serotype 3 utilizes CD80
(B7.1) and CD86 (B7.2) as cellular attachment receptors. Virology.
322:349–359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tuve S, Wang H, Ware C, Liu Y, Gaggar A,
Bernt K, Shay-akhmetov D, Li Z, Strauss R, Stone D and Lieber A: A
new group B adenovirus receptor is expressed at high levels on
human stem and tumor cells. J Virol. 80:12109–12120. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Krasnykh V, Belousova N, Korokhov N,
Mikheeva G and Curiel DT: Genetic targeting of an adenovirus vector
via replacement of the fiber protein with the phage T4 fibritin. J
Virol. 75:4176–4183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van Beusechem VW, van Rijswijk AL, van Es
HH, Haisma HJ, Pinedo HM and Gerritsen WR: Recombinant adenovirus
vectors with knobless fibers for targeted gene transfer. Gene Ther.
7:1940–641. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma J, Zhao J, Lu J, Jiang Y, Yang H, Li P,
Zhao M, Liu K and Dong Z: Coxsackievirus and adenovirus receptor
promotes antitumor activity of oncolytic adenovirus H101 in
esophageal cancer. Int J Mol Med. 30:1403–1409. 2012.PubMed/NCBI
|
|
43
|
Kitazono M, Goldsmith ME, Aikou T, Bates S
and Fojo T: Enhanced adenovirus transgene expression in malignant
cells treated with the histone deacetylase inhibitor FR901228.
Cancer Res. 61:6328–6330. 2001.PubMed/NCBI
|
|
44
|
Yoo GH, Piechocki MP, Oliver J, Lonardo F,
Zumstein L, Lin HS, Kim H, Shibuya TY, Shehadeh N and Ensley JF:
Enhancement of Ad-p53 therapy with docetaxel in head and neck
cancer. Laryngoscope. 114:1871–1879. 2004. View Article : Google Scholar : PubMed/NCBI
|