Introduction
The immune system provides the host with defense
against foreign antigens, and ensures limiting activation against
self-antigens during immune surveillance (1). Immunomodulation either stimulates or
suppresses the host immune system, in order to enable it to succeed
against perturbing conditions. Immunomodulators are biological
agents that improve the host defense mechanism against disease, by
striking a balance between regulatory and effector cells (2). Nutrients and other spice constituents
have the potential to affect almost all aspects of the immune
system (3).
Garlic (Allium sativum) is a member of the
lily family, which has been widely used as an ancient folk medicine
in India, Egypt, Greece, Rome and China to treat various
sicknesses, including abdominal pain, parasitic infections and
rheumatism (4). Its high vitamin C
content and antimicrobial properties make it a significant and
potent immune system booster. In addition, it is very effective
against bacterial, viral, fungal and parasitic infections (5). Garlic enhances various immune
factors, such as macrophage and T-lymphocyte phagocytic activities
(6). The immunomodulatory effects
of garlic are due to garlic lectin or agglutinin proteins (7). In a previous study, protein fractions
purified from fresh garlic bulbs were reported to augment
CD8+ T-cell infiltration into the tumor site and inhibit
tumor growth (8).
Levamisole (LEVA) is a synthetic broad-spectrum
anthelmintic widely used in veterinary practice. LEVA has attracted
attention due to its use as an immunomodulator, and it has been
shown to support anti-carcinogenic activity in skin diseases and
improve weight gain in animals (9,10).
In some countries, LEVA is used as an immunomodulatory agent to
treat human cancer (11).
LEVA is able to modulate the immune system by
resetting the immune balance towards a T helper (Th)1 response
(11,12). It has been reported to reconstitute
the histological integrity of the thymus in malnutrition (13). Furthermore, its influence on the
course of allergic diseases has been demonstrated to cause a shift
from Th2-dominant immunity towards a Th1-mediated response in
BALB/c mice (14). LEVA has been
reported to induce sufficient dendritic cell maturation, in order
to promote the activation of naïve T cells toward a Th1 response
(15). LEVA has also been reported
to increase serum complement activity and leukocyte functions,
including phagocytosis and/or lymphokine production by lymphocytes
(16). LEVA-induced suppression of
the Th2 immune response and stimulation of the Th1 immune response
are clinically important in the prevention and treatment of atopic
diseases (17). LEVA stimulates
the production of several cytokines, including interferon (IFN)-γ,
interleukin (IL)-6, IL-12, IL-18 and IL-1 (12,15).
The immunohistochemical study of CD4 and CD8 is important; however,
few studies have been conducted.
The present study aimed to investigate the
biochemical, immunohistochemical and molecular effects of garlic
extract and LEVA alone or in combination on the immune response in
Wistar rats.
Materials and methods
Chemicals and kits
LEVA, ethidium bromide and agarose were purchased
from Sigma-Aldrich (St. Louis, MO, USA). The Wistar rats were
purchased from King Fahd Center for Scientific Research (King
Abdel-Aziz University, Jeddah, Saudi Arabia). Serologic mouse
interferon gamma (cat. no. MBS289298), IL-5 (mouse) (cat. no.
MBS260096) and rat TNF-alpha (cat. no. MBS2882073) enzyme-linked
immunosorbent assay (ELISA) kits were purchased from My Biosource,
Inc. (San Diego, CA, USA). The DNA 100 bp ladder was purchased from
Fermentas (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
QIAzol for RNA extraction and oligo dT primers were purchased from
Qiagen, Inc. (Valencia, CA, USA). Anti-rat CD4 (cat. no. sc-7219)
and CD8 (cat. no. sc-18860) primary antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Animals, experimental design and
sampling
All animal procedures were approved by the Ethical
Committee Office of the dean of scientific affairs of Taif
University (Taif, Saudi Arabia). A total of 24 male Wistar rats
(age, 3 months; weight, 200–280 g) were used in the present study.
For acclimation, the rats were handled daily and kept under
observation for 1 week prior to onset of the experiment. The rats
were maintained under a 12-h light-dark cycle, and were given ad
libitum access to food and water. The 24 rats were divided into
four groups (n=6/group): Control group, which received only corn
oil as treatment; LEVA group, which were orally administered LEVA
[2.5 mg/kg body weight (BW)] every 2 days for 4 weeks; garlic oil
(GO; Sigma-Aldrich) group, which were orally administered GO (5
ml/kg BW) daily for 4 weeks (18);
and the LEVA plus GO group, which were administered LEVA and GO (5
ml GO plus 2.5 mg/kg BW LEVA) for 4 weeks.
A total of 24 h after administration of the final
medication, all rats were sacrificed following anesthetization by
diethyl ether inhalation. Blood samples were subsequently taken
from the medial canthus of the eye. The rat heads were then
dislocated, and tissue samples were collected under sterile
conditions. Serum samples were extracted following centrifugation
of the blood at 4,000 × g for 10 min at 4°C. For gene expression
analysis, thymus tissues and blood samples were maintained in
QIAzol reagent at −80°C for RNA extraction. Spleen samples were
maintained in 10% neutral buffered formalin (NBF) at room
temperature for 24 h for histopathological and immunohistochemical
analysis.
Serum cytokine and immunoglobulin (Ig)
assays
IgG and IgM were measured in the serum samples
obtained from rats using a radial immunodiffusion assay purchased
from First Clinical Laboratory (Cairo, Egypt). Levels of the
following serum cytokines: IFN-γ, IL-5 and tumor necrosis factor
(TNF)-α were measured using ELISA kits, according to the
manufacturer's protocols.
Total blood counts and lysozyme
activity
Total blood counts of EDTA blood were measured
automatically using a cell counter set (Bio-Rad Laboratories Inc.,
Hercules, CA, USA). Lysozyme enzymatic activity in the serum
samples was determined by measuring the diameters of the zones of
clearance relative to lysozyme (19).
Histological examination
Samples for histological examination were taken from
the spleen, were fixed in 10% NBF solution, washed in tap water,
dehydrated using various grades of alcohol, cleared using xylene,
and embedded in paraffin. The paraffin-embedded tissue blocks were
cut into 5 µm sections. The sections were then routinely
stained with hematoxylin and eosin (20). Tissue slides were visualized using
a Wolfe S9-0982 microscope (Carolina Biological Supply Co.,
Burlington, NC, USA) and images were captured using a Canon Power
Shot SX500 IS digital camera (Canon, Inc., Tokyo, Japan).
Immunohistochemistry
Immunohistochemical reactions were carried out using
the streptavidin-biotin-peroxidase method. Primary antibodies
against CD4 and CD8 (Santa Cruz Biotechnology, Inc.) were diluted
to 1:500 in phosphate-buffered saline (PBS). As positive controls,
normal tissue (spleen and/or thymus) was tested for CD4 and
CD8.
Spleen sections were deparaffinized in xylene,
washed in PBS (pH 7.4), and rehydrated in a graded series of
ethanol solutions. Endogenous peroxidase activity was blocked
following incubation with 3% hydrogen peroxide in methanol for 10
min. After washing in PBS, the specimens were saturated with 10%
normal goat serum (Histofine SAB-PO kit; Nichirei Corporation) for
5 min and were incubated at room temperature for 30 min with the
primary antibodies. After washing in PBS, the slides were incubated
with biotinylated goat anti-mouse Ig antibody (Histofine SAB-PO
kit; Nichirei Corporation) for 60 min at room temperature.
Immunohistochemical reactions were visualized using freshly
prepared 3,3β-diaminobenzidine tetrahydrochloride (Histofine SAB-PO
kit; Nichirei Corporation). Subsequently, slides were
counterstained with hematoxylin and were mounted on cover-slips
(21). Tissue slides were
visualized using a Wolfe S9-0982 microscope and images were
captured using a Canon Power Shot SX500 IS digital camera.
RNA extraction, cDNA synthesis and
semi-quantitative polymerase chain reaction (PCR) analysis
All rats were anesthetized by diethyl ether
inhalation until they lost consciousness. Subsequently, blood
samples were taken from the medial canthus of the eye. Total RNA
was extracted from the thymus as previously discussed (22). For leukocyte RNA extraction, 1 ml
QIAzol was added to 350 ml total blood collected in tubes
containing an anticoagulant. RNA concentration and purity were
determined spectrophotometrically by measuring optical density at
260 and 280 nm. RNA integrity was confirmed after running on a 1.5%
denatured agarose gel stained with ethidium bromide. The ratio of
260/280 optical density of all RNA samples was 1.7–1.9. A mixture
of 3 µg total RNA and 0.5 ng oligo dT primer was used for
cDNA synthesis, in a total volume of 11 µl sterilized DEPC
water. The mixture was incubated in a Bio-Rad T100™ Thermal Cycler
(Bio-Rad Laboratories, Inc.) at 65°C for 10 min for denaturation.
Subsequently, 2 µl 10X reverse transcription-buffer, 2
µl 10 mM dNTPs and 100 units Moloney Murine Leukemia Virus
Reverse Transcriptase (SibEnzyme Ltd., Novosibirsk, Russia) were
added, and the total volume was made up to 20 µl using DEPC
water. The mixture was then re-incubated in the Bio-Rad thermal
cycler at 37°C for 1 h, and at 90°C for 10 min in order to
inactivate the enzyme. For semi-quantitative PCR analysis, specific
primers for the examined genes (Table
I) were designed using Oligo-4 computer program and were
synthesized by Macrogen (Macrogen Inc., Seoul, Korea). PCR was
conducted in a final volume of 25 µl, which consisted of 1
µl cDNA, 1 µl 10 pM of each primer (forward and
reverse), and 12.5 µl PCR master mix (Promega Corporation,
Madison, WI, USA); the volume was brought up to 25 µl using
sterilized, deionized water. PCR was carried out using the Bio-Rad
T100™ Thermal Cycler, and the cycling conditions were as follows:
One cycle at 94°C for 5 min, followed by 27 cycles (Table I), each of which consisted of
denaturation at 94°C for 1 min, annealing at the specific
temperature corresponding to each primer, and extension at 72°C for
1 min, with an additional final extension step at 72°C for 7 min.
As a reference, expression of glyceraldehyde-3-phosphate
dehydrogenase (G3PDH) mRNA was examined. PCR products were
visualized under UV light following 1.5% agarose gel
electrophoresis (Bio Basic Inc., Markham, ON, Canada). The gel was
stained with ethidium bromide in Tris-Borate-EDTA buffer. Images of
the PCR products were captured using a gel documentation system
(GelDoc-It Imaging system; UVP, LLC, Upland, CA, USA). The
intensity of the bands was quantified densitometrically using
ImageJ software version 1.47 (http://imagej.en.softonic.com/).
 | Table IPolymerase chain reaction conditions
and primer sequences of examined genes. |
Table I
Polymerase chain reaction conditions
and primer sequences of examined genes.
| Gene | Product size
(bp) | Annealing (°C) | Direction | Sequence
(5′–3′) |
|---|
| IL-2 | 324 | 57 | Forward |
AAGGAAACACAGCAGCACCT |
| | | Reverse |
CACAGTTGCTGGCTCATCAT |
| IL-4 | 327 | 57 | Forward |
AGGTCAACACCACGGAGAAC |
| | | Reverse |
AGGACATGGAAGTGCAGGAC |
| IL-5 | 320 | 57 | Forward |
TGAGGATGCTTCTGTGCTTG |
| | | Reverse |
CCTCTCTTCGCCACACTTCT |
| IL-12α | 447 | 57 | Forward |
GCTTACCACTGGAACTCCACA |
| | | Reverse |
TCCTACAGGAGCTGAAGGTCA |
| G3PDH | 309 | 52 | Forward |
AGATCCACAACGGATACATT |
| | | Reverse |
TCCCTCAAGATTGTCAGCAA |
Statistical analysis
Results are presented as the mean ± standard error
of the mean. Data were analyzed using one-way analysis of variance
followed by the least significant difference test for multiple
comparisons among groups. Statistical analysis was conducted using
SPSS software version 11.5 for Windows (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of LEVA and GO on serum IFN-γ,
IL-5 and TNF-α levels in Wistar rats
Treatment with LEVA elevated serum cytokine levels
of IFN-γ, TNF-α and IL-5 to 5-, 4- and 2-fold that of the control,
respectively. Treatment with GO elevated levels of IFN-γ and TNF-α
to 2- and 3-fold that of the control, respectively. In addition, GO
induced a slight increase in IL-5 levels. Co-administration of GO
and LEVA decreased LEVA-induced IFN-γ, TNF-α and IL-5 levels;
however, GO and LEVA co-administration induced a 1.5-fold increase
in serum IFN-γ levels and a ~3-fold increase in serum TNF-α levels
compared with the control group (Table II).
 | Table IISerum changes in IFN-γ, IL-5 and
TNF-α in Wistar rats following separate or combined administration
of LEVA and GO for 4 weeks. |
Table II
Serum changes in IFN-γ, IL-5 and
TNF-α in Wistar rats following separate or combined administration
of LEVA and GO for 4 weeks.
| Group | IFN-γ (pg/ml) | IL-5 (pg/ml) | TNF-α (pg/ml) |
|---|
| Control | 22.9±2.7 | 12.9±4.2 | 9.1±1.6 |
| LEVA | 117.7±14.1a | 22.6±0.8a | 36.4±2.8a |
| GO | 48.2±7.3a,b | 14.5±3.01b | 26.4±2.9a,b |
| LEVA + GO | 33.8±6.9b | 14.3±1.3b | 25.03±3.9a,b |
Effects of LEVA and GO on IgG and IgM
levels in Wistar rats
Treatment with LEVA induced a 2-fold increase in IgG
and IgM serum levels. In addition, GO administration induced a ~60%
elevation in IgG levels, but had no significant effects on IgM.
Co-administration of LEVA and GO decreased LEVA-stimulated IgG and
IgM levels, and returned them to normal (Table III).
 | Table IIISerum changes in immunoglobulin
levels (IgG and IgM) in Wistar rats after separate or combined
administration of LEVA and GO for 4 weeks. |
Table III
Serum changes in immunoglobulin
levels (IgG and IgM) in Wistar rats after separate or combined
administration of LEVA and GO for 4 weeks.
| Group | IgG (mg/ml) | IgM (ng/ml) |
|---|
| Control | 1.8±0.2 | 7.2±1.1 |
| LEVA | 4.0±0.8a | 15.2±0.9a |
| GO | 3.0±0.3a | 8.6±1.5b |
| LEVA+GO | 2.2±0.1b | 5.5±1.1b |
Effects of LEVA and GO on red blood cell
(RBC), white blood cell (WBC) and differential leukocyte count, and
serum lysozyme activity in Wistar rats
Treatment with LEVA increased the number of total
RBCs and WBCs, and various types of leukocytes compared with the
control. GO administration increased the total WBC count and the
various types of leukocytes compared with the control.
Co-administration of LEVA and GO decreased LEVA-induced total WBC
count and reduced the monocyte count. Lysozyme activity was
increased in the LEVA treatment group and was higher than in the GO
group. However, co-administration of LEVA and GO decreased
LEVA-induced lysozyme activity (Table
IV).
 | Table IVChanges in RBC count, total WBC count
and leukocyte types, and serum lysozyme activity in Wistar rats
after separate or combined administration of LEVA and GO for 4
weeks. |
Table IV
Changes in RBC count, total WBC count
and leukocyte types, and serum lysozyme activity in Wistar rats
after separate or combined administration of LEVA and GO for 4
weeks.
| Cell type | Control | LEVA | GO | LEVA+GO |
|---|
| WBCs
(×103/µl) | 10.2±1.75 | 12.07±0.63a | 10.7±0.62b | 9.82±0.10c |
| Neutrophils
(×103/µl) | 2.32±0.31 | 3.303±0.29a | 2.58±0.18b | 2.25±0.27c |
| Lymphocytes
(×103/µl) | 7.5±0.35076 | 8.7±0.87a | 8.7±0.75b | 7.29±0.13c |
| Monocytes
(×103/µl) | 0.9±0.09 | 1.12±0.10a | 1.01±0.16b | 0.2±0.09c |
| Eosinophils
(×103/µl) | 0.2±0.15 | 0.33±0.035a | 0.23±0.005b | 0.37±0.18c |
| Basophils
(×103/µl) | 0.02±0.0006 | 0.18±0.23a | 0.03±0.005b | 0.023±0.01c |
| RBCs
(×106/µl) | 8.9±0.17 | 9.4±0.31a | 9.01±1.38b | 9.5±0.5c |
| Lysozyme
activity | 255.3±19.5 | 387.3±23.6a | 303.2±19.3a | 217.6±21.2a |
Effects of LEVA and GO on spleen
immunohistochemical expression of CD4 and CD8 in Wistar rats
The spleen samples consisted of white pulp and red
pulp surrounded by a connective tissue capsule. The white pulp
consisted of circumscribed oval areas of B lymphocytes around a
central vein. The central vein was surrounded by a periarterial
lymphocytic sheath, which consisted of T lymphocytes. In addition,
the white pulp contained a faint area called the germinal center.
The red pulp consisted of numerous B lymphocytes, plasma cells,
macrophages and blood cells in the sinuses, which separated the
white pulp from the red pulp (Fig.
1A). The immunohistochemical expression of CD4 in the
lymphocytes of the control group was positive (Fig. 1B); however, the immunoreactivity
was stronger in the LEVA group (Fig.
1C). The expression was diminished in the GO group compared
with the LEVA group (Fig. 1D), and
the levels returned to control levels in the LEVA and GO co-treated
rats (Fig. 1E). The
immunohistochemical expression of CD8 was positive in the spleen of
the control group (Fig. 1F), and
was increased in the LEVA group (Fig.
1G). CD8 expression was decreased in the GO group (Fig. 1H). In addition, in the LEVA plus GO
group the expression of CD8 almost returned to control levels
(Fig. 1J).
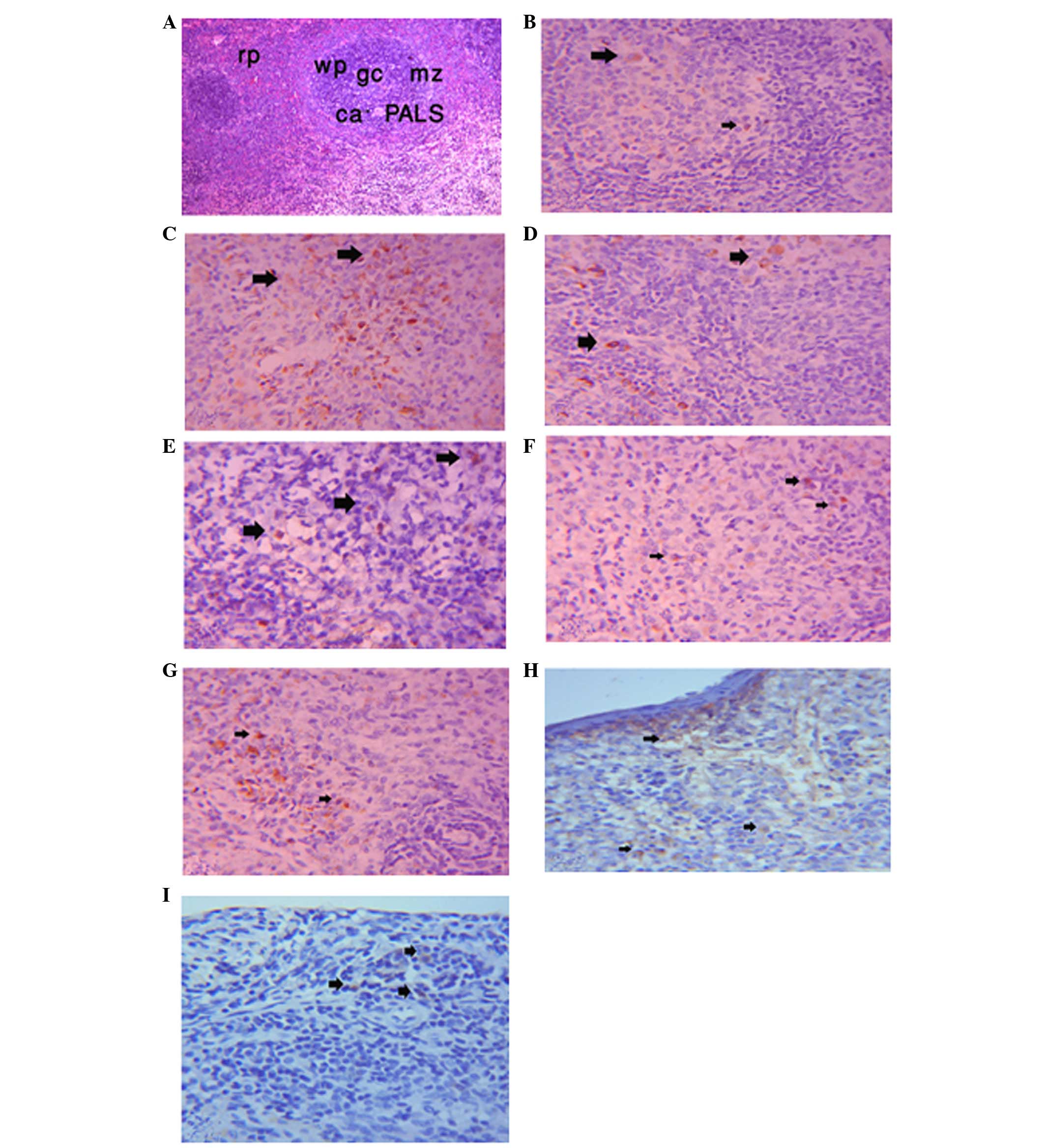 | Figure 1CD4 expression in the spleen.
Photomicrograph of the spleen in male rats showing (A) white pulp
(wp), red pulp (rp), germinal center (gc), marginal zone (mz),
central artery (ca) and periarterial lymphocytic sheath (PALS).
Hematoxylin & eosin staining; magnification, ×10. (B)
Immunohistochemical expression of CD4 in lymphocyte cells of the
spleen of the control group (as indicated by arrows; magnification,
×10). (C) Immunohistochemical expression of CD4 in lymphocyte cells
of the spleen of the levamisole (LEVA) group (as indicated by
arrows; magnification, ×10). (D) Immunohistochemical expression of
CD4 in lymphocyte cells of the spleen of the garlic oil (GO) group
(as indicated by arrows; magnification, ×10). (E)
Immunohistochemical expression of CD4 in lymphocyte cells of the
spleen of the LEVA plus GO group (as indicated by arrows;
magnification, ×10). (F) Immunohistochemical expression of CD8 in
lymphocyte cells of the spleen of the control group (as indicated
by arrows; magnification, ×10). (G) Immunohistochemical expression
of CD8 in lymphocyte cells of the spleen of the LEVA group (as
indicated by arrows; magnification, ×10). (H) Immunohistochemical
expression of CD8 in lymphocyte cells of the spleen of the GO group
(as indicated by arrows; magnification, ×10). (I)
Immunohistochemical expression of CD8 in lymphocyte cells of the
spleen of the LEVA plus GO group (as indicated by arrows;
magnification, ×10). |
Effects of LEVA and GO on the mRNA
expression levels of IL-2, IL-4 and IL5 in rat leukocytes
The mRNA expression levels of the cytokines IL-2,
IL-4 and IL-5 were upregulated in rat leukocytes (3-, 6- and
9-folds, respectively) in the LEVA group compared with the control
group. In addition, GO administration downregulated the expression
levels of IL-2, IL-4 and IL-5 compared with the control group.
Co-administration of LEVA and GO decreased the expression of the
examined cytokines by 50% compared with in the LEVA group (Fig. 2).
Effects of LEVA and GO on the mRNA
expression levels of IL-4, IL-5 and IL-12 in the thymus gland of
rats
The mRNA expression levels of IL-4 were increased by
7-fold in the thymus of the LEVA group compared with the control
group, and were also increased, but to a lesser extent, in the GO
group. Co-administration of LEVA and GO decreased IL-4 gene
expression to 20% that in the LEVA-treated group (Fig. 3). IL-5 mRNA expression was
upregulated in the LEVA and GO groups compared with the control.
Co-administration of LEVA and GO exhibited an additive regulatory
effect on IL-5 expression (Fig.
3). IL-12α mRNA expression was not affected by LEVA treatment
alone; however, it was upregulated following GO treatment alone or
in combination with LEVA, compared with the control group (Fig 3).
Discussion
The results of the present study demonstrated that
LEVA and GO can modulate the immune response in rats.
Co-administration of LEVA and GO modulated the rats' immune
response towards a Th1/Th2 balance; therefore, maintaining the
body's defensive mechanisms within normal ranges.
IFN-γ is an essential cytokine for immunity against
intracellular pathogens and cancer (23). Targeted disruptions of the IFN-γ
gene or IFN-γ receptor 1 gene in mice render them highly
susceptible to bacterial, protozoan and viral infections (24). The role of IFN-γ in CD8 T cell
cellular immunity is to enhance the major histocompatibility
complex class I antigen presentation pathway, which facilitates
cytotoxic T cells to recognize infected cells (23). In the present study, the elevated
levels of IFN-γ detected following LEVA treatment indicated its
immunostimulatory effect towards induction and activation of Th1
cells, as reported in a previous study (12). The reduction of LEVA-elevated serum
IFN-γ levels by GO administration indicated the balancing effect of
GO against immune deviation. Autoimmune and inflammatory diseases
may be associated with a Th1/Th2 bias. For example,
insulin-dependent diabetes mellitus (type 1 diabetes) (25) features a predominant Th1 response,
whereas asthma is characterized by a predominant Th2 response
(26). The induction of TNF-α with
either LEVA or GO suggested that these two agents have a macrophage
stimulatory effect. Recently, garlic extract has been reported to
exert a stimulatory effect on TNF-α gene expression in murine
macrophages (27). However, in the
combined group in the present study, GO reduced LEVA-induced serum
TNF-α elevation, thus implying that GO has a stimulatory effect on
basal macrophage activity, which is concordant with the results of
a previous study (28).IL-5 has a
central role not only in the stimulation of B-cell growth and Ig
production, but also in the production, mobilization, activation,
recruitment, proliferation and survival of eosinophils at the site
of inflammation (29,30). The downregulating effect of GO on
LEVA-induced serum IL-5 levels and gene expression in circulating
leukocytes and the thymus is concordant with its recently reported
anti-allergic effects (31).
LEVA-induced IgG and IgM elevation provided evidence for the
immunostimulatory effect of LEVA, and is in agreement with its
effect on calves (32). The
normalizing effects of GO on LEVA-induced IgG and IgM elevation
indicated its immunomodulatory effect, and is in agreement with its
lowering effect on IgG and IgM in human patients (33).
The effects of LEVA on the upregulation of
peripheral leukocytes is in line with its effects on serum cytokine
and Ig levels, and coincided with its effect in rats (34). The lowering effect of GO on the
LEVA-induced upregulation of circulating leukocytes may be
attributed to its major sulfur component, diallyl disulfide, which
has been reported to decrease the number of circulating cells,
particularly lymphocytes, eosinophils and monocytes (35).
Lysozyme enzymes are required for antigen
presentation by T cells, and the removal of tumor cells and
infected cells by cytotoxic T lymphocytes (36). Therefore the LEVA-induced
upregulation of lysozyme activity is consistent with its
immunostimulatory effect. In addition, prolonged use of proton pump
inhibitors (PPI) has been associated with the development of
gastric neuroendocrine carcinoma (37), which has recently been attributed
to the inhibitory effects of PPI on lysozyme enzyme activity and
may result in immunosuppression (36). The stimulatory effect of GO on
lysozyme activity when administered alone, and its inhibitory
effect when administered with LEVA, indicated that GO has an
immunomodulatory balancing effect, which may counteract any immune
system deviation. The effects of GO on reduction of lysozyme
activity may be attributed to the inhibitory effects of allicin, a
constituent of GO (38).
LEVA-induced elevation of the CD4+
population in the spleen is consistent with its elevating effect on
the number of circulating lymphocytes, serum Igs and cytokine
levels. CD4+ cells have been reported to be increased in
response to LEVA used as an adjuvant to hepatitis B vaccine in
patients with human immunodeficiency virus/acquired immune
deficiency syndrome (39). The
administration of LEVA in children with malnutrition, has also been
reported to increase the mean CD4 count by 20% (40). These results indicated that LEVA
may augment the ability of B cells to produce antibodies through
increasing the CD4+ population (41). The GO-induced reduction in the
CD4+ population may indicate its balancing effect on the
immune reaction; lowering B cell activation and antibody
production, particularly when B cells are highly activated. This
effect may be attributed to the presence of diallyl trisulfide in
GO, which has been reported to exert anti-inflammatory effects and
reduce lipopolysaccharide-induced IL-6, IL-12 and TNF-α (42).
The upregulatory effect of LEVA on the population of
CD8+ cells in the thymus and spleen modulated the
cytotoxic cell response through the modulation of cytokine
expression and secretion. In the present study, GO increased IL-12
expression, which is known to mediate the CD8+ cytotoxic
resopnse; however, in the present study GO decreased the CD8
cytotoxic response. Conversely, LEVA alone did not alter IL-12α
gene expression; however, when co-administered with GO, LEVA
induced partial suppression of GO-induced IL-12α gene expression.
In addition, human conventional myeloid dendritic cells have been
described to be the most potent producers of bioactive IL-12, which
can promote cytotoxic CD8+ T-cell responses (43). The LEVA-induced suppression of
GO-induced IL-12α detected in the present study is in accordance
with a previous report indicating LEVA-induced suppression of
IL-12α gene expression after 3 days administration in rats
(12). Furthermore, the induction
of IL-12α gene expression following GO administration is in
concordance with the previously reported activation of IL-12 using
garlic extract (44). These
effects may be mediated by garlic protein, which has been reported
to augment CD8+ T-cell infiltration into the tumor site
and inhibit tumor growth (8).
IL-4 stimulates the differentiation of naïve helper
T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2
cells subsequently produce additional IL-4 in a positive feedback
loop. IL-4 expression is upregulated in hypersensitive immune
responses and allergies (45). The
suppressive effect of GO on the LEVA-induced upregulation of IL-4
indicates the anti-allergic effects of GO. These results are
consistent with those of a previous study that revealed the
anti-allergic effects of garlic extract (31). IL-2 supports the growth and
expansion of B cells, as well as other immune cells, such as T
cells, at varying stages of development or activation (46). In the present study, LEVA
induced-IL-2 expression as it stimulated the immune system towards
a type 1 response, and GO downregulated the expression of IL-2,
which is consistent with its lowering effects on serum Ig and
cytokine levels, and is in accordance with previously reported data
when both LEVA and GO are administered (47).
In conclusion, the present study demonstrated that
LEVA acts by directing the immune balance towards a type 1 response
via induction of Th1 activation and upregulation of IL-2, IL-4,
IL-5, IFN-γ, TNF-α and IL-12. Treatment with GO was shown to direct
the immune response towards the induction and activation of Th2,
thus increasing humoral immunity against extracellular
microorganisms. The combination of LEVA and GO acted in a
countercurrent manner to maintain the Th1/Th2 balance and enforce
the body defensive mechanism. The present study provided a novel
perspective for the use of medicinal plants as adjuvant therapy,
which may be added to food to prevent disease. Further in
vitro studies focusing on the mechanism of action of GO are
required.
References
|
1
|
Page DB, Postow MA, Callahan MK, Allison
JP and Wolchok JD: Immune modulation in cancer with antibodies.
Annual Review Med. 65:185–202. 2014. View Article : Google Scholar
|
|
2
|
Agrawal SS, Khadase SC and Talele GS:
Studies on immunomodulatory activity of Capparis zeylanica leaf
extracts. International Journal of Pharmaceutical Sciences and
Nanotechnology. 3:887–892. 2010.
|
|
3
|
Kandil OM, Abdellah TH and Elkadi A:
Garlic and the immune system in humans: Its effects on natural
killer cells. Federal Procedures. 46:4411987.
|
|
4
|
Butt MS, Sultan MT, Butt MS and Iqbal J:
Garlic nature's protection against physiological threats. Crit Rev
Sci Nutr. 49:538–551. 2009. View Article : Google Scholar
|
|
5
|
Kyo E, Uda N, Suzuki A, Kakimoto M,
Ushijima M, Kasuga S and Itakura Y: Immunomodulatory effects of
aged garlic extract. J Nutr. 131:1075S–1079S. 2001.PubMed/NCBI
|
|
6
|
Ghazanfari T, Hassan ZM, Ebtekar M,
Ahmadiani A, Naderi G and Azar A: Garlic induces a shift in
cytokine pattern in leishmania major-infected BALB/c mice. Scand J
Immunol. 52:491–495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chandrashekar PM and Venkatesh YP:
Identification of the protein components displaying
immunomodulatory activity in aged garlic extract. J
Ethnopharmacolol. 124:384–390. 2009. View Article : Google Scholar
|
|
8
|
Ebrahimi M, Mohammad Hassan Z, Mostafaie
A, Zare Mehrjardi N and Ghazanfari T: Purified protein fraction of
garlic extract modulates cellular immune response against breast
transplanted tumors in BALB/c mice model. Cell J. 15:65–75.
2013.PubMed/NCBI
|
|
9
|
Chadwick RG, Jain S, Cohen BJ, Scott GM,
Thomas HC and Sherlock S: Levamisole therapy for HbsAg-positive
chronic liver disease. Scand J Gastroenterol. 15:973–978. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fox MT, Jacobs DE, Campling RC, Pocknee
BR, Clampitt R and Hart IC: Effect of thiabendazole treatment on
feed intake, digetability and selected blood values in lactating
dairy cows. Vet Rec. 116:257–260. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitchell MS: Immunotherapy as part of
combinations for the treatment of cancer. Int Immunopharmacol.
3:1051–1059. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szeto C, Gillespie KM and Mathieson PW:
Levamisole induces interleukin-18 and shifts type 1/type 2 cytokine
balance. Immunology. 100:217–224. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olusi SO, Jessop WJE and Shoroye A:
Effects of levamisole on the immune response of experimentally
malnourished rats. Pediatric Res. 13:1237–1239. 1979. View Article : Google Scholar
|
|
14
|
Kocabas CN, Sekerel BE, Firat PA, Okur H
and Adahoglu G: Levamisole: Might it be used in treatment and
prevention of atopic diseases? J Asthma. 41:547–551. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen LY, Lin YL and Chiang BL: Levamisole
enhances immune response by affecting the activation and maturation
of human monocyte-derived dendritic cells. Clin Exp Immunol.
151:174–181. 2008. View Article : Google Scholar
|
|
16
|
Mulero V, Esteban MA, Munxoz J and
Meseguer J: Dietary intake of levamisole enhances the immune
response and disease resistance of the marine teleost gilthead
seabream (Sparus aurata L). Fish and Shellfish Immunology. 8:49–62.
1998. View Article : Google Scholar
|
|
17
|
Ali HS, El-Sanosi YA and Shaimaa A:
Biochemical, immunomodulatory and antioxidant proportes of
levamisole at different storage conditions and administration
routes. Pak J Biol Sci. 15:986–991. 2012. View Article : Google Scholar
|
|
18
|
Hassan HA, Hafez HS and Zeghebar FE:
Garlic oil as a modulating agent for oxidative stress and
neurotoxicity induced by sodium nitrite in male albino rats. Food
Chem Toxicol. 48:1980–1985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Osserman EF and Lawlor DP: Serum and
urinary lysozyme (muramidase) in monocytic and monomyelocytic
leukemia. J Exp Med. 124:921–952. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bancroft JD and Gamble M: Theory and
practice of histological techniques. 6th ed. Churchill Livingstone
Elsevier; Philadelphia: pp. 126–127. 2008
|
|
21
|
Cho Y, Miyamoto M, Kato K, Fukunaga A,
Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki
M, et al: CD4+ and CD8+ T cells cooperate to improve prognosis of
patients with esophageal squamous cell carcinoma. Cancer Res.
63:1555–1559. 2003.PubMed/NCBI
|
|
22
|
Soliman MM, Nassan MA and Ismail TA:
Immunohistochemical and molecular study on the protective effect of
curcumin against hepatic toxicity induced by paracetamol in Wistar
rats. BMC Complement Altern Med. 14:4572014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Araújo-Souza PS, Hanschke SC and Viola
JPJ: Epigenetic control of interferon-gamma expression in CD8 T
cells. J Immunol Res. 2015:8495732015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schroder K, Hertzog PJ, Ravasi T and Hume
DA: Interferon-gamma: An overview of signals, mechanisms and
functions. J Leukoc Biol. 75:163–189. 2004. View Article : Google Scholar
|
|
25
|
Heurtier AH and Boitard C: T-cell
regulation in murine and human autoimmune diabetes: The role of TH1
and TH2 cells. Diabetes Metab. 23:377–385. 1997.
|
|
26
|
Kay AB: TH2-type cytokines in asthma. Ann
N Y Acad Sci. 796:1–8. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sung J, Harfouche Y, De La Cruz M, Zamora
MP, Liu Y, Rego JA and Buckley NE: Garlic (Allium sativum)
stimulates lipopoly-saccharide-induced tumor necrosis factor-alpha
production from J774A.1 murine macrophages. Phytother Res.
29:288–294. 2015. View
Article : Google Scholar
|
|
28
|
Nair P: What is an 'eosinophilic
phenotype' of asthma? J Allergy Clin Immunol. 132:81–83. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gleich GJ: Mechanisms of
eosinophil-associated inflammation. J Allergy Clin Immuol.
105:651–663. 2000. View Article : Google Scholar
|
|
30
|
Garcia G, Taillé C, Laveneziana P, Bourdin
A, Chanez P and Humbert M: Antiinterleukin-5 therapy in severe
asthma. Eur Respir Rev. 22:251–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoo JM, Sok DE and Kim MR: Anti-allergic
action of aged black garlic extract in RBL-2H3 cells and passive
cutaneous anaphylaxis reaction in mice. J Med Food. 17:92–102.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pekmezci D and Cakiroglu D: Investigation
of immunmodulatory effects of levamisole and vitamin E on immunity
and some blood parameters in newborn Jersey calves. Vet Res Commun.
33:711–721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Budoff MJ, Ahmadi N, Gul KM, Liu ST,
Flores FR, Tiano J, Takasu J, Miller E and Tsimikas S: Aged garlic
extract supplemented with B vitamins, folic acid and L-arginine
retards the progression of subclinical atherosclerosis: A
randomized clinical trial. Prev Med. 49:101–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chekwube AI, Onyema EI, Ikenna UE and
Ezeokonkwo RC: Effect of diminazene aceturate, levamisole and
vitamin C combination therapy in rats experimentally infected with
Trypanosoma brucei brucei. Asian Pac Trop Med. 7:438–445. 2014.
View Article : Google Scholar
|
|
35
|
Dong Diegane N and Fall Jean: The effect
of garlic (Allium sativum) on growth and immune responses of hybrid
tilapia (Oreochromis niloticus × Oreochromis aureus. Journal of
Clinical Immnunology and Immunopathology Research. 3:1–9. 2011.
|
|
36
|
Jollès P and Jollès J: What's new in
lysozyme research? Always a model system, today as yeas day. Mol
Cell Biochem. 63:165–189
Jorgensen J and Robertsen B:
1995.Yeast_-glucan stimulates respiratory burst activity of
Atlantic salmon (Salmo salar L) macrophages. Dev Comp Immunol.
19:43–157. 1984. View Article : Google Scholar
|
|
37
|
Jianu CS, Lange OJ, Viset T, Qvigstad G,
Martinsen TC, Fougner R, Kleveland PM, Fossmark R, Hauso Ø and
Waldum HL: Gastric neuroendocrine carcinoma after long-term use of
proton pump inhibitor. Scan J Gastroenterol. 47:64–67. 2012.
View Article : Google Scholar
|
|
38
|
Mayeux PR, Agrawal KC, Tou JS, King BT,
Lippton HL, Hyman AL, Kadowitz PJ and McNamara DB: The
pharmacological effects of allicin, a constituent of garlic oil.
Agents Actions. 25:182–190. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sayad B, Alavian SM, Najafi F, Soltani B,
Shirvani M, Janbakhsh A, Mansouri F, Afsharian M, Vaziri S,
Alikhani A and Bashiri H: Effects of Oral Levamisole as an adjuvant
to hepatitis B vaccine in HIV/AIDS patients: A randomized
controlled trial. Hepat Mon. 12:e62342012. View Article : Google Scholar
|
|
40
|
Prakash MS, Rao VM and Reddy V: Effect of
levamisole on the immune status of malnourished children. J Trop
Pediatr. 44:165–166. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cantor HEA and Boyse: Regulation of
cellular and humoral immune responses by T-cell subclasses. Cold
Spring Harb Symp Quant Biol. 41:23–32. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
You S, Nakanishi E, Kuwata H, Chen J,
Nakasone Y, He X, He J, Liu X, Zhang S, Zhang B and Hou DX:
Inhibitory effects and molecular mechanisms of garlic organosulfur
compounds on the production of inflammatory mediators. Mol Nutr
Food Res. 57:2049–2060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nizzoli G1, Krietsch J, Weick A,
Steinfelder S, Facciotti F, Gruarin P, Bianco A, Steckel B, Moro M,
Crosti M, et al: Human CD1c+ dendritic cells secrete high levels of
IL-12 and potently prime cytotoxic T-cell responses. Blood.
122:932–942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gharavi M, Nobakht M, Khademvatan S, Fani
F, Bakhshayesh M and Roozbehani M: The Effect of aqueous garlic
extract on interleukin-12 and 10 levels in leishmania major
(MRHO/IR/75/ER) infected macrophages. Iran J Public Health.
40:105–111. 2011.
|
|
45
|
Burton OT, Darling AR, Zhou JS,
Noval-Rivas M, Jones TG, Gurish MF, Chatila TA and Oettgen HC:
Direct effects of IL-4 on mast cells drive their intestinal
expansion and increase susceptibility to anaphylaxis in a murine
model of food allergy. Mucosal Immunol. 6:740–750. 2013. View Article : Google Scholar :
|
|
46
|
Liao W, Lin JX and Leonard WJ:
Interleukin-2 at the crossroads of effector responses, tolerance
and immunotherapy. Immunity. 38:13–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mantawy MM, Ali HF and Rizk MZ:
Therapeutic effects of Allium sativum and Allium cepa in
Schistosoma mansoni experimental infection. Revista Inst Med Trop
Sao Paulo. 53:155–163. 2011. View Article : Google Scholar
|

















