Introduction
Head and neck squamous cell carcinoma (HNSCC) is one
of the most common malignancies worldwide, and is characterized by
high invasiveness, early metastasis, recurrence and difficult early
detection (1,2). Hypopharyngeal cancer is a distinct
type of malignant head and neck tumor currently treated by surgical
procedures, radiotherapy and chemotherapy, which have numerous side
effects, including loss of larynx function that severely affects
quality of life. Despite the apparent advances in surgery in recent
years, distant metastases and recurrence have remained concerns
(3).
Tumor development is a multi-step process involving
multiple genes, which includes the activation of proto-oncogenes
and inactivation of tumor suppressor genes (4). With improved understanding of the
molecular mechanisms underlying this process, gene therapy exhibits
increasing potential for use as a novel cancer treatment.
Hypopharyngeal cancer exhibits low sensitivity to anti-cancer
drugs. Its strong resistance to various anti-tumor therapies and
the unknown underlying mechanism lead to unfavorable prognosis and
a low five-year survival rate for patients (5). Therefore, the importance of
developing methods for avoiding chemotherapy resistance, and
improving and enhancing prognosis has been emphasized, and is
considered a challenge for effective clinical treatment of
hypopharyngeal cancer.
Previous studies have demonstrated that gene therapy
using a combination of two genes can promote tumor cell apoptosis.
For example, Luo et al (6)
reported that the adenovirus-mediated CD/TK double suicide gene,
driven by a survivin promoter, specifically inhibits gastric cancer
cells to a greater extent than that observed with a single suicide
gene. Therefore, the effect of combined gene therapy on
hypopharyngeal tumor survival was investigated.
The inhibitor of growth protein 4 (ING4) gene was
originally identified by Shiseki et al (7) and later recognized as an important
factor in tumor growth inhibition (8). It is expressed in all cells,
including normal tissues. Extensive studies have demonstrated that
ING4 is critical for gene transcription, cell proliferation,
apoptosis and senescence, cell contact inhibition, DNA damage
repair, and tumor invasion and metastasis (9–12).
Recent studies have packaged the ING4 gene into an
adenoviral vector (Ad-ING4) for introduction into various human
tumor cells, including malignant melanoma (13), breast cancer (14), human lung adenocarcinoma (15) and osteosarcoma (16). These studies demonstrated
significantly higher growth inhibition and apoptosis in tumor cells
with Ad-ING4 compared with cells infected with the empty vector,
indicating that ING4 inhibits the growth of tumor cells and induces
their apoptosis. A previous study demonstrated that there were
decreased expression levels of ING4 in HNSCC and concluded that it
is important in cancer cell apoptosis and is anti-proliferative
(17). However, its function and
mechanism of action remain unknown and require further study.
P53, the first member of the P53 family to be
identified, is associated with various types of cancer, including
sporadic cancers, which are correlated with mutations in somatic
cells (18). Although wild-type
P53 inhibits tumor development and progression, a previous study
demonstrated that P53 is generally mutated in tumor cells to induce
and promote tumor development (19). Gene therapy with wild-type P53 and
other P53 family members was observed to be effective and safe for
the treatment of pulmonary metastatic tumors from hepatocellular
carcinoma (20) and inhibited
proliferation and apoptosis in osteosarcoma cell lines (21). Furthermore, previous investigation
demonstrated that ING4 induces apoptosis through a P53-dependent
pathway (22,23).
The present study used combination gene therapy with
ING4 and P53 to treat hypopharyngeal cancer in vivo.
Mutations identified in 40–70% of HNSCC tumors occur in P53
(24), providing a theoretical
basis for adenovirus-mediated combination gene therapy to inhibit
hypopharyngeal cancer cell growth and proliferation. Thus, it was
hypothesized that combination treatment with ING4 and P53 tumor
suppressors would enhance tumor chemosensitivity.
Materials and methods
Cell lines
The FADU human hypopharyngeal cancer cell line was
purchased from American Type Culture Collection (Manassas, VA, USA)
and cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal
bovine serum (Zhejiang Tianhang Biotechnology Co., Ltd. (Huzhou,
China) and 1% antibiotic solution (100X penicillin and
streptomycin; Beyotime Institute of Biotechnology, Haimen, China)
at 37°C in a humidified atmosphere composed of 95% air and 5%
CO2.
Virus propagation and purification
Adenoviral vectors with the ING4 and P53 genes were
provided by Professor Jicheng Yang (Soochow University, Suzhou,
China). QBI 293A cells (American Type Culture Collection) with the
adenoviral vectors to amplify the recombinant virus promoter, and
specific steps and titers were measured by conventional methods as
described previously (16). The
QBI 293A cells were cultured as described for the FADU cells,
however, in RPMI 1640 (Gibco; Thermo Fisher Scientific, Inc.)
rather than DMEM.
Animals
Specific-pathogen free BALB/c nu/nu nude mice (25
males; weight, 22–25 g; age, 5–6 weeks) were obtained from the
Changzhou Cavens Laboratory Animal Co. [Changzhou, China;
certificate no. SCXK (Su) 2011-0003 Su regulatory certificate no.
201403734]. They were maintained and used for the experiments
performed at the Laboratory Animal Center of Bengbu Medical
University (Anhui, China; certificate no. Wan SYXK 2012-002). The
current study was approved by the appropriate ethical review boards
for the use of laboratory animals. The animals were fed as standard
with access to drinking water. The temperature was maintained at
23–28°C and relative humidity was 40–60%, with a natural light/dark
cycle.
Establishment of tumor models
Each mouse was injected subcutaneously with
1×106 human FADU hypopharyngeal cancer cells in the
axilla of the right anterior limb. The tumor dimensions were
measured 2–3 times per week with a caliper, and the tumor volume
was calculated as follows: Tumor size = a×b2/2, where a
and b represent the larger and smaller of the two dimensions,
respectively.
Experimental design and preparation of
cisplatin
The mice were randomly assigned to five groups (five
mice per group) when the tumors developed to a mean volume of 60–80
mm3 after ~20 days. The xenograft tumor-bearing mice
were intra-tumorally and peritumorally injected with
phosphate-buffered saline (PBS control), empty adenoviral vector
[Ad; 0.1 ml, 1×108 plaque forming units (pfu)],
Ad-ING4-P53 (0.1 ml, 1×108 pfu), cisplatin (0.1 ml, 300
µg), or Ad-ING4-P53 (0.1 ml, 1×108 pfu) and
cisplatin (0.1 ml, 300 µg) every other day for five days.
Cisplatin (3 mg; Qilu Pharmaceutical Co., Ltd., Jinan, China) was
dissolved in 1 ml normal saline and used at 3 µg/µl
in all experiments.
Five days after treatment, the mice were sacrificed
by cervical dislocation. The transplanted tumors were removed and
weighed to calculate the inhibition rate as follows: Inhibition
rate = (1-mean experimental tumor weight/mean control tumor
weight)×100%. Based on the inhibition rate, the combined effect was
evaluated from the Q values (25)
as follows: Q value = E (A + B)/(EA + EB − EA × EB), where EA and
EB are the effects of molecules A and B alone, respectively, E (A +
B) is the combined effect, and the denominator represents the
expected effects when the two are combined. Molecules A and B are
interpreted as having additive effects when Q=1±0.15, synergistic
effects when Q>1.15, and antagonistic effects when
Q<0.85.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Gene transcript levels were detected by RT-PCR
analysis. Total RNA was extracted from the xenografted tumors using
TRIzol according to the manufacturer's instructions (Invitrogen;
Thermo Fisher Scientific, Inc.). The RT-PCR reactions were
performed using a RevertAid First Strand cDNA Synthesis kit (Thermo
Fisher Scientific, Inc.) to generate cDNA and then a PCR Master mix
(2X; Thermo Fisher Scientific, Inc.) in an ABI StepOne™ Real-Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.) by
using degenerate primers (Sangon Biotech Co., Ltd., Shanghai,
China) (Table I). The
thermocycling conditions were as follows: 95°C for 3 min; 40 cycles
of 95°C for 30 sec, 61.5°C, 52.5°C, 59°C or 58°C for 30 sec (for
ING4, P53, Bax and Bcl-2, respectively) and 72°C for 1 min;
followed by 72°C for 10 min. GAPDH served as the control. The PCR
products were separated on 1.5% agarose gel.
 | Table IReverse transcription-polymerase
chain reaction primers. |
Table I
Reverse transcription-polymerase
chain reaction primers.
| Name | Primer
sequence | Size (bp) |
|---|
| GADPH | | 240 |
| F |
5′-TGATGACATCAAGAAGGTGGTGAA-3′ | |
| R |
5′-TCCTTGGAGGCCATGTGGGCC-3′ | |
| ING4 | | 750 |
| F |
5′-TAGAGATCTACCATGGCTGCTGGGATGTATTTGG-3′ | |
| R |
5′-ACCGTCGACCCTATTTCTTCTTCCGTTCTTG-3′ | |
| P53 | | 259 |
| F |
5′-CCTCCTCAGCATCTTATCCG-3′ | |
| R |
5′-CACAAACACGCACCTCAAA-3′ | |
| Bcl-2 | | 304 |
| F |
5′-TTCTTTGAGTTCGGTGGGGTC-3′ | |
| R |
5′-TGCATATTTGTTTGGGGCAGG-3′ | |
| Bax | | 257 |
| F |
5′-TCCACCAAGAAGCTGAGCGAG-3′ | |
| R |
5′-GTCCAGCCCATGATGGTTCT-3′ | |
Hematoxylin and eosin (H&E)
staining
The mice were sacrificed by cervical dislocation 5
days after drug administration, and the xenografted tumors, livers
and kidneys were removed to observe the distribution of tumor
cells, and the tumor metastasis and cytotoxicity of the livers and
kidneys. Tissue sections were incubated overnight in neutral
formalin buffer (10%) and then stored in ethanol and embedded in
paraffin. Cross-sections (4 µm) were stained with H&E
and observed under a microscope (BX43; Olympus Corporation, Tokyo,
Japan), and tumor cells were identified by the following
characteristics: Small volume, condensed cytoplasm, small nuclei,
condensed and fractured chromatin, nuclei migrated to the cell
edge, or apoptotic bodies with karyorrhexis.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
Sections of tumor tissue were fixed in 10% neutral
formalin buffer according to the standard procedure and then
stained using an In Situ Cell Apoptosis Detection Kit IV
following the manufacturer's instructions (Sigma-Aldrich, St.
Louis, MO, USA) using conventional methods as described previously
(26). The sections were analyzed
under a confocal microscope (×200). Yellow granules in the nucleus
indicated apoptotic cells.
Immunohistochemistry in xenografted
tumors
The expression of ING4, P53, B-cell lymphoma-2
(Bcl-2), and Bcl-2 associated X protein (Bax) in the xenografted
tumors was analyzed by immunohistochemistry using an
Ultrasensitive™ SP kit (Fuzhou Maixin Biotech Co., Ltd., Fuzhou,
China). Tissue sections were deparaffinized in dimethyl benzene and
dehydrated by an alcohol gradient. The sections were incubated in
3% H2O2 for 10 min to block inactivated
endogenous peroxidase. For antigen retrieval, the sections were
placed in 0.01 M citrate buffer (pH 6.0) and boiled at 95°C for
15–20 min, cooled at room temperature for 20 min, and washed with
cold water to facilitate cooling. Then sections were sealed in
normal bovine serum (Zhejiang Tianhang Biotechnology Co., Ltd.) for
10 min at 37°C, and then the bovine serum was removed. The
polyclonal rabbit anti-mouse antibodies were incubated for 1 h at
room temperature and were as follows: Anti-ING4 (1:50; cat. no.
16188-1-AP), anti-P53 (1:100; cat. no. 10442-1-AP), anti-Bcl-2
(1:50; cat. no. 12789-1-AP) and Bax (1:50; cat. no. 23931-1-AP; all
from Proteintech Group, Inc., Chicago, IL, USA) Biotinylated goat
anti-rabbit secondary antibodies (1:40; Proteintech Group, Inc.;
cat. no. SA00001-2) were then incubated for 20 min at room
temperature. Sections were subsequently incubated in peroxidase
substrate mixing liquid (Fuzhou Maixin Biotech Co., Ltd.) and
washed in deionized water. Finally, the sections were
counterstained with hematoxylin, dehydrated with deionized water,
dried and sealed. They were observed by microscopy (BX43). Positive
gene expression was indicated by the presence of yellow
diaminobenzidine precipitates.
Statistical analysis
Data were analyzed by SPSS for Windows (version
17.0; SPSS, Inc., Chicago, IL, USA) and are presented as the mean ±
standard error of the mean. Differences between groups were
evaluated by one-way analysis of variance (ANOVA), two-way ANOVA,
and Dunnett's multiple comparisons test. P<0.05 was considered
statistically significant.
Results
Establishment of tumor models
Subcutaneous solid nodules developed gradually for
6–7 days post-injection. At ~20 days later, the mean tumor volume
was 60–80 mm3. All 25 nude mice were tumorigenic, with a
tumor formation rate of 100%. During treatment, the mice exhibited
dry skin, however there were no significant changes in their stool
color, appetite or behavior. The mice treated with cisplatin and a
combination of Ad-ING4-P53 and cisplatin (Ad-ING4-P53 + cisplatin)
exhibited weight loss, which was most notable in the cisplatin
group. Following sacrifice of the mice, no significant macroscopic
or microscopic lesions were observed in the organs.
Effect of Ad-ING4-P53 + cisplatin on FADU
cell inhibition
Following establishment of the tumor models, the
xenograft tumor-bearing mice were intratumorally and peritumorally
injected with PBS, Ad, Ad-ING4-P53, cisplatin, or Ad-ING4-P53 +
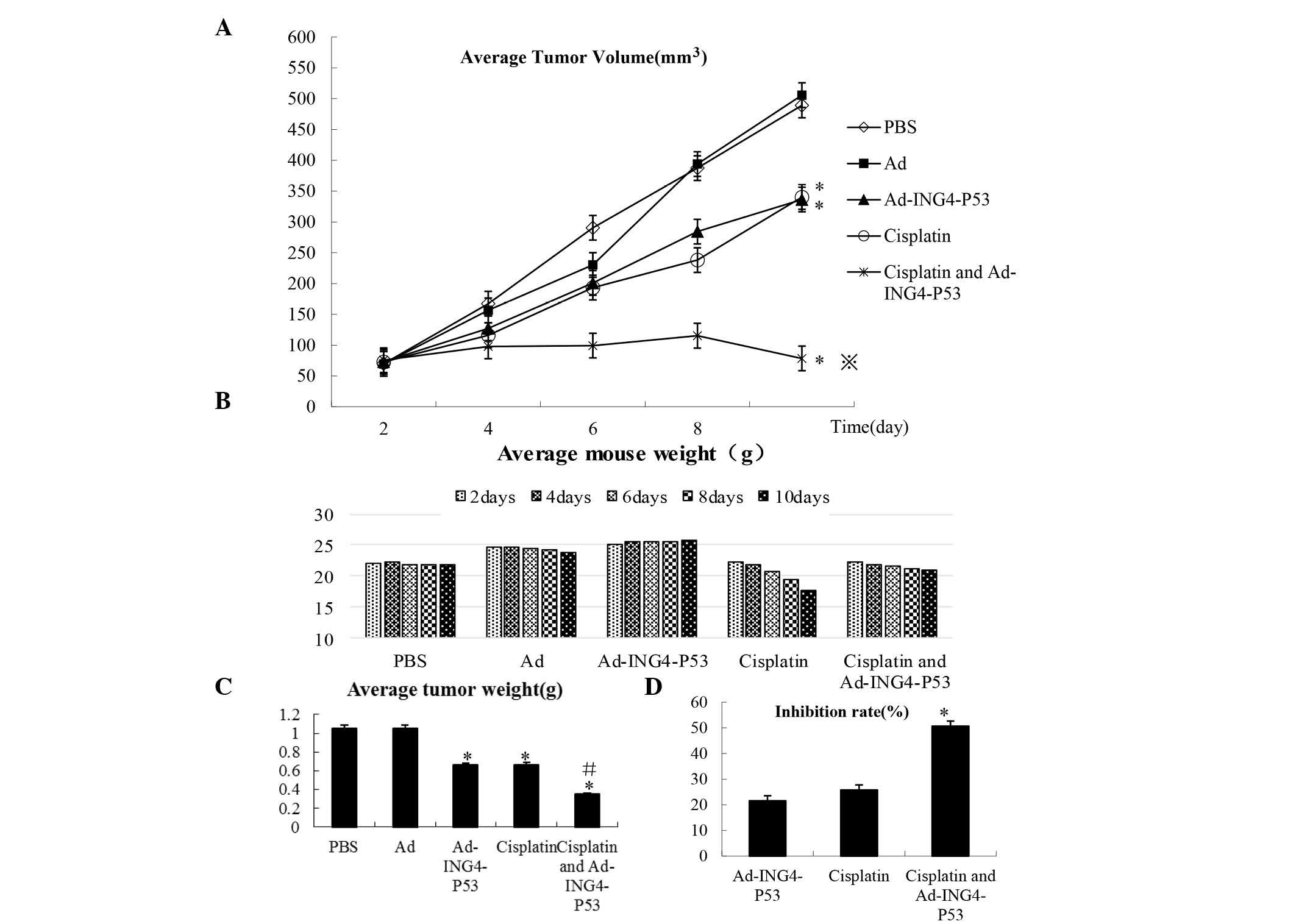
cisplatin every other day for five days. We measured the xenograft
tumor volumes and weighed the mice every other day (Fig. 1A and B) and removed and weighed the
tumors five days after treatment (Fig.
1C).
Compared with the cisplatin and Ad-ING4-P53 groups,
Ad-ING4-P53 + cisplatin significantly inhibited the growth of FADU
hypopharyngeal cancer cells in nude mice bearing transplantation
tumors (P<0.05; Fig. 1D) and
exerted a synergistic effect (Q=1.19).
Expression of ING4 and P53
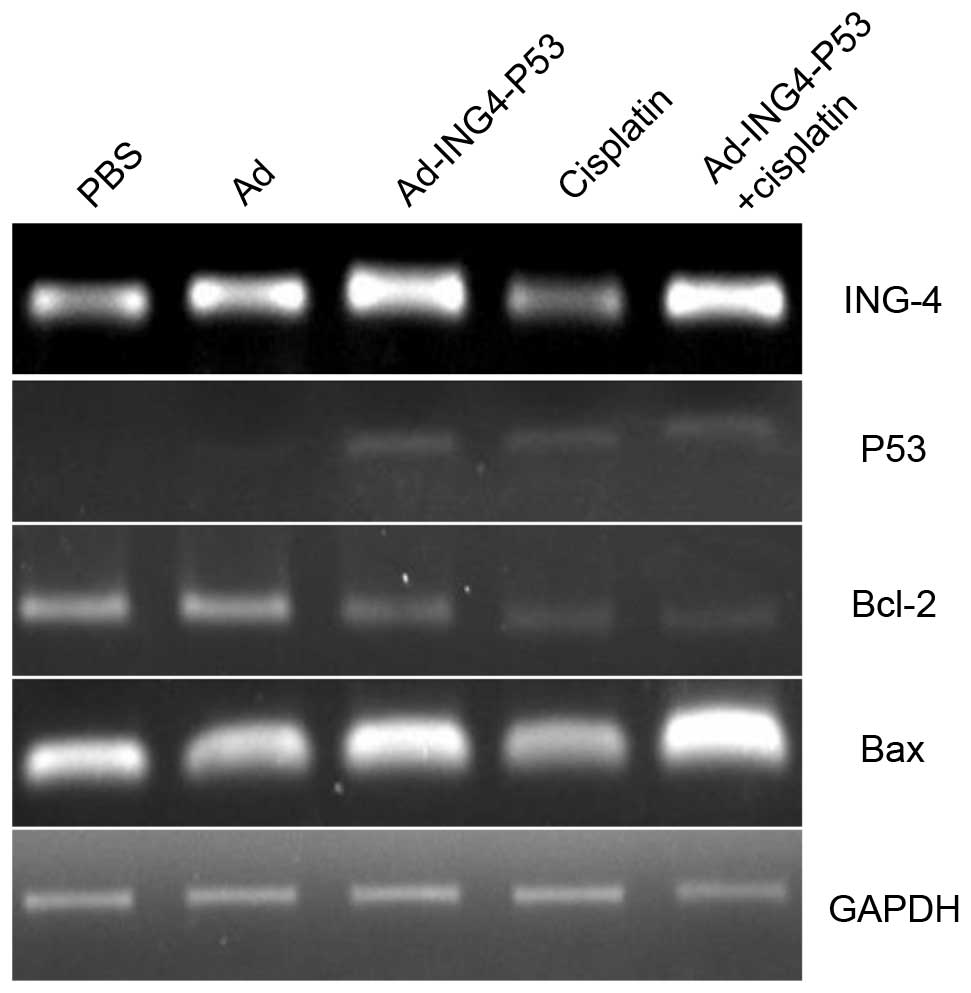
Results of the RT-PCR analysis of ING4 and P53 gene
expression levels are demonstrated in Fig. 2. GADPH was used as a reference
gene. The Ad-ING4-P53 and Ad-ING4-P53 + cisplatin groups
demonstrated strong positive ING4/P53 bands compared with the PBS,
Ad and cisplatin groups. Furthermore, immunohistochemical analysis
demonstrated no obvious expression of ING4/P53 in the PBS, Ad and
cisplatin groups, whereas the Ad-ING4-P53 and Ad-ING4-P53 +
cisplatin groups demonstrated positive ING4 and P53 expression
(Fig. 3).
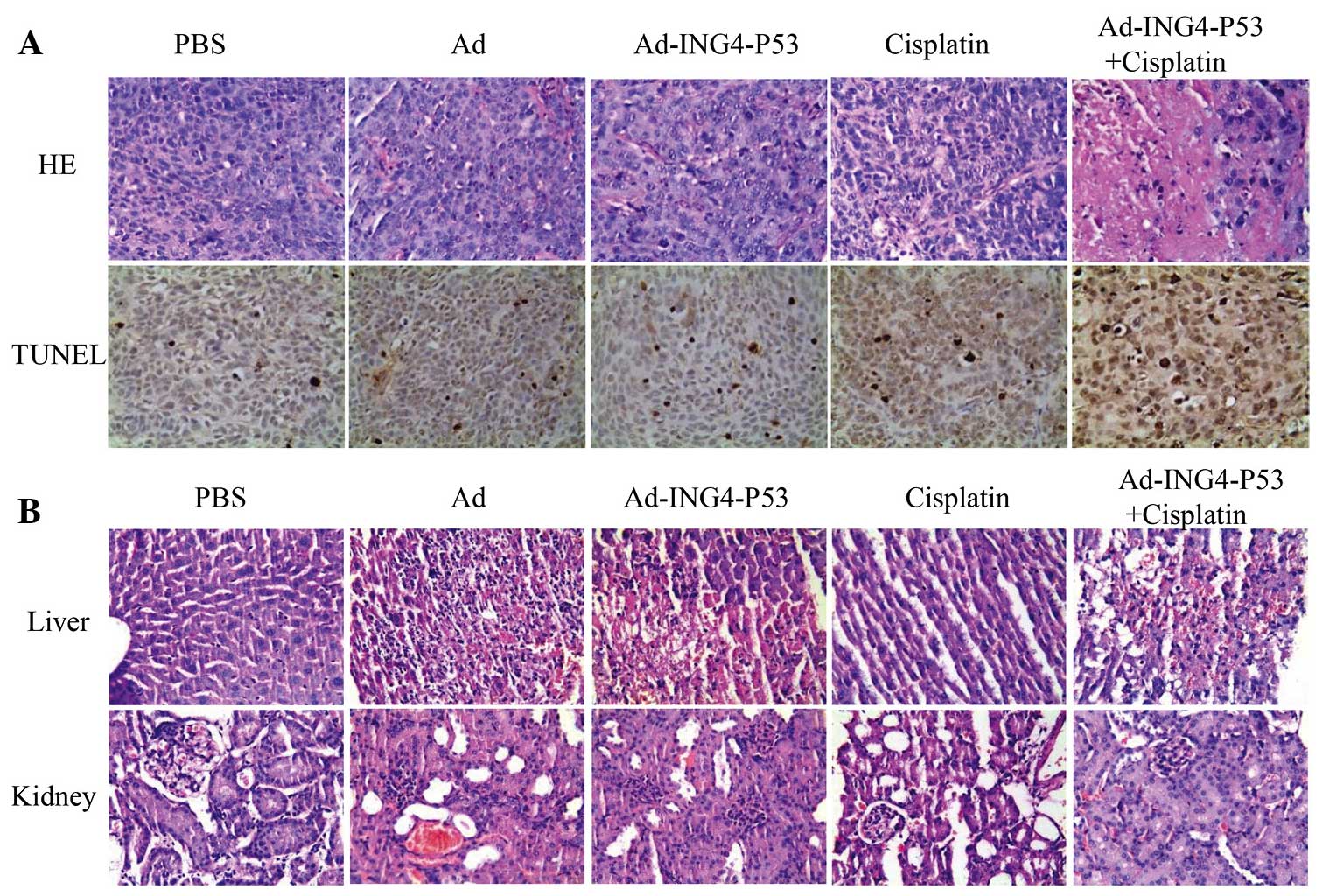
 | Figure 3Immunohistochemical analysis of
xenografted tumors. Immunohistochemistry was used to detect the
expression levels of ING4, P53, Bcl-2, and Bax. The Ad-ING4-P53 +
cisplatin group showed increased ING4, P53, and Bax expression and
significantly decreased Bcl-2 expression. PBS, phosphate-buffered
saline; Ad, adenovirus; ING4, inhibitor of growth protein 4; Bcl-2,
B-cell lymphoma-2; Bax, Bcl-2 associated X protein. |
Apoptotic gene expression changes in
xenografted tumors
To determine the potential molecular mechanisms of
apoptosis induction, Bcl-2 and Bax expression levels were detected
in the tumor tissue using RT-PCR. Compared with the PBS group, the
gene expression of the Ad group changed only marginally, whereas
the Ad-ING4-P53 and Ad-ING4-P53 + cisplatin groups demonstrated
strongly positive Bax expression and negative Bcl-2 expression,
with a greater change in the Ad-ING4-P53 + cisplatin group
(Fig. 2) compared with the PBS and
Ad groups. These results were further supported by
immunohistochemical analysis which demonstrated the Ad-ING4-P53 +
cisplatin group exhibited increased ING4, P53 and Bax expression
levels and decreased Bcl-2 expression compared to other groups
(Fig. 3).
Enhanced tumor apoptosis by Ad-ING4-P53 +
cisplatin
Sections of xenografted hypopharyngeal tumors from
nude mice were stained by H&E (Fig. 4A), and the tumor cells exhibited a
nest-like distribution and disordered arrangement. In the PBS and
Ad groups, the tumor cells were closely arranged with complete and
atypical structures. By contrast, the Ad-ING4-P53, cisplatin and
Ad-ING4-P53 + cisplatin groups exhibited a greater degree of tumor
cell apoptosis, characterized by incomplete cell membranes,
condensed cytoplasm, pyknotic or cracking nuclei, and cavity-shaped
organization. The Ad-ING4-P53 group and cisplatin group
demonstrated similar characteristic, whereas the apoptotic effect
was more obvious in the Ad-ING4-P53 + cisplatin group.
TUNEL analysis demonstrated the apoptotic cells as
small, with condensed nuclei, circumscribed nuclear membranes and
yellow granules in the nuclei (Fig.
4A). Comparison of the five treatment groups indicated that
Ad-ING4-P53, cisplatin and Ad-ING4-P53 + cisplatin accelerated
apoptosis relative to PBS and Ad, with the greatest effect exerted
by treatment with Ad-ING4-P53 + cisplatin.
Toxicity of gene therapy and
cisplatin
H&E-stained liver and kidney sections from human
hypopharyngeal tumor-bearing nude mice were observed
microscopically (Fig. 4B). The PBS
and cisplatin groups exhibited normal liver tissue, with clear
lobule structures and orderly hepatic cords. However, the Ad,
Ad-ING4-P53, and Ad-ING4-P53 + cisplatin groups exhibited
irregularly bleeding necrotic areas, and their liver cell
structures did not demonstrate marked inflammatory cell
infiltration. However, the kidney microstructures were in good
condition for all groups, with no pathological changes, such as
blood extravasation or cell necrosis. Thus, the adenoviral vector
induced liver toxicity.
Discussion
Cisplatin is the most commonly used chemotherapeutic
drug, particularly for the treatment of head and neck cancer.
However, the development of cisplatin resistance has limited its
widespread clinical use (27–29).
Therefore, identification of high-efficiency chemosensitization
drugs to improve the efficiency of chemotherapy is an important
direction of current research.
The ING4 gene was identified as an important tumor
growth inhibition factor (8).
Further studies demonstrated that ING4 exerts specific anti-tumor
effects through various pathways and can induce tumor cell
apoptosis. Zhang et al (30) established several HepG-2
hepatocellular carcinoma cell lines stably expressing ING4 and
observed that ING4 inhibited HepG-2 cell growth and improved the
sensitivity of liver cancer cells to DNA damage reagents, including
adriamycin. Another important factor, P53, is regarded as an
important factor in tumor activation and an essential gene for
genome integrity (31). Kraljević
Pavelić et al (32)
introduced the P53 gene into Hep-2 and CAL27 HNSCC cell lines and
demonstrated that p53 overexpression at sub-cytotoxic levels
enhanced the activity of low doses of cisplatin and methotrexate
through changes in the cell cycle.
Resistance to multiple anti-tumor therapies arises
through complex mechanisms, and tumor cell apoptosis resistance is
generally understood to be a major mechanism of multi-drug
resistance. The majority of chemotherapy drugs act by inducing
tumor cell apoptosis. The Bcl-2 gene inhibits apoptosis caused by
various factors, including carcinogens and radioactive rays,
abnormally extends cell survival time, promotes the accumulation of
mutations and increases resistance to immune system monitoring
(33). A previous study
demonstrated an association between excessive expression of Bcl-2,
and expression of tumor cell drug resistance genes and inhibition
of apoptosis, which leads to drug resistance (34). Bax is an apoptosis-promoting gene
in the Bcl-2 family. In vivo, Bax/Bax cognate dimer
formation promotes cell apoptosis, whereas Bcl-2/Bax heterologous
dimers inhibit apoptosis (35).
The present study demonstrated that the Ad,
Ad-ING4-P53, and Ad-ING4-P53 + cisplatin groups exhibited liver
toxicity, however they exerted no effect on kidney cells,
consistent with previous demonstrations that adenoviral vectors
damage liver function (36,37).
Haisma et al (38)
demonstrated that liver toxicity arises from removal of Kupffer
cells by adenoviral vectors, potentially as an immune response to
the virus and its transduction gene products (39). Future studies must investigate
whether changes in the adenovirus vector structure or simultaneous
of administration liver-protection drugs would abrogate the
observed liver toxicity.
The current study was designed to investigate the
chemo-sensitivity of a recombinant adenovirus co-expressing ING4
and P53, and to analyze the potential mechanisms underlying this
process. The results of the present study demonstrated that
Ad-ING4-P53 + cisplatin significantly inhibited the growth of FADU
hypopharyngeal cancer cells in nude mice bearing transplanted
tumors compared with the effect of cisplatin or Ad-ING4-P53 alone
(P<0.05) and a synergistic effect between Ad-ING4-P53 and
cisplatin was observed (Q=1.19). Immunohistochemical analysis of
the expression of associated factors in transplanted tumors further
indicated that Ad-ING4-P53 + cisplatin strongly increased Bax
expression and decreased Bcl-2 expression compared with the levels
observed with cisplatin or Ad-ING4-P53 alone, which is consistent
with previous findings by Zhu et al (40). Thus, the combination therapy may
increase chemosensitivity through the Bcl-2/Bax pathway; however,
elucidation of the specific underlying mechanism requires further
research.
In conclusion, the results of the current study
demonstrated that Ad-ING4-P53 improves the chemosensitivity of
transplanted human hypopharyngeal tumors, potentially by increasing
and decreasing the expression levels of Bax and Bcl-2,
respectively, to induce tumor cell apoptosis. The co-expression of
ING4 and P53 genes via a recombinant adenovirus synergistically
enhanced the anti-tumor effects with simultaneous chemotherapy for
human hypopharyngeal cancer in vivo. Further investigation
is required to elucidate the specific underlying mechanism of
action in vitro and in vivo, however, this work
provides an experimental basis for these future studies and for
clinical applications.
Acknowledgments
This study was supported by the Natural Science
Research Project of Colleges and Universities of Anhui Province
(grant no. KJ2013A193).
Abbreviations:
|
Ad
|
adenoviral vector
|
|
ING4
|
inhibitor of growth protein 4
|
References
|
1
|
Genden EM, Ferlito A, Bradley PJ, Rinaldo
A and Scully C: Neck disease and distant metastases. Oral Oncol.
39:207–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keereweer S, Kerrebijn JD, Al-Mamgani A,
Sewnaik A, Baatenburg de Jong RJ and van Meerten E: Chemoradiation
for advanced hypopharyngeal carcinoma: A retrospective study on
efficacy, morbidity and quality of life. Eur Arch Otorhinolaryngol.
269:939–946. 2012. View Article : Google Scholar :
|
|
4
|
Hanna NN, Mauceri HJ, Wayne JD, Hallahan
DE, Kufe DW and Weichselbaum RR: Virally directed cytosine
deaminase/5-fluo-rocytosine gene therapy enhances radiation
response in human cancer xenografts. Cancer Res. 57:4205–4209.
1997.PubMed/NCBI
|
|
5
|
Thurfjell N, Coates PJ, Boldrup L,
Lindgren B, Bäcklund B, Uusitalo T, Mahani D, Dabelsteen E,
Dahlqvist A, Sjöström B, et al: Function and importance of p63 in
normal oral mucosa and squamous cell carcinoma of the head and
neck. Adv Otorhinolaryngol. 62:49–57. 2005.
|
|
6
|
Luo XR, Li JS, Niu Y and Miao L:
Adenovirus-mediated double suicide gene selectively kills gastric
cancer cells. Asian Pac J Cancer Prev. 13:781–784. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shiseki M, Nagashima M, Pedeux RM,
Kitahama-Shiseki M, Miura K, Okamura S, Onogi H, Higashimoto Y,
Appella E, Yokota J and Harris CC: p29ING4 and p28ING5 bind to p53
and p300, and enhance p53 activity. Cancer Res. 63:2373–2378.
2003.PubMed/NCBI
|
|
8
|
Garkavtsev I, Kozin SV, Chernova O, Xu L,
Winkler F, Brown E, Barnett GH and Jain RK: The candidate tumour
suppressor protein ING4 regulates brain tumour growth and
angiogenesis. Nature. 428:328–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Unoki M, Shen JC, Zheng ZM and Harris CC:
Novel splice variants of ING4 and their possible roles in the
regulation of cell growth and motility. J Biol Chem.
281:34677–34686. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Colla S, Tagliaferri S, Morandi F, Lunghi
P, Donofrio G, Martorana D, Mancini C, Lazzaretti M, Mazzera L,
Ravanetti L, et al: The new tumor-suppressor gene inhibitor of
growth family member 4 (ING4) regulates the production of
proangiogenic molecules by myeloma cells and suppresses
hypoxia-inducible factor-1 alpha (HIF-1alpha) activity: Involvement
in myeloma-induced angio-genesis. Blood. 110:4464–4475. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen JC, Unoki M, Ythier D, Duperray A,
Varticovski L, Kumamoto K, Pedeux R and Harris CC: Inhibitor of
growth 4 suppresses cell spreading and cell migration by
interacting with a novel binding partner, liprin alpha1. Cancer
Res. 67:2552–2558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Martinka M and Li G: Role of ING4 in
human melanoma cell migration, invasion and patient survival.
Carcinogenesis. 29:1373–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai L, Li X, Zheng S, Wang Y, Wang Y, Li
H, Yang J and Sun J: Inhibitor of growth 4 is involved in
melanomagenesis and induces growth suppression and apoptosis in
melanoma cell line M14. Melanoma Res. 19:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei Q, He W, Lu Y, Yao J and Cao X: Effect
of the tumor suppressor gene ING4 on the proliferation of MCF-7
human breast cancer cells. Oncol Lett. 4:438–442. 2012.PubMed/NCBI
|
|
15
|
Huang J, Yang J, Ling C, Zhao D, Xie Y and
You Z: The mechanism of inhibition effect of adenovirus-mediated
ING4 on human lung adenocarcinoma xenografts in nude mice. Zhongguo
Fei Ai Za Zhi. 17:142–147. 2014.In Chinese. PubMed/NCBI
|
|
16
|
Xu M, Xie Y, Sheng W, Miao J and Yang J:
Adenovirus-mediated ING4 gene transfer in osteosarcoma suppresses
tumor growth via induction of apoptosis and inhibition of tumor
angiogenesis. Technol Cancer Res Treat. 14:369–378. 2015.
View Article : Google Scholar
|
|
17
|
Gunduz M, Nagatsuka H, Demircan K, Gunduz
E, Cengiz B, Ouchida M, Tsujigiwa H, Yamachika E, Fukushima K,
Beder L, et al: Frequent deletion and down-regulation of ING4, a
candidate tumor suppressor gene at 12p13, in head and neck squamous
cell carcinomas. Gene. 356:109–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vijayaraman KP, Veluchamy M, Murugesan P,
Shanmugiah KP and Kasi PD: p53 exon 4 (codon 72) polymorphism and
exon 7 (codon 249) mutation in breast cancer patients in southern
region (Madurai) of Tamil Nadu. Asian Pac J Cancer Prev.
13:511–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levine AJ, Finlay CA and Hinds PW: P53 is
a tumor suppressor gene. Cell. 116(Suppl 2): S67–S69, 1 p following
S69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu M, Chen W and Zhang J: p53 gene therapy
for pulmonary metastasis tumor from hepatocellular carcinoma.
Anticancer Drugs. 21:882–884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oshima Y, Sasaki Y, Negishi H, Idogawa M,
Toyota M, Yamashita T, Wada T, Nagoya S, Kawaguchi S, Yamashita T
and Tokino T: Antitumor effect of adenovirus-mediated p53 family
gene transfer on osteosarcoma cell lines. Cancer Biol Ther.
6:1058–1066. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Russell M, Berardi P, Gong W and Riabowol
K: Grow-ING, Age-ING and Die-ING: ING proteins link cancer,
senescence and apoptosis. Exp Cell Res. 312:951–961. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soliman MA and Riabowol K: After a decade
of study-ING, a PHD for a versatile family of proteins. Trends
Biochem Sci. 32:509–519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sozzi G, Miozzo M, Donghi R, Pilotti S,
Cariani CT, Pastorino U, Della Porta G and Pierotti MA: Deletions
of 17p and p53 mutations in preneoplastic lesions of the lung.
Cancer Res. 52:6079–6082. 1992.PubMed/NCBI
|
|
25
|
Jin ZJ: Addition in drug combination
(author's transl). Zhongguo Yao Li Xue Bao. 1:70–76. 1980.In
Chinese. PubMed/NCBI
|
|
26
|
Yin W, Zhang J, Jiang Y and Juan S:
Combination therapy with low molecular weight heparin and
Adriamycin results in decreased breast cancer cell metastasis in CH
mice. Exp Ther Med. 8:1213–1218. 2014.PubMed/NCBI
|
|
27
|
Chan DA, Sutphin PD, Nguyen P, Turcotte S,
Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, et al:
Targeting GLUT1 and the Warburg effect in renal cell carcinoma by
chemical synthetic lethality. Sci Transl Med. 3:94ra702011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimanishi M, Ogi K, Sogabe Y, Kaneko T,
Dehari H, Miyazaki A and Hiratsuka H: Silencing of GLUT-1 inhibits
sensitization of oral cancer cells to cisplatin during hypoxia. J
Oral Pathol Med. 42:382–388. 2013. View Article : Google Scholar
|
|
29
|
Wang YD, Li SJ and Liao JX: Inhibition of
glucose transporter 1 (GLUT1) chemosensitized head and neck cancer
cells to cisplatin. Technol Cancer Res Treat. 12:525–535.
2013.PubMed/NCBI
|
|
30
|
Zhang X, Xu LS, Wang ZQ, Wang KS, Li N,
Cheng ZH, Huang SZ, Wei DZ and Han ZG: ING4 induces G2/M cell cycle
arrest and enhances the chemosensitivity to DNA-damage agents in
HepG2 cells. FEBS Lett. 570:7–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou X, Gu Y and Zhang SL: Association
between p53 codon 72 polymorphism and cervical cancer risk among
Asians: A HuGE review and meta-analysis. Asian Pac J Cancer Prev.
13:4909–4914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kraljević Pavelić S, Marjanović M, Poznić
M and Kralj M: Adeno-virally mediated p53 overexpression diversely
influence the cell cycle of HEp-2 and CAL 27 cell lines upon
cisplatin and methotrexate treatment. J Cancer Res Clin Oncol.
135:1747–1761. 2009. View Article : Google Scholar
|
|
33
|
Rudin CM and Thompson CB: Apoptosis and
disease: Regulation and clinical relevance of programmed cell
death. Ann Rev Med. 48:267–281. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tse C, Shoemaker AR, Adickes J, Anderson
MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, et
al: ABT-263: A potent and orally bioavailable Bcl-2 family
inhibitor. Cancer Res. 68:3421–3428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cosulich SC, Savory PJ and Clarke PR:
Bcl-2 regulates amplification of caspase activation by cytochrome
c. Curr Biol. 9:147–150. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lozier JN, Csako G, Mondoro TH, Krizek DM,
Metzger ME, Costello R, Vostal JG, Rick ME, Donahue RE and Morgan
RA: Toxicity of a first-generation adenoviral vector in rhesus
macaques. Hum Gene Ther. 13:113–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Shao JY, Liu RY, Zhou L, Chai LP, Li
HL, Han HY, Huang BJ, Zeng MS, Zhu XF, et al: Evaluation of
long-term toxicity of Ad/hIFN-, an Adenoviral vector encoding the
human interferon-gamma gene, in nonhuman primates. Hum Gene Ther.
19:827–839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haisma HJ, Boesjes M, Beerens AM, van der
Strate BW, Curiel DT, Plüddemann A, Gordon S and Bellu AR:
Scavenger receptor A: A new route for adenovirus 5. Mol Pharm.
6:366–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lieber A, He CY, Meuse L, Schowalter D,
Kirillova I, Winther B and Kay MA: The role of Kupffer cell
activation and viral gene expression in early liver toxicity after
infusion of recombinant adenovirus vectors. J Virol. 71:8798–8807.
1997.PubMed/NCBI
|
|
40
|
Zhu Y, Lv H, Xie Y, Sheng W, Xiang J and
Yang J: Enhanced tumor suppression by an ING4/IL-24 bicistronic
adenovirus-mediated gene cotransfer in human non-small cell lung
cancer cells. Cancer Gene Ther. 18:627–636. 2011. View Article : Google Scholar : PubMed/NCBI
|