Introduction
Titanium (Ti) implants are considered to be the
optimal material for teeth replacements and orthopedic devices
(1). However, osseointegration of
Ti implants remain a challenge in type 2 diabetes mellitus (T2DM)
patients. With economic growth and an aging population, the T2DM
patient population is increasing rapidly; therefore, there is a
high demand for improving osseointegration in T2DM.
Improvement of osseointegration in patients with
T2DM has been widely investigated. A previous study reported that
the systematic administration of vitamin D3 and insulin improves Ti
osseointegration in diabetes mellitus rats (2). Local delivery of basic fibroblast
growth factor may also enhance the osseointegration of implants
(3). However, the drug delivery
method (systematic or local) appears unimportant, as certain drugs
work solely in a non-specific manner around the Ti implant, lack
targeted intervention and may result in unknown side effects. It is
considered that hyperglycemia in T2DM has a negative influence on
the differentiation of bone marrow stromal cells (BMSCs) and thus
results in deficiencies of these cells during osseointegration
(4,5). The introduction of exogenous healthy
stem cells into the implant cavity may alleviate this problem. A
stem cell sheet consists of numerous cells and bioactive growth
factors. In addition, the stem cell sheet is semi-differentiated in
order to allow for more effective osseointegration by providing a
pre-organized microenvironment for bone formation (6,7). A
previous study used BMSCs for this purpose and prepared
cell-implant complexes to improve osseointegration in a T2DM model
(6). However, the collection of
BMSCs requires complex procedures; therefore, the possible
application of this treatment is limited by the ability to source
BMSCs (8,9). Adipose-derived mesenchymal stem cells
(ASCs) are considered as an attractive candidate to replace BMSCs
in various areas, including bone tissue regeneration due to their
abundant availability and clear expansion capacity (10–13).
Therefore, it is possible that an ASCs sheet-wrapped Ti implant may
promote osseointegration in a T2DM model. Previous studies have
determined that the bone matrix mineralization and calcium
deposition abilities of ASCs are weaker compared with BMSCs
(14,15). Therefore, it is necessary to
further investigate the osteogenic ability of ASCs prior to
implantation.
Bone homeostasis requires a balance between bone
formation and resorption, which is crucial for bone metabolism
(16). Certain drugs are able to
promote bone formation (17),
however, they may also activate bone resorption (18) as a result of dysfunctional bone
homeostasis. Semaphorin 3A (Sema3A) was the first member to be
identified from the large semaphorin family and has been determined
to be a novel osteoprotective protein (19,20).
This protein is able to affect osteogenic promotion and bone
resorption inhibition (19).
Therefore, Sema3A may be effective for use in ASC modification.
The present study assessed the osteogenic capacity
of ASCs in vitro by Sema3A modification. The constructed ASC
sheet was modified by Sema3A prior to being wrapped around a Ti
implant and inserted into a T2DM rat to evaluate the
osseointegration under T2DM conditions.
Materials and methods
Cell isolation and culture
A total of 20 adult male Sprague-Dawley rats (age,
6–8 weeks; weight, 150–200 g) were obtained from the Laboratory
Animal Center of Fourth Military Medical University (FMMU; Xi'an,
China). They were maintained under a 12-h light/dark cycle with
access to a normal diet, at 18–26°C and a humidity of 30–70%. The
animal experiment procedures were approved by the Animal Welfare
Committee of the FMMU. Primary rat ASCs were isolated as previously
described (21). Briefly, the
subcutaneous adipose tissue was harvested from the inguinal fat pad
and washed with phosphate-buffered saline (PBS). The tissue was
finely minced into pieces and digested at 37°C with 0.1% type I
collagenase (MP Biomedicals LLC, Santa Ana, CA, USA) for 40 min.
The digestion was terminated by growth medium (GM) composed of
Dulbecco's modified Eagle's medium, Ham's F12 nutrient mixture, 10%
fetal bovine serum and 1% penicillin/streptomycin (all from
Hyclone; GE Healthcare, Logan, UT, USA). The digested product was
centrifuged at 120 × g for 5 min at room temperature and the pellet
was resuspended in GM for culturing. Cells at passage 3–6 were used
for the further investigation. In order to perform the osteogenic
differentiation in the presence of Sema3A, conditioned medium (CM)
was prepared by supplementing 10 mM β-glycerophosphate, 50
μg/ml ascorbic acid, 10 nM dexamethasone (all from MP
Biomedicals LLC) and different concentrations (0, 0.25, 0.5 and 1.0
μg/ml) of Sema3A (PeproTech, Inc., Rocky Hill, NJ, USA) into
the GM. The treatment groups were then named ASCs, ASCs-0.25,
ASCs-0.5 and ASCs-1.0.
Morphological observations
The ASCs were seeded onto coverslips (5×5 mm) at a
density of 2.0×104 cells/well in a 24-well plate and
incubated for 24 h. Subsequently, the samples were fixed in 2.5%
glutaraldehyde and dehydrated gradually in ethanol (from 30–100%).
The sample was further dried under critical point conditions and
sputter coated with platinum. Observations were then performed
using a scanning electron microscope (SEM; S-4800; Hitachi, Ltd.,
Tokyo, Japan; magnification, ×200).
Cell proliferation analysis
The ASCs were seeded in 96-well plates at a density
of 1.0×104 cells/well. Cell viability was continuously
monitored each day using Cell Counting Kit-8 (CCK-8; MP Biomedicals
LLC) according to the manufacturer's protocol. Briefly, 10
μl CCK-8 solution was gently mixed with phenol red-free
medium in each well and incubated for 3 h at 37°C. The medium was
then transferred into a new 96-well plate, and the absorbance was
detected at 450 nm by a microplate reader (Epoch; BioTek
Instruments, Inc., Winooski, VT, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The ASCs were seeded in 24-well plates at a density
of 2.0×104 cells/well. Subsequent to expansion for 3
days in GM, the cells were induced with CM. Following 3 and 7 day
induction periods, total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1 μg total RNA was reverse transcribed to complementary DNA
(cDNA) using PrimeScript RT reagent kit (Takara Biotechnology Co.,
Ltd., Dalian, China). Osteogenesis-associated genes, including
alkaline phosphatase (ALP) and collagen type I α1
(COL1A1), were amplified using a SYBR Premix Ex Taq II
RT-PCR kit (Takara Biotechnology Co., Ltd.). A 10 μl system
containing 5μl SYBR Premix, 1 μl forward primer, 1
μl reverse primer and 3 μl cDNA was used. The
thermocycling conditions were 40 cycles of 95°C for 15 sec and 60°C
for 30 sec. The PCR was analyzed using CFX Manager software version
3.1 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The primers
used are presented in Table I and
were as previously described (22). The mRNA expression was calculated
based on the Cq value (23), and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the
endogenous reference.
 | Table IPrimer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward
(5′–3′) | Reverse
(5′–3′) |
|---|
| ALP |
CCTTGTAGCCAGGCCCATTG |
GGACCATTCCCACGTCTTCAC |
| COL1A1 |
CCTACAGCACGCTTGTGGAT |
ATTGGGATGGAGGGAGTTTA |
| GAPDH |
CAAGTTCAACGGCACAGTCA |
CCATTTGATGTTAGCGGGAT |
Osteogenesis staining
ALP production, collagen secretion and extracellular
matrix (ECM) mineralization were visualized using
5-bromo-4-chloro-3-indolyl-phosphate (BCIP)/nitro blue tetrazolium
(NBT) alkaline phosphatase, Sirius red and Alizarin red S (all from
Leagene Biotech Co., Ltd., Beijing, China) staining. Following
induction in CM for 7, 14 and 21 days, the cells were fixed in 2.5%
glutaraldehyde for 15 min at room temperature. Next, the specimens
were stained by BCIP/NBT alkaline phosphatase solution, Sirius red
and Alizarin red according to the manufacturer's protocol. The
cells were rinsed with PBS in order to remove any excess dye and
images were captured using a stereo microscope (Leica Microsystems,
Inc., Buffalo Grove, IL, USA; magnification, ×20).
Cell sheet construction and surgical
implantation
The construction of the ASC sheets was performed as
previously described (24).
Briefly, cells were seeded into 6-well plates at a density of
3.0×105 cells/well and allowed to expand for 24 h to
reach 100% confluence. Following in vitro observation, it
was determined that Sema3A at the highest concentration (1.0
μg/ml) and ascorbic acid (50 μg/ml) should be added
into the GM to perform the cell sheet engineering. The control
group was induced without Sema3A. The cell sheet was formulated
after 1 week and was allowed to shrink instantly subsequent to
detachment of the forceps. The sheet wrapped closely around the
implant (L=5 mm; D=1.5 mm; provided by Northwest Institute for
Nonferrous Metal Research, Xi'an, China).
A high-fat diet and a low-dose (30 mg/kg)
streptozotocin (MP Biomedicals LLC) intraperitoneal injection was
administered to induce T2DM as described previously (6). Animals were anesthetized by an
intra-abdominal injection of a pentobarbital sodium solution
(Sigma-Aldrich, St. Louis, MO, USA) at a dose of 50 mg/kg.
Following shaving and sterilization, the implant cavity (D=1.8 mm)
was prepared at approximately 7 mm below the knee joint using a
twist drill. Subsequently, the cell sheet-imbedded implant was
inserted into the cavity. A post-operative antibiotic treatment,
based on body weight, was injected once a day for seven days.
Micro-computed tomography (CT) scanning
and histological evaluation
The healing process was 4–8 weeks, then the rats
were subsequently sacrificed by intraperitoneal injection of
overdose (>100 mg/kg) of pentobarbital sodium solution and the
specimens were scanned by micro-CT (scanning resolution, 50
μm) in order to determine alterations in the peri-implant
tissue. The region of interest (ROI), including the trabecular
compartment surrounding the implant, was defined as a ring with a
radius of 1.25 mm from the implant surface. In the
three-dimensional level, the Hounsfield unit of this newly formed
bone area was determined using the Inveon Research Workplace
software package, version 2.2.0 (Siemens Healthcare GmbH, Erlangen,
Germany). The trabecular thickness, trabecular number and bone
volume ratio were determined and recorded. The tibia tissue was
isolated and fixed in 10% formalin (pH=6.7) at 4°C. The samples
were dehydrated in a graded ascending series of ethanol solutions
(70–100%), infiltrated and embedded in methyl methacrylate. Serial
sections (150 μm) along the implant axis were obtained using
a high-speed precision microtome (SP1600; Leica Microsystems,
Inc.). All sections were ground and polished to a thickness of 35
μm. The sections were surface-stained using a Van Gieson
solution and observed using an optical microscope (Olympus
Corporation, Tokyo, Japan).
Statistical analysis
The quantitative data were expressed as the mean ±
standard deviation. A one-way analysis of variance followed by a
Student-Newman-Keuls post-hoc test was used to compare the means.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cell morphology and proliferation remains
unchanged with Sema3A treatment
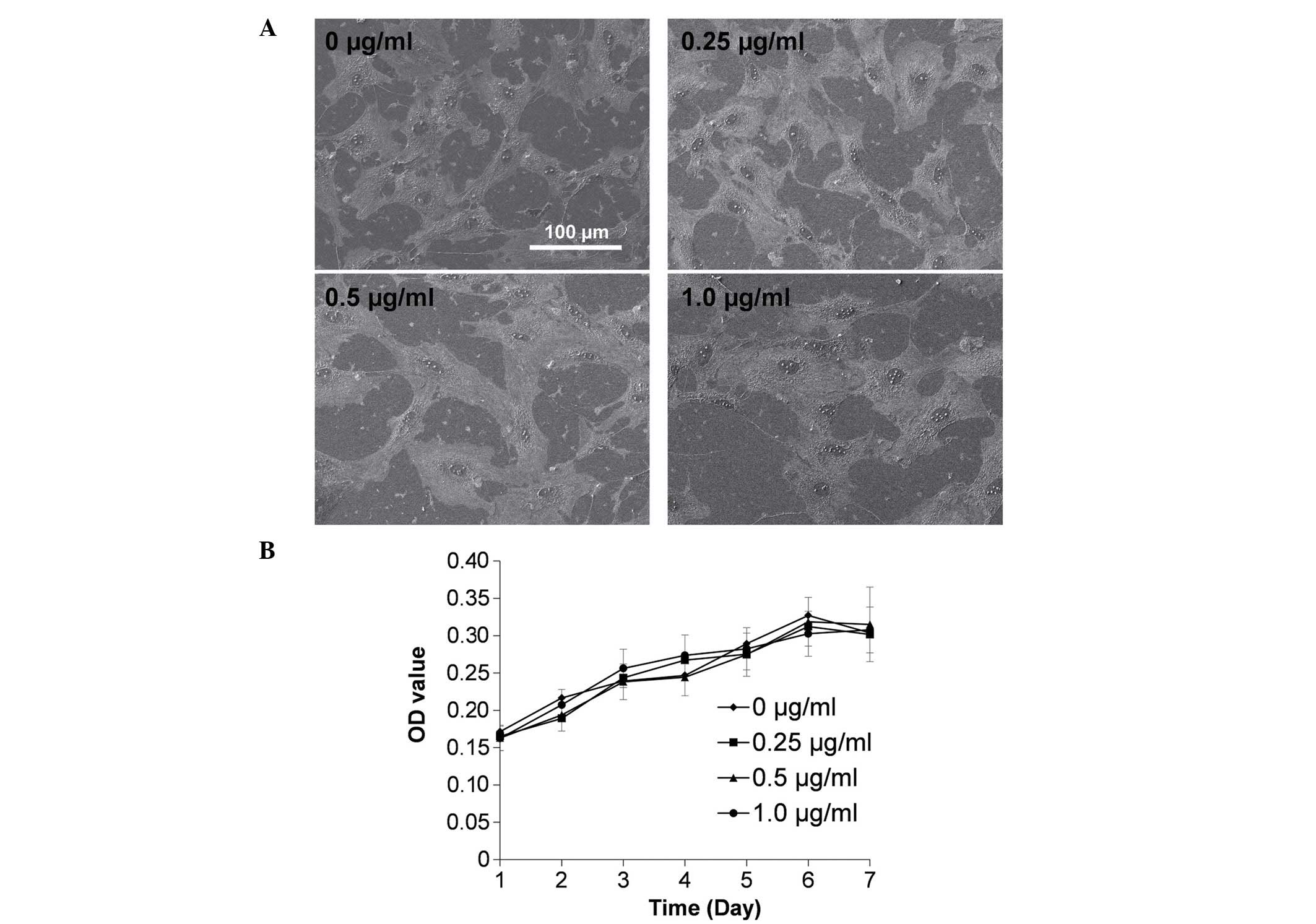
The morphology and proliferation of ASCs under
different concentrations of Sema3A were observed (Fig. 1). The cells spread widely with
abundant cell-cell connections and no significant differences were
identified under SEM observation (Fig.
1A). Additionally, no significant difference in cell
proliferation was identified subsequent to treatment with various
concentrations of Sema3A (Fig.
1B).
Sema3A upregulates expression levels of
osteogenic-associated genes
The RT-qPCR analysis determined that ALP and
COL1A1 mRNA expression levels were significantly
upregulated, mostly in line with the increase of Sema3A
concentration (P<0.05; Fig. 2).
Specifically, the mRNA levels of ALP and COL1A1
increased by 5-fold and 7-fold, respectively, following treatment
with Sema3A from 0.25 to 1.0 μg/ml at day 3 (Fig. 2A). At day 7, the mRNA levels
increased significantly when compared with the control ASCs group
by 7-fold and 5-fold, respectively, in the ALP and
COL1A1 groups (P<0.05; Fig.
2B).
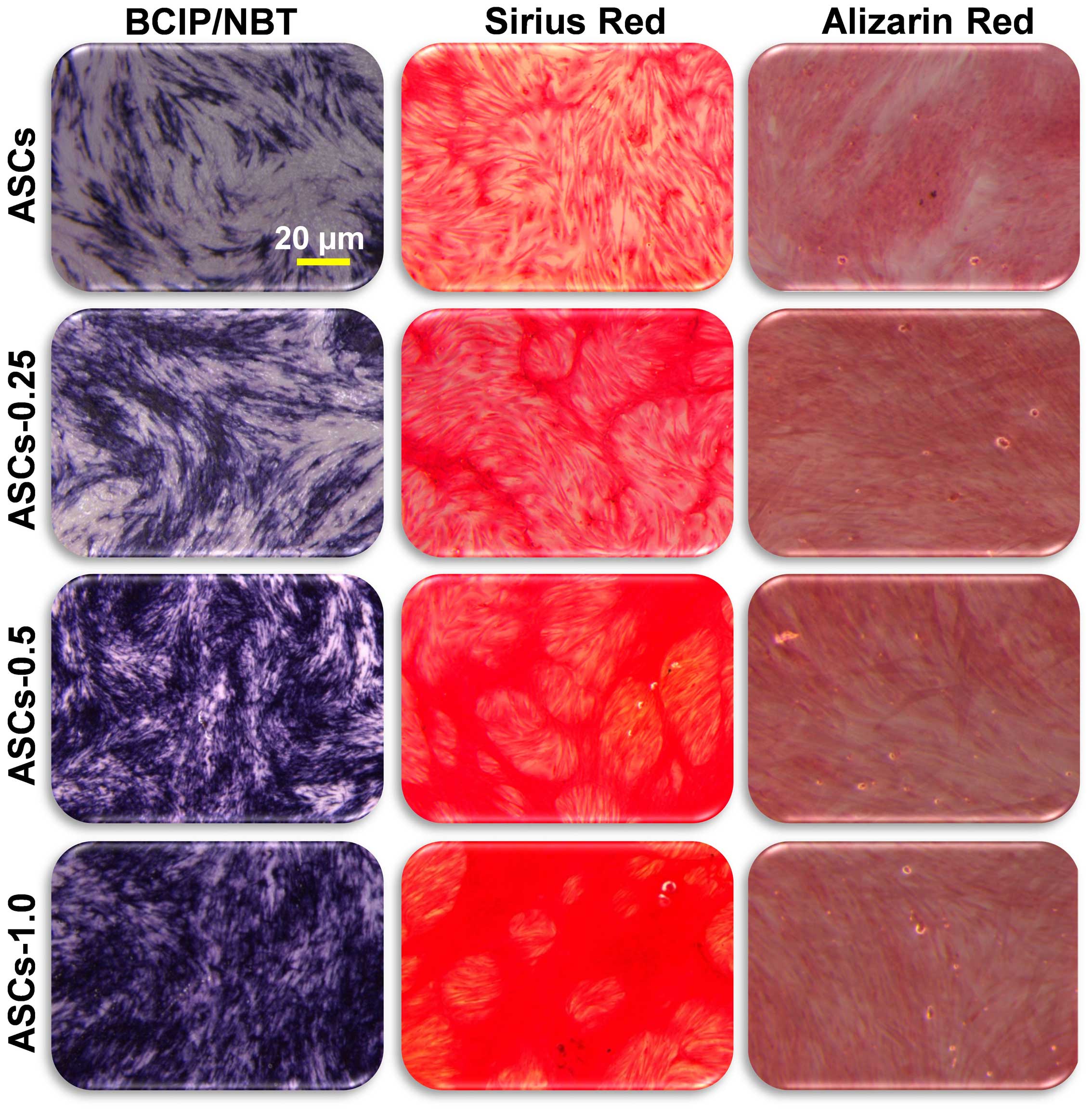
Osteogenesis staining is altered by
Sema3A
The osteogenic differentiation was further analyzed
by staining for ALP production, collagen secretion and ECM
mineralization using BCIP/NBT, Sirius red and Alizarin red
staining, respectively. In agreement with the gene expression data,
the ALP production and collagen secretion were observed to be
greater with the increase of Sema3A concentration (Fig. 3). No differences between the
treatment groups were identified from the ECM mineralization of the
samples, however this may be due to insufficient induction
time.
Osseointegration is greater in rats
implanted with Sema3A ASC sheets
To confirm that Sema3A may improve osseointegration
in vivo, ASC sheets were produced and modified with Sema3A
in order to coat Ti implants. The formation of new bone around the
implant was examined by micro-CT scanning (Fig. 4). The Sema3A-modified ASC sheets
had formulated more new bone (green section) around the implant
(red section), 4 weeks and 8 weeks subsequent to insertion
(Fig. 4A). The quantitative
analysis of the ROI demonstrated that the new bone volume and
number (4 weeks) and the new bone volume, thickness and number (8
weeks) were significantly greater in the rats implanted with the
Sema3A-modified ASC sheet compared with the ASCs only sheet
(P<0.05; Fig. 4B–D).
Bone formation is increased in rats with
Sema3A-modified ASC sheets compared with ASC-only rats
In the ASC sheet-only group, 4 weeks after
implantation, the new bone formation observed was thin compared
with the intermittent but thicker new bone formation around the
implant surface observed in the Sema3A-modified ASCs sheet group
(Fig. 5). At 8 weeks
post-implantation, fragmented new bone formation was observed in
the ASC sheet-only group, whereas continuous and thick new bone
formation was evident in the Sema3A-modified ASC sheet group
(Fig. 5).
Discussion
The proportion of the human population diagnosed
with T2DM has increased rapidly worldwide, resulting in a clear
health problem. Bone tissue is primarily damaged by the high
glucose levels, which lead to the suppression of differentiation in
osteoblastic lineage cells (25).
To improve osseointegration in patients with T2DM, the current
study introduced exogenous stem cells into the Ti implant
periphery. The osteogenic capacity of ASCs was significantly
increased with increases in Sema3A concentration. Therefore, ASCs
are suggested to be suitable for bone engineering following Sema3A
modification.
According to a previous study (19), the binding of Sema3A to
neuropilin-1 may stimulate osteoblast and inhibit adipocyte
differentiation via the canonical Wnt/β-catenin signaling pathway.
However, whether the same molecular mechanism is active in ASCs
remains to be elucidated. It is of note that an association between
Sema3A and mesenchymal stem cells has been demonstrated. BMSCs have
been identified to express Sema3A in order to regulate T-cell
immunosuppression (26). The
stimuli of Sema3A has been reported to induce mesenchymal stem
cell-like properties in human periodontal ligament cells (27). Therefore, although the exact
osteogenesis mechanism in ASCs remains unclear, it may be inferred
that Sema3A and ASCs interact closely.
The morphology of stem cells may allow for
determination of differentiation status. The spindle
fibroblast-like shape indicates a low differentiation status,
whereas the polygonal osteoblast-like shape indicates potential
osteogenic differentiation (28).
However, the present study has determined that all cells spread
widely without clear morphological alterations subsequent to Sema3A
treatment. This may be due to short incubation time; therefore the
cells were unable to exhibit a morphological difference. In
addition, it is possible that cell shape may be predominantly
defined by substrate topography. Although Sema3A has been
associated with cytoskeleton reorganization (29), the present study did not identify
any alterations in the morphology of ASCs treated with Sema3A.
Therefore, when the cells are grown on a glass surface, the
ultra-smooth topography may give cells the opportunity to spread
freely in all directions without obstacles. Proliferation is
primarily recognized as the opposite of differentiation, as
differentiated cells have demonstrated low proliferation and
mitotic cells are not terminally differentiated (30). However, a recent study identified
that proliferation and differentiation are two distinct
translational programs (31). The
current study confirmed that Sema3A may improve the osteogenic
ability of ASCs without inducing their proliferation.
In order to determine the formation of new bone
in vivo, micro-CT scanning and super-rigidity slicing were
performed. Similar to the results of the in vitro
experiments, the Sema3A pre-treated ASC sheet significantly
improved the formation of new bone around the Ti implant. It is of
note that Sema3A is only used during the ASC sheet formulation
in vitro, while the in vivo bone formation
enhancement occurs several weeks later. Therefore, the effect of
Sema3A is evident in the differentiation process of the ASCs in the
sheet long after its administration. A previous study observed that
the osteogenic promotion effect of Sema3A is effective at the
initial stage (27). Therefore,
Sema3A is convenient to use in conjunction with Ti implants. There
is a variety of bioactive molecules that may promote bone
metabolism and previous studies have aimed to establish a sustained
drug delivery system to acquire a prolonged effect. Sema3A allows
for treatment at the initial stage and avoids the problems
surrounding long-term delivery. Another important advantage of the
current study is that the systematic infusion of ASCs has been
validated as a novel therapy for T2DM (32). Sema3A-modified ASCs may be a
reliable choice for improving osseointegration in T2DM
patients.
The present study demonstrated that Sema3A may
significantly improve the osteogenesis ability of the ASC sheet and
may be able to facilitate osseointegration under T2DM
conditions.
Acknowledgments
The present study was supported by National Natural
Science Foundation of China (grant nos. 81170984, 81470775 and
81300918). The manuscript was been revised by the Elsevier English
Language Service. The authors would like to thank the Department of
Oral and Maxillofacial Surgery, School of Stomatology, Fourth
Military Medical University (Xi'an, China) for the technical
assistance.
References
|
1
|
Pjetursson BE, Brägger U, Lang NP and
Zwahlen M: Comparison of survival and complication rates of
tooth-supported fixed dental prostheses (FDPs) and
implant-supported FDPs and single crowns (SCs). Clin Oral Implants
Res. 18(Suppl 3): 97–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu YY, Yu T, Yang XY, Li F, Ma L, Yang Y,
Liu XG, Wang YY and Gong P: Vitamin D3 and insulin combined
treatment promotes titanium implant osseointegration in diabetes
mellitus rats. Bone. 52:1–8. 2013. View Article : Google Scholar
|
|
3
|
Zou GK, Song YL, Zhou W, Yu M, Liang LH,
Sun DC, Li DH, Deng ZX and Zhu WZ: Effects of local delivery of
bFGF from PLGA microspheres on osseointegration around implants in
diabetic rats. Oral Surg Oral Med Oral Pathol Oral Radio.
114:284–289. 2012. View Article : Google Scholar
|
|
4
|
Mellado-Valero A, Ferrer García JC,
Herrera Ballester A and Labaig Rueda C: Effects of diabetes on the
osseointegration of dental implants. Med Oral Patol Oral Cir Bucal.
12:E38–E43. 2007.PubMed/NCBI
|
|
5
|
Gopalakrishnan V, Vignesh RC, Arunakaran
J, Aruldhas MM and Srinivasan N: Effects of glucose and its
modulation by insulin and estradiol on BMSC differentiation into
osteoblastic lineages. Biochem Cell Biol. 84:93–101. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu M, Zhou W, Song Y, Yu F, Li D, Na S,
Zou G, Zhai M and Xie C: Development of mesenchymal stem
cell-implant complexes by cultured cells sheet enhances
osseointegration in type 2 diabetic rat model. Bone. 49:387–394.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan J, Zhang C, Zhao Y, Cao C, Wu K, Zhao
L and Zhang Y: Non-viral oligonucleotide antimiR-138 delivery to
mesenchymal stem cell sheets and the effect on osteogenesis.
Biomaterials. 35:7734–7749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Auquier P, Macquart-Moulin G, Moatti JP,
Blache JL, Novakovitch G, Blaise D, Faucher C, Viens P and
Maraninchi D: Comparison of anxiety, pain and discomfort in two
procedures of hematopoietic stem cell collection: Leukacytapheresis
and bone marrow harvest. Bone marrow Transplant. 16:541–547.
1995.PubMed/NCBI
|
|
9
|
Nishimori M, Yamada Y, Hoshi K, Akiyama Y,
Hoshi Y, Morishima Y, Tsuchida M, Fukuhara S and Kodera Y:
Health-related quality of life of unrelated bone marrow donors in
Japan. Blood. 99:1995–2001. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hiwatashi N, Hirano S, Mizuta M, Tateya I,
Kanemaru S, Nakamura T and Ito J: Adipose-derived stem cells versus
bone marrow-derived stem cells for vocal fold regeneration.
Laryngoscope. 124:E461–E469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Semon JA, Maness C, Zhang X, Sharkey SA,
Beuttler MM, Shah FS, Pandey AC, Gimble JM, Zhang S, Scruggs BA, et
al: Comparison of human adult stem cells from adipose tissue and
bone marrow in the treatment of experimental autoimmune
encephalomyelitis. Stem Cell Res Ther. 5:22014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin YC, Grahovac T, Oh SJ, Ieraci M, Rubin
JP and Marra KG: Evaluation of a multi-layer adipose-derived stem
cell sheet in a full-thickness wound healing model. Acta Biomater.
9:5243–5250. 2013. View Article : Google Scholar
|
|
13
|
Romagnoli C and Brandi ML: Adipose
mesenchymal stem cells in the field of bone tissue engineering.
World J Stem Cells. 6:144–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao HT and Chen CT: Osteogenic potential:
Comparison between bone marrow and adipose-derived mesenchymal stem
cells. World J Stem Cells. 6:288–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niemeyer P, Fechner K, Milz S, Richter W,
Suedkamp NP, Mehlhorn AT, Pearce S and Kasten P: Comparison of
mesenchymal stem cells from bone marrow and adipose tissue for bone
regeneration in a critical size defect of the sheep tibia and the
influence of platelet-rich plasma. Biomaterials. 31:3572–3579.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kini U and Nandeesh BN: Physiology of bone
formation, remodeling and metabolism. Radionuclide and Hybrid Bone
Imaging. Fogelman I, Gnanasegaran G and van der Wall H: Springer
Berlin; Heidelberg: pp. 29–57. 2012, View Article : Google Scholar
|
|
17
|
Hodsman AB, Bauer DC, Dempster DW, Dian L,
Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski
WP, et al: Parathyroid hormone and teriparatide for the treatment
of osteoporosis: A review of the evidence and suggested guidelines
for its use. Endocr Rev. 26:688–703. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neer RM, Arnaud CD, Zanchetta JR, Prince
R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S,
Genant HK, et al: Effect of parathyroid hormone (1-34) on fractures
and bone mineral density in postmenopausal women with osteoporosis.
N Engl J Med. 344:1434–1441. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hayashi M, Nakashima T, Taniguchi M,
Kodama T, Kumanogoh A and Takayanagi H: Osteoprotection by
semaphorin 3A. Nature. 485:69–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukuda T, Takeda S, Xu R, Ochi H, Sunamura
S, Sato T, Shibata S, Yoshida Y, Gu Z, Kimura A, et al: Sema3A
regulates bone-mass accrual through sensory innervations. Nature.
497:490–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pieri F, Lucarelli E, Corinaldesi G,
Aldini NN, Fini M, Parrilli A, Dozza B, Donati D and Marchetti C:
Dose-dependent effect of adipose-derived adult stem cells on
vertical bone regeneration in rabbit calvarium. Biomaterials.
31:3527–3535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang G, Guo B, Wu H, Tang T, Zhang BT,
Zheng L, He Y, Yang Z, Pan X, Chow H, et al: A delivery system
targeting bone formation surfaces to facilitate RNAi-based anabolic
therapy. Nat Med. 18:307–314. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Cerqueira MT, Pirraco RP, Santos TC,
Rodrigues DB, Frias AM, Martins AR, Reis RL and Marques AP: Human
adipose stem cells cell sheet constructs impact epidermal
morphogenesis in full-thickness excisional wounds.
Biomacromolecules. 14:3997–4008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hamann C, Goettsch C, Mettelsiefen J,
Henkenjohann V, Rauner M, Hempel U, Bernhardt R, Fratzl-Zelman N,
Roschger P, Rammelt S, et al: Delayed bone regeneration and low
bone mass in a rat model of insulin-resistant type 2 diabetes
mellitus is due to impaired osteoblast function. Am J Physiol
Endocrinol Metab. 301:E1220–E1228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lepelletier Y, Lecourt S, Renand A, Arnulf
B, Vanneaux V, Fermand JP, Menasché P, Domet T, Marolleau JP,
Hermine O and Larghero J: Galectin-1 and semaphorin-3A are two
soluble factors conferring T-cell immunosuppression to bone marrow
mesenchymal stem cell. Stem Cells Dev. 19:1075–1079. 2010.
View Article : Google Scholar
|
|
27
|
Wada N, Maeda H, Hasegawa D, Gronthos S,
Bartold PM, Menicanin D, Fujii S, Yoshida S, Tomokiyo A, Monnouchi
S and Akamine A: Semaphorin 3A induces mesenchymal-stem-like
properties in human periodontal ligament cells. Stem Cells Dev.
23:2225–2236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao L, Liu L, Wu Z, Zhang Y and Chu PK:
Effects of micropitted/nanotubular titania topographies on bone
mesenchymal stem cell osteogenic differentiation. Biomaterials.
33:2629–2641. 2012. View Article : Google Scholar
|
|
29
|
Nakamura F, Kumeta K, Hida T, Isono T,
Nakayama Y, Kuramata-Matsuoka E, Yamashita N, Uchida Y, Ogura K,
Gengyo-Ando K, et al: Amino- and carboxyl-terminal domains of
Filamin-A interact with CRMP1 to mediate Sema3A signalling. Nat
Commun. 5:53252014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klochendler A, Weinberg-Corem N, Moran M,
Swisa A, Pochet N, Savova V, Vikeså J, Van de Peer Y, Brandeis M,
Regev A, et al: A transgenic mouse marking live replicating cells
reveals in vivo transcriptional program of proliferation. Dev Cell.
23:681–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gingold H, Tehler D, Christoffersen NR,
Nielsen MM, Asmar F, Kooistra SM, Christophersen NS, Christensen
LL, Borre M, Sørensen KD, et al: A dual program for translation
regulation in cellular proliferation and differentiation. Cell.
158:1281–1292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu J, Fu Z, Chen Y, Tang N, Wang L, Wang
F, Sun R and Yan S: Effects of autologous adipose-derived stem cell
infusion on type 2 diabetic rats. Endocr J. 62:339–352. 2015.
View Article : Google Scholar : PubMed/NCBI
|