Introduction
The renin-angiotensin-aldosterone system (RAAS) has
an important role in homeostasis of the cardiovascular system
(1). Angiotensin II (AngII) has an
indispensable role in the RAAS, and is well recognized for its key
function in cardiovascular and renal physiology (2). AngII not only mediates immediate
physiological effects, including vasoconstriction and blood
pressure regulation, but is also involved in inflammation,
atherosclerosis, endothelial injury and heart failure (3). Heart failure is a complex clinical
syndrome that lowers quality of life. It can be caused by
hypertension, myocardial disease, coronary artery disease, or
numerous other related heart disorders. RAAS inhibitors are widely
used in the clinical therapy of heart failure. However, the exact
mechanism underlying their effects remains to be fully elucidated.
As a part of the RAAS, the effects of AngII are mediated by its
interaction with angiotensin II type 1 receptor (AT1R) and
angiotensin II type 2 receptor (AT2R) (4,5). The
majority of the effects of AngII are mediated by AT1R, including
extracellular matrix formation, increased vasopressin secretion,
vasoconstriction, cardiac hypertrophy and cardiac contractility
(6). AT1R is widespread in various
organs and tissues, such as the liver, adrenal cortex and some
areas of the brain. Vascular and myocardial tissues express more
AngII compared with other tissues (7,8).
Notably, a previous study revealed that angiotensin AT1
receptor-associated protein (ARAP1), which is also referred to as
angiopoietin-like protein 2 (Angptl2), was suppressed in the kidney
vasculature by AngII, which was mediated by AT1R (9). In this previous study, localization
of Angptl2 was detected in the kidney of mice and humans, in order
to confirm that Angptl2 expression can be regulated by AngII.
Angptl2 was initially cloned, expressed and
characterized in 1999 (10). It is
a member of the angiopoietin-like family, which contains eight
members of glycoproteins. It is a secreted circulating protein with
a molecular weight of 57 kDa, which consists of 493 amino acids
(10–12). Members of the angiopoietin-like
family possess a structure containing a typical N-terminal helical
coiled-coil domain, a short linker peptide and a C-terminal
globular fibrinogen-like domain, which is analogous to
angiopoietins (10). Angptl2 has
been detected in numerous mouse organs, with the highest expression
levels found in the heart, aorta, kidney and adrenal gland
(9). In addition, it has been
reported that the distribution of Angptl2 is consistent with AT1R.
The Angptl2 protein interacts with AT1R, and promotes AT1R
translocation to the plasma membrane, facilitating increased AT1R
surface expression in vitro (13). However, the expression levels of
Angptl2 have not been detected in vitro following
stimulation with AngII.
In the present study, a 48 h stimulation with 100
nmol/l AngII was initially conducted to observe alterations in
Angptl2 expression; these conditions are considered the most
appropriate for use in the kidney in vitro (9). The present study hypothesized that
Angptl2 expression would be suppressed in rat cardiomyocytes
following AngII treatment via AT1R. The most appropriate conditions
(100 nmol/l AngII, 24 h) were identified in the present study,
which were shown to significantly suppress Angptl2 expression.
Several experiments were subsequently conducted to explore the role
of AT1R in this process. Finally, a conclusion was drawn that the
expression of Angptl2 may be suppressed by AngII via AT1R in rat
cardiomyocytes.
Materials and methods
Isolation and culture of
cardiomyocytes
Male, 3-day-old Wistar rats used in the present
study were provided by the Animal Center of Shandong University
(Jinan, China). All animal experiments were performed in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals, and were approved by the ethics committee of
Shandong University. Primary neonatal rat cardiomyocyte cultures
were prepared as described in a previous study (14). Briefly, cardiomyocytes from Wistar
rats were cultured in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc., Shanghai, China) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Brisbane, Australia), 100 U/ml penicillin-streptomycin (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) at
37°C in an atmosphere containing 5% CO2. The
cardiomyocytes were incubated at a density of 5×105
cells/ml. Subsequent experiments were conducted on first generation
primary cardiomyocytes.
Preparation of cultured cells
To illustrate the effects of AngII on Angptl2
expression, AngII (Sigma-Aldrich Chemie GmbH, Hamburg, Germany) was
added to the culture medium in six-well plates containing
cardiomyocytes (5×105 cells/ml) at the following final
concentrations: 0, 50, 100 and 200 nmol/l for 24 h. In addition,
cells were incubated with 100 nmol/l AngII for 0, 6, 24 or 48 h.
The culture medium from each condition was collected. Total protein
was extracted from the rat cardiomyocytes using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Beijing, China), which contained 0.1 M
phenylmethylsulfonyl fluoride. After washing with
phosphate-buffered saline (PBS), grinding, lysis and centrifugation
(at 4°C, 13,000 × g, 15 min), the supernatant was collected.
Protein concentrations were measured using the bicinchoninic acid
assay (Protein Assay kit; Beyotime Institute of Biotechnology), in
which bovine serum albumin was used as a standard. After adding 5×
loading buffer [sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) Loading Buffer/Lane Markers; Beyotime
Institute of Biotechnology], the mixture was boiled in water at
100°C for 8 min, and was stored at −80°C.
Immunofluorescence
α-Smooth muscle actin (α-SMA) was used as a marker
to identify cardiomyocytes. The extracted cells were identified as
cardiomyocytes by observing cellular morphology using
immunofluorescent staining (14–16).
Briefly, the cells were rinsed with PBS and were fixed with 4%
paraformaldehyde for 15 min. Subsequently, they were permeabilized
with 0.3% Triton X-100 for 20 min. Nonspecific antibody binding
sites were blocked by incubating the cells in normal goat serum
(Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China)
for 30 min. The cells were then incubated with α-SMA (1:200; cat.
no. 6487; Cell Signaling Technology, Inc., Danvers, MA, USA) and
Angptl2 antibodies (1:400; cat. no. sc-292811; Santa Cruz
Biotechnology, Inc.) overnight in a humidified incubator at 4°C.
The following day cells were incubated with Alexa Fluor 488 goat
anti-rabbit immunoglobulin G secondary antibody (cat. no. A-11034;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 35 min at
37°C and were washed three times with PBS. After the final wash,
nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 5
min. Images were acquired under an inverted fluorescence microscope
(Olympus Corporation, Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the rat cardiomyocytes
following various treatment conditions using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). RNA concentration was
determined using the Thermo Scientific Nano Drop 2000/2000c
Spectrometer (Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
cDNA was obtained by RT using Takara PrimeScript RT Reagent kit
with gDNA Eraser (Takara Biotechnology Co., Ltd., Dalian, China)
according the manufacturer's specifications. qPCR was then
performed to detect Angptl2 and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) expression using the Applied Biosystems 7300
fast real-time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The mRNA expression levels of
Angptl2 were normalized to GAPDH mRNA expression levels. qPCR
amplification was carried out in a total volume of 20 μl,
containing 10 ml SYBR Premix Ex Taq (Takara Biotechnology Co.,
Ltd.), 0.4 ml each of forward and reverse primers, 6.8 ml PCR-grade
sterile water and 2 ml DNA templates.
The oligonucleotide primers used were as follows:
Rat Angptl2, forward 5′-gaagcatgaagccctgctc-3′, reverse,
5′-cagcagtccaagccaccagta-3′; and rat GAP DH, forward
5′-gcaagagagaggccctcag-3′, and reverse 5′-tgtgagggagatgctcagtg-3′.
The cycling conditions were as follows: Initial denaturation at
95°C for 30 sec, 40 cycles at 95°C for 5 sec, 60°C for 34 sec and
95°C for 15 sec, followed by 60°C for 1 min and 95°C for 15 sec.
The ΔCq values were obtained by subtracting the quantification
cycle (Cq) value of GAPDH from the Cq value of Angptl2. The ΔCq
values of each treatment group were further compared with those of
the controls (ΔΔCq) to obtain relative quantification of the target
gene expression (17).
SDS-PAGE and western blotting
Protein samples (100 μg) extracted from rat
cardiomyocytes were separated by 10% SDS-PAGE and were transferred
onto polyvinylidene fluoride membranes (EMD Millipore, Billerica,
MA, USA). The membranes were blocked in 5% non-fat milk at room
temperature for 1 h. The membranes were then immunoblotted with
Angptl2 (1:400; cat. no. sc-292811; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and GAPDH antibodies (1:10,000; cat. no.
60004-1-Ig; Wuhan Sanying Biotechnology, Wuhan, China) at 4°C
overnight, followed by goat anti-rabbit (1:3,000; cat. no. LK2001;
Sanjian, Tianjin, China) and rabbit anti-mouse (1:5,000; cat. no.
GTX29170; GeneTex, Inc., Irvine, CA, USA) secondary antibodies for
1 h. Subsequently, the images were detected using Luminol Reagent
(EMD Millipore) and FluorChem E (ProteinSimple, San Jose, CA USA).
Analysis of western blotting was conducted using ImageJ software
(version 2.1.4.7; National Institutes of Health, Bethesda, MD,
USA).
Regulation of Angptl2 expression using
AT1R and AT2R antagonists
To further explore the role of AT1R in Angptl2
expression regulation, additional experiments were conducted. AT1R
antagonist (losartan; 10−4mol/l; Sigma-Aldrich, St.
Louis, MO, USA) and AT2R antagonist (PD123319;
10−4mol/l; Abcam, Cambridge, UK) were added to the cells
45 min prior to treatment with AngII (100 nmol/l). After 24 h,
protein, RNA and culture media were extracted. The normal +
losartan/PD123319 groups were used as negative controls.
Enzyme-linked immunosorbent assay
(ELISA)
The culture medium was harvested from the culture
plates of various treatment groups. Angptl2 expression was measured
using an Angptl2 detection kit (Human ANGPTL2 ELISA kit; R&D
Systems, Inc., Minneapolis, MN, USA), according to the
manufacturer's protocol. Angptl2 levels were calculated according
to a standard curve and were corrected using an blank well.
Statistical analysis
Student's t test was used to analyze differences
between two groups. One-way analysis of variance with Bonferroni's
tests was used to analyze more than two groups. All analyses were
performed using GraphPad Prism v 5.01 software (GraphPad Software,
Inc., La Jolla, CA, USA). All the experimental data are presented
as the mean ± standard deviation and the mean values were
calculated according to at least three independent experiments.
P<0.05 was considered to indicate a statistically significant
difference.
Results
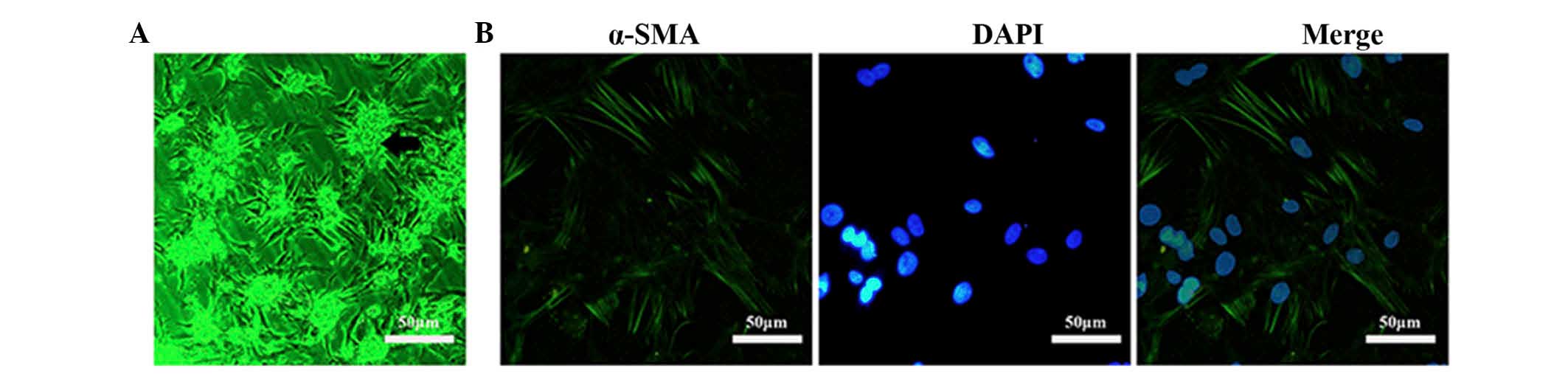
Identification of cardiomyocytes
Primary cardiomyocytes were isolated from 3-day-old
rats. The cellular morphology was observed under a microscope.
Cardiomyocytes were shown to grow in a group, which adhered to the
bottom of the dish (black arrow, Fig.
1A). In addition, the cardiomyocytes were detected to produce a
certain beating rhythm under the microscope. The identification of
cardiomyocytes was also verified by detecting α-SMA expression,
which was chosen as a label of cardiomyocytes. Following incubation
with α-SMA antibodies, the cells were stained with a secondary
antibody, which was equipped with fluorescence (green), and DNA was
stained with DAPI (blue). Fibroblasts cultured with cardiomyocytes
exhibited negative staining for α-SMA. Images were captured at a
magnification of ×200 (Fig. 1B).
Stained cells were counted under a microscope in four fields. The
purity of cardiomyocytes in the isolated primary cells was
~94%.
Effects of various AngII concentrations
on cardiomyocytes
To explore the effects of AngII on cardiomyocytes,
the cells were incubated with various concentrations of AngII (0,
50, 100 or 200 nmol/l) for 24 h, or were incubated with 100 nmol/l
AngII for various durations (0, 6, 24 or 48 h). As shown in
Fig. 2A, the mRNA expression
levels of Angptl2 were initially quantified in the cardiomyocyte
samples, which were divided into two groups: The normal group and
the cardiomyocyte group, which was stimulated with 100 nmol/l AngII
for 48 h. The expression levels of Angptl2 were measured by RT-qPCR
and were normalized to GAPDH. Treatment with 100 nmol/l AngII
resulted in a decrease in Angptl2 mRNA expression (0.604±0.061) in
rat cardiomyocytes compared with in the normal group (1.069±0.047;
P<0.05; Fig. 2A). As shown in
Fig. 2B and C, the protein
expression levels of Angptl2 were measured following incubation for
various durations with various AngII concentrations, respectively.
The results demonstrated that following incubation with 100 nmol/l
AngII for 24 h, the protein expression levels of Angptl2
(0.59±0.044) were more obviously decreased than under any of the
other conditions, as compared with the normal group (1.78±0,029;
P<0.05; Fig. 2B and C). An
ELISA was also conducted to detect Angptl2 expression, in order to
verify this trend. Culture media samples were used, which were
collected from cells treated under the same conditions as for
western blotting (Fig. 2D and E).
The results of the ELISA were consistent with those of western
blotting. Immunofluorescence was used to further verify the trend;
DNA was stained with DAPI (blue) and Angptl2 was stained with green
fluorescence (Fig. 2F). Blue and
green fluorescence were assessed simultaneously under a
magnification of ×400. Representative images were captured under
identical exposure parameters. ImageJ software was used to analyze
the fluorescence (Fig. 2G). The
expression levels of Angtpl2 were markedly decreased following
treatment with 100 nmol/l AngII for 24 h.
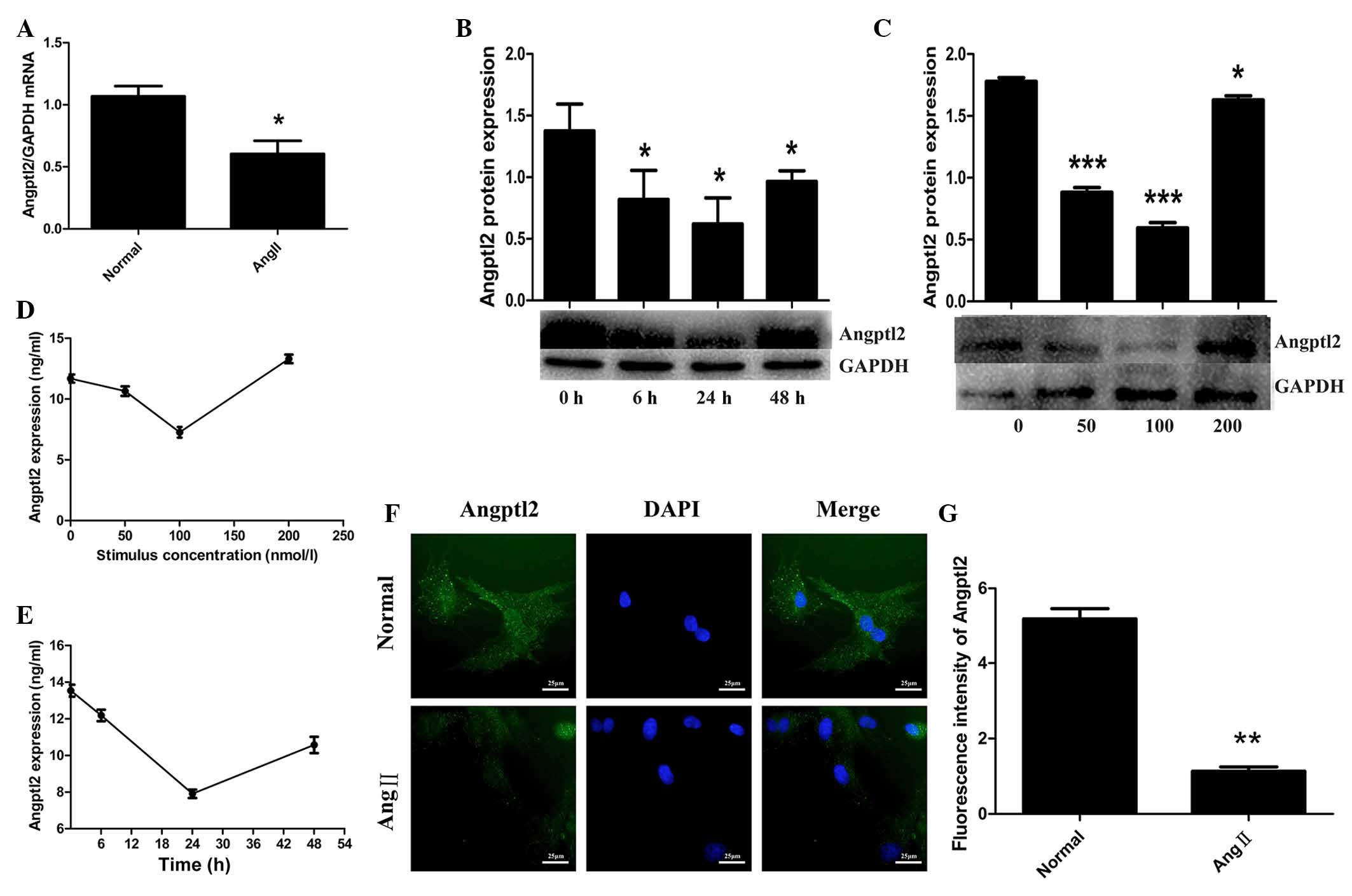 | Figure 2Effects of various angiotensin II
(AngII) concentrations on cardiomyocytes. (A) mRNA expression
levels of angiopoietin-like protein 2 (Angptl2) were measured by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and were normalized to glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) following treatment with 100 nmol/l AngII for
48 h. cDNA sample size was 100 ng. Protein expression levels of
Angptl2 were detected by western blotting (B) following treatment
with 100 nmol/l AngII for various durations (0, 6, 24 and 48 h;
protein sample size, 100 μg), (C) or with various
concentrations of AngII (0, 50, 100 or 200 nmol/l) for 24 h
(protein sample size, 40 μg). Enzyme-linked immunosorbent
assay for Angptl2 was used to verify the trend detected by RT-qPCR
and western blotting following treatment of cells with (D) various
concentrations of AngII (E) for various durations (sample size of
each group, 10 μl). (F) Immunofluorescence was used to
confirm the conclusions of these experiments. Green staining,
Angptl2; blue staining, 4′,6-diamidino-2-phenylindole (DAPI);
magnification, ×400. (G) Fluorescence intensity was analyzed using
ImageJ software. *P<0.05, **P<0.01 and
***P<0.001, vs. the corresponding control group (0 h
or 0 nmol/l). |
Role of AT1R and AT2R antagonists
Additional experiments were conducted to determine
the possible role AT1R had with regards to this phenomenon.
Following treatment with losartan (10−4mol/l) and
PD123319 (10−4mol/l), the alterations in Angptl2
expression were detected in cardiomyocytes using western blotting,
ELISA, RT-qPCR and immunofluorescence. Analysis of western blotting
revealed that the protein expression levels of Angtpl2 were
significantly decreased in the AngII group (0.17±0.012) compared
with in the normal group (0.52±0.018; P<0.05; Fig. 3A). Conversely, the AngII + losartan
group exhibited increased Angptl2 expression (0.55±0.016) compared
with the AngII group (P<0.05; Fig.
3A). Angptl2 expression in the normal + losartan group
(0.61±0.0091) was similar to that in the normal group (P>0.05;
Fig. 3A). The Angptl2 expression
level in each group is shown in Fig.
3B. The expression level of Angptl2 in the AngII group was
decreased significantly compared with the normal group (P<0.05),
whereas the AngII + PD123319 group exhibited no difference compared
with the AngII group. Additionally, the normal + PD123319 exhibited
no significant difference compared with the normal group. RT-qPCR
was also performed to detect Angptl2 expression, and the results
are displayed in Fig. 3C.
Furthermore, the secretion of Angptl2 was detected using an ELISA.
The ELISA results were consistent with those of western blotting
(Fig. 3D). Immunofluorescence was
performed concurrently and the results are presented in Fig. 3E. DNA was stained with DAPI (blue),
and Angptl2 was stained using an antibody equipped with
fluorescence (green). Representative images were captured under
identical exposure parameters, and ImageJ was used to analyze
fluorescence intensity (Fig. 3F).
The RT-PCR and immunofluorescence results were in accoradance with
the western blot analysis. These data suggest that Angptl2 is
suppressed by AngII (100 nmol/l, 24 h), and this effect may be
reversed by losartan (AT1R antagonist) but not by PD123319 (AT2R
antagonist).
 | Figure 3Role of angiotensin II type 1 receptor
(AT1R) and angiotensin II type 2 receptor (AT2R) antagonists. To
determine the possible underlying mechanisms associated with the
effects of angiotensin II (AngII) on angiopoietin-like protein 2
(Angptl2), the cells were divided into six groups: Normal, AngII
(100 nmol/l), AngII (100 nmol/l) + losartan (10−4mol/l),
AngII (100 nmol/l) + PD123319 (10−4mol/l), normal +
losartan (10−4mol/l) and normal + PD123319
(10−4mol/l). (A and B) Angptl2 protein expression was
detected in each group by western blotting (protein sample size, 40
μg). (C) Angptl2 mRNA expression was detected in each group
by reverse transcription-quantitative polymerase chain reaction
(cDNA sample size, 100 ng), (D) enzyme-linked immunosorbent assay
(culture media volume, 10 μl) and (E) immunofluorescence.
(F) ImageJ software was used to analyze fluorescence intensity;
blue and green fluorescence were assessed simultaneously
(magnification, ×200). Data are presented as the mean ± standard
deviation. *P<0.05, **P<0.01 and
***P<0.001, comparison indicated by brackets. ns, not
significant. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
Cardiac hypertrophy is an adaptive response that
occurs in physical and pathological conditions, which ultimately
results in heart failure. Pathological cardiac hypertrophy, which
is caused by hypertension, myocardial infarction, cardiomyopathy or
structural heart diseases, is associated with cardiac structural
remodeling (18). Higher plasma
levels of AngII have been detected in the heart of patients with
cardiac hypertrophy and heart failure (19). AngII is involved in the RAAS and
has vital roles in several diseases; the majority of effects of
AngII are mediated by AT1R. Angptl2 is a member of the Angptl
family, which has a vital role in AT1R recycling (20). Furthermore, Huang et al
(21) revealed that Angptl2 serum
levels in patients with heart failure are independently associated
with heart failure. Therefore, Angptl2 may be related to cardiac
hypertrophy; however, there is currently no evidence to verify its
relationship with heart failure.
The results of the present study suggested that
Angptl2 is suppressed by AngII in cardiomyocytes in vitro.
The study speculated that AT1R may be the key factor mediating the
effects of AngII in this process. To test this assumption an
antagonist of AT1R was used, and an antagonist of AT2R was used as
a negative control. The normal + losartan/PD123319 groups were also
the negative control groups to testify the conclusion of this
study. Analysis of the experimental data supported the
aforementioned hypothesis.
Angptl2 is well recognized in several chronic
diseases, including atherosclerosis, diabetes, metabolic disorders
and cancer, due to its harmful proinflammatory properties (22). Angptl2 has been reported to act as
a key inflammatory factor in activating adhesion molecule
expression, thus increasing circulating cholesterol levels and
accelerating the development of atherosclerotic plaques in
dyslipidemic and pre-atherosclerotic mice (23). Angptl2 expression may increase
following stimulation with AngII according to its harmful
properties (21). However, the
present study demonstrated that AngII had the opposite effect on
Angptl2 expression. Furthermore, a previous study reported that
Angptl2 expression in the heart and aorta remained unchanged
following stimulation with AngII (9). This contradiction is probably due to
the use of chronic infusion of AngII in vivo, whereas the
present study was conducted in vitro, with cardiomyocytes
being treated with 100 nmol/l AngII for 24 h. Various exposure
times and concentrations may also account for this discrepancy.
This phenomenon can be explored by further in vivo and in
vitro studies.
The expression levels of ARAP1 have been reported to
be suppressed by AngII in mice kidney vasculature (9). ARAP1, is an alternative name for
Angptl2; this name is typically only used when referring to the
interaction with AngII (22). The
present study focused on the interaction of Angptl2 with AT1R.
Previous studies have proposed the possibility of additional
intracellular proteins that may interact with AT1R, and have an
imperative role in receptor recycling (24–30).
Angptl2 can modulate AT1R expression at the cell surface, enhancing
its sensitivity to AngII (31,32).
It has also been reported that Angptl2 is downregulated during
sepsis, causing decreased renal sensitivity to AngII and eventually
contributing to hypotension, demonstrating that Angptl2 takes part
in AT1R recycling (33). These
results suggested that Angptl2 promotes AT1R recycling to the
plasma membrane (34) and enhances
sensitivity to AngII in various pathophysiological processes. In
the present study, following stimulation with 100 nmol/l AngII for
24 h, Angptl2 expression levels were decreased; however, Angptl2
expression was increased following treatment with an AT1R
antagonist. Therefore, it may be hypothesized that under AngII
stimulation, AngII promotes the hypertrophy of cardiomyocytes by
interacting with active AT1R, thus cardiomyocytes express less
Angptl2 in order to decrease AT1R sensitivity so as to adapt to the
excessive amount of active AT1R.
In conclusion, the results of the present in
vitro study suggested that Angptl2 expression is suppressed by
AngII, which may be mediated by AT1R activation. In normal
cardiomyocytes, treatment with AT1R or AT2R antagonist exerted no
significant effect on Angptl2 expression. Therefore, Angptl2 may
serve as a local modulator of AT1R function and may contribute to
cardiomyocyte adaptations in response to increased levels of
circulating AngII. In the present study, Angptl2 expression was
demonstrated to be more markedly suppressed following treatment
with 100 nmol/l AngII for 24 h, as compared with any other
conditions. Following stimulation with 200 nmol/l AngII, Angptl2
expression was increased compared with treatment with 100 nmol/l
AngII for 24 h; therefore, the suppression of Angptl2 by AngII was
shown to be attenuated by time extension. The results of the
present study also suggested that Angptl2 expression is suppressed
by AngII at lower concentrations, and this effect, which is
mediated by AT1R, may have a role at a certain concentration range.
Another possibility is that, as time prolongs and concentration
increases, AT1R may saturate, and Angptl2 would increase to improve
the sensitivity of AT1R to AngII. Further studies may explore the
possibility that Angptl2 expression is altered under lower and
higher concentrations of AngII in vitro and in vivo,
and it is hypothesized that various mechanisms will lead to this
effect. Since AT1R, which is an indispensable component of the
RAAS, has a central role in several pathophysiological processes,
Angptl2 may be involved in the process of cardiovascular diseases
and function. The results of the present study may be used to
propose a novel therapeutic method for the treatment of
cardiovascular disease by decreasing Angptl2. Undoubtedly, numerous
aspects of the present study require verification. The
concentration range of AngII that suppressed Angptl2 in
cardiomyocytes is not definite, and the explicit mechanism
underlying this phenomenon is not certain. AngII is able to
suppress Angptl2 in cardiomyocytes, and its effects on
cardiomyocytes require further research. In addition, although
Angptl2 and ARAP1 are encoded by the same gene, this does not
necessarily mean that the matching proteins are entirely identical.
More extensive experiments and further clinical proof are required
to support this speculation in future studies.
Acknowledgments
The authors would like to thank the Department of
Cardiology and Central Lab of Jinan Central Hospital for technical
assistance. The present study was supported by grants from the
Natural Science Foundation of Shandong Province (grant no.
ZR2013HM003), the Natural Science Foundation of China (grant no.
81170087), the National Natural Science Funds for Young Scholar
(grant no. 81200211) and the National Natural Science Foundation of
China Grant (grant no. 81270175).
References
|
1
|
Cai Y, Wang Y, Xu J, Zuo X and Xu Y:
Down-regulation of ether-a-go-go-related gene potassium channel
protein through sustained stimulation of AT1 receptor by
angiotensin II. Biochem Biophys Res Commun. 452:852–857. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Welter H, Huber A, Lauf S, Einwang D,
Mayer C, Schwarzer JU, Köhn FM and Mayerhofer A: Angiotensin II
regulates testicular peritubular cell function via AT1 receptor: A
specific situation in male infertility. Mol Cell Endocrinol.
393:171–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsoukas MT, Potamitis C, Plotas P,
Androutsou ME, Agelis G, Matsoukas J and Zoumpoulakis P: Insights
into AT1 receptor activation through AngII binding studies. J Chem
Inf Model. 53:2798–2811. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reid IA: Interactions between ANG II,
sympathetic nervous system, and baroreceptor reflexes in regulation
of blood pressure. Am J Physiol. 262:E763–E778. 1992.PubMed/NCBI
|
|
5
|
Siragy HM: AT(1) and AT(2) receptors in
the kidney: Role in disease and treatment. Am J Kidney Dis. 36(3
Suppl 1): S4–S9. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmieder RE, Hilgers KF, Schlaich MP and
Schmidt BM: Renin-angiotensin system and cardiovascular risk.
Lancet. 369:1208–1219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Messerli FH, Weber MA and Brunner HR:
Angiotensin II receptor inhibition. A new therapeutic principle.
Arch Intern Med. 156:1957–1965. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goodfriend TL, Elliott ME and Catt KJ:
Angiotensin receptors and their antagonists. N Engl J Med.
334:1649–1654. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doblinger E, Höcherl K, Mederle K, Kattler
V, Walter S, Hansen PB, Jensen B and Castrop H: Angiotensin AT1
receptor-associated protein Arap1 in the kidney vasculature is
suppressed by angiotensin II. Am J Physiol Renal Physiol.
302:F1313–F1324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SS, Choi IG, Kim SH and Yu YG:
Molecular cloning, expression, and characterization of a
thermostable glutamate racemase from a hyperthermophilic bacterium,
Aquifex pyrophilus. Extremophiles. 3:175–183. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tabata M, Kadomatsu T, Fukuhara S, Miyata
K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, et al:
Angiopoietin-like protein 2 promotes chronic adipose tissue
inflammation and obesity-related systemic insulin resistance. Cell
Metab. 10:178–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kadomatsu T, Tabata M and Oike Y:
Angiopoietin-like proteins: Emerging targets for treatment of
obesity and related metabolic diseases. FEBS J. 278:559–564. 2011.
View Article : Google Scholar
|
|
13
|
Guo DF, Chenier I, Tardif V, Orlov SN and
Inagami T: Type 1 angiotensin II receptor-associated protein ARAP1
binds and recycles the receptor to the plasma membrane. Biochem
Biophys Res Commun. 310:1254–1265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsui H, Yokoyama T, Tanaka C, Sunaga H,
Koitabashi N, Takizawa T, Arai M and Kurabayashi M: Pressure
mediated hypertrophy and mechanical stretch up-regulate expression
of the long form of leptin receptor (ob-Rb) in rat cardiac
myocytes. BMC Cell Biol. 13:372012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tao T, Wang X, Liu M and Liu X:
Myofibrillogenesis regulator-1 attenuates
hypoxia/reoxygenation-induced injury by repairing microfilaments in
neonatal rat cardiomyocytes. Exp Cell Res. 337:234–242. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cao T, Gao Z, Gu L, Chen M, Yang B, Cao K,
Huang H and Li M: AdipoR1/APPL1 potentiates the protective effects
of globular adiponectin on angiotensin II-induced cardiac
hypertrophy and fibrosis in neonatal rat atrial myocytes and
fibroblasts. PLoS One. 9:e1037932014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Oka T, Akazawa H, Naito AT and Komuro I:
Angiogenesis and cardiac hypertrophy: Maintenance of cardiac
function and causative roles in heart failure. Circ Res.
114:565–571. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Serneri GG, Boddi M, Cecioni I, Vanni S,
Coppo M, Papa ML, Bandinelli B, Bertolozzi I, Polidori G, Toscano
T, et al: Cardiac angiotensin II formation in the clinical course
of heart failure and its relationship with left ventricular
function. Circ Res. 88:961–968. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo DF, Chenier I, Lavoie JL, Chan JS,
Hamet P, Tremblay J, Chen XM, Wang DH and Inagami T: Development of
hypertension and kidney hypertrophy in transgenic mice
overexpressing ARAP1 gene in the kidney. Hypertension. 48:453–459.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang CL, Wu YW, Wu CC, Hwang JJ and Yang
WS: Serum Angiopoietin-like protein 2 concentrations are
independently associated with heart failure. PLoS One.
10:e01386782015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thorin-Trescases N and Thorin E:
Angiopoietin-like-2: A multifaceted protein with physiological and
pathophysiological properties. Expert Rev Mol Med. 16:e172014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Farhat N, Thorin-Trescases N, Mamarbachi
M, Villeneuve L, Yu C, Martel C, Duquette N, Gayda M, Nigam A,
Juneau M, et al: Angiopoietin-like 2 promotes atherogenesis in
mice. J Am Heart Assoc. 2:e0002012013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ali MS, Sayeski PP, Dirksen LB, Hayzer DJ,
Marrero MB and Bernstein KE: Dependence on the motif YIPP for the
physical association of Jak2 kinase with the intracellular carboxyl
tail of the angiotensin II AT1 receptor. J Biol Chem.
272:23382–23388. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sano T, Ohyama K, Yamano Y, Nakagomi Y,
Nakazawa S, Kikyo M, Shirai H, Blank JS, Exton JH and Inagami T: A
domain for G protein coupling in carboxyl-terminal tail of rat
angiotensin II receptor type 1A. J Biol Chem. 272:23631–23636.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Venema RC, Ju H, Venema VJ, Schieffer B,
Harp JB, Ling BN, Eaton DC and Marrero MB: Angiotensin II-induced
association of phospholipase Cgamma1 with the G-protein-coupled AT1
receptor. J Biol Chem. 273:7703–7708. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith RD, Hunyady L, Olivares-Reyes JA,
Mihalik B, Jayadev S and Catt KJ: Agonist-induced phosphorylation
of the angiotensin AT1a receptor is localized to a
serine/threonine-rich region of its cytoplasmic tail. Mol
Pharmacol. 54:935–941. 1998.PubMed/NCBI
|
|
28
|
Smith RD, Baukal AJ, Zolyomi A, Gaborik Z,
Hunyady L, Sun L, Zhang M, Chen HC and Catt KJ: Agonist-induced
phosphorylation of the endogenous AT1 angiotensin receptor in
bovine adrenal glomerulosa cells. Mol Endocrinol. 12:634–644. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chaki S, Guo DF, Yamano Y, Ohyama K, Tani
M, Mizukoshi M, Shirai H and Inagami T: Role of carboxyl tail of
the rat angiotensin II type 1A receptor in agonist-induced
internalization of the receptor. Kidney Int. 46:1492–1495. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hunyady L, Bor M, Balla T and Catt KJ:
Identification of a cytoplasmic Ser-Thr-Leu motif that determines
agonist-induced internalization of the AT1 angiotensin receptor. J
Biol Chem. 269:31378–31382. 1994.PubMed/NCBI
|
|
31
|
Castrop H: Angiotensin receptor-associated
proteins: Local modulators of the renin-angiotensin system.
Pflugers Arch. 465:111–119. 2013. View Article : Google Scholar
|
|
32
|
Higuchi S, Ohtsu H, Suzuki H, Shirai H,
Frank GD and Eguchi S: Angiotensin II signal transduction through
the AT1 receptor: Novel insights into mechanisms and
pathophysiology. Clin Sci (Lond). 112:417–428. 2007. View Article : Google Scholar
|
|
33
|
Mederle K, Schweda F, Kattler V, Doblinger
E, Miyata K, Höcherl K, Oike Y and Castrop H: The angiotensin II
AT1 receptor-associated protein Arap1 is involved in sepsis-induced
hypotension. Crit Care. 17:R1302013. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo DF, Sun YL, Hamet P and Inagami T: The
angiotensin II type 1 receptor and receptor-associated proteins.
Cell Res. 11:165–180. 2001. View Article : Google Scholar : PubMed/NCBI
|