Introduction
Preeclampsia (PE) is a common complication during
pregnancy, which is characterized by hypertension, high urea
protein and abnormalities in other systems (1). PE is a key cause of fatality of
pregnant women and perinatal fetuses. It has been demonstrated that
PE is associated with immunity, uterus-placental ischemia,
endothelin and nitrogen oxide (NO) dysfunction (2); however, the precise etiology of PE
remains to be determined. Recent studies demonstrated that PE
development is accompanied with reduced placental infusion due to
extensive damage in the endothelium, which is predominantly caused
by oxidative stress and inflammation (3,4).
Moreover, ischemia can induce the production of free radicals and
decrease the activity of antioxidative proteins, resulting in
damage to the endothelium (5,6). It
is well-known that inflammation can cause immune imbalance,
endothelial damage, cytokine production, activation of neutrophils
and local oxidative stress in the placenta, eventually inducing PE
(6,7).
Currently it is difficult to prevent the early
stages of PE, clinical treatments predominantly focus on relieving
the symptoms, such as reducing blood pressure and seizures, and
supplying albumin (8). However,
the therapeutic effects of these treatments are not satisfactory
and over supplementation of albumin can put a strain on the kidney.
Thus, development of novel therapeutic agents to treat PE is
required. Harma et al (9)
reported that supplementation of antioxidants can reduce the
incidence of PE by 2/3 in high-risk pregnant women, which provides
direction for PE treatment. Raijmakers et al (10) observed that the combination of
vitamins C and E is a promising prophylactic strategy for
prevention of preeclampsia.
Astaxanthin, 3,3′-dihydroxy-β-carotene-4,4′-dione,
is extensively present in aquatic biology. The major feature of
astaxanthin is its antioxidative activity is 100–550 times stronger
than that of vitamin E. The biological activities of astaxanthin
include clearing cellular reactive oxygen species (ROS), reducing
oxidative stress, inflammation and blood pressure, and increasing
NO utilization (11–14). Thus, according to the biological
activities of astaxanthin, it was predicted that astaxanthin may
effectively reduce PE. Thus, the present study aimed to investigate
the effect of astaxanthin on the antioxidative activity of
endothelial cells, and assess the therapeutic effects of
astaxanthin on rats with Nω-nitro-L-arginine methyl ester
(L-NAME)-induced preeclampsia.
Materials and methods
Cell lines and cell culture
Human umbilical vein endothelial cells (HUVECs) were
obtained from China Center for Type Culture Collection (Wuhan,
China). The cells were maintained in minimal essential medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and streptomycin at 37°C in
a humidified atmosphere of 5% CO2, and subcultured upon
reaching 80% confluence.
Cell viability assay
Viability of HUVECs following exposure to
astaxanthin isolated from Pluvialis algae (Wako Pure Chemical
Industries, Ltd., Wako, Japan) and H2O2 was
assessed using an MTT
(3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide)
cytotoxicity assay. Briefly, 50–60% confluent cell monolayers
(5×105/ml) were exposed to different concentrations of
astaxanthin (0.1, 1 or 10 µmol/l) for 48 h. Cells were
washed for three times and then cultured in medium containing 500
µmol/l or 2 mmol/l H2O2 for 1 or 24 h.
Cells were then incubated with 20 µl MTT for 4 h at 37°C.
After removing MTT, 200 µl dimethyl formamide was added.
Absorbance readings were taken at 490 nm using a microplate reader.
Results were expressed as the percentage of control (untreated
cells).
Determination of ROS production
Intracellular oxidant stress was monitored by
measuring the changes in fluorescence after intracellular probe
oxidation. HUVECs were treated with different concentrations of
astaxanthin (0.1, 1 or 10 µmol/l) for 48 h and 2 mmol/l
H2O2 for 1 h, then trypsinized and washed
twice in phosphate-buffered saline (PBS). The fluorometric probe,
2′,7′-dichlorofluorescein diacetate (DCFH-DA) (20 µM) was
added to the cells and incubated at 37°C for 45 min. Cells were
washed with PBS and ROS measurement was conducted using a
FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA). In total, 10,000 events were counted in each run using
CellQuest software (BD Biosciences) and all the experiments were
repeated 3 times.
Determination of the mitochondrial
membrane potential (MMP) by JC-1 fluorescence
MMP was measured with the lipophilic cationic probe
JC-1. HUVECs were treated with different concentrations of
astaxanthin for 48 h and then 2 mmol/l H2O2
for 1 h. Cells were washed twice in PBS and incubated with 2.5
µg/ml JC-1 in the dark for 20 min at 37°C. Cells were washed
twice with PBS and resuspended in 400 µl PBS and analyzed by
flow cytometry. A 488-nm filter was used for excitation of JC-1.
Emission filters of 535 and 595 nm were used to quantify the
population of HUVECs with green (JC-1 monomer) and orange (JC-1
aggregates) fluorescence, respectively. All samples were examined
by fluorescence microscopy to confirm JC-1 labeling patterns.
Animals and treatments
A total of 120 mature Sprague-Dawley (SD) rats
(weight, 250–260 g; age, 75 days) were purchased from Zhejiang
Provincial Experimental Animal Center and housed in the animal
center of Ningbo University Medical College (Ningbo, China). All
rats were maintained under a 12-h light/dark cycle (08:00 AM lights
on) and provided with food and water ad libitum. Housing and
experimental environments were temperature- and humidity-controlled
(21±2°C and ~60% humidity, respectively). All experimental
procedures were performed in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals and approved by the Ethical Committee of Animal Use and
Protection of Ningbo University (Ningbo, China).
Male rats and female rats in estrous cycle were
mated at a ratio of 1:3. The day of appearance of a vaginal plug
was regarded as day 1 of pregnancy. All pregnant rats were randomly
assigned to the following 5 groups (n=10): Blank (no treatment),
control (L-NAME treatment only) and three astaxanthin groups that
were treated with 5, 15, 25 mg/kg body weight (bw)/day astaxanthin,
respectively, by gavage from day 5 until the end of experiment. The
rats in the control and astaxanthin groups were subcutaneously
injected with 125 mg/kg bw/day L-NAME, while the rats in the blank
group were injected with same volume of saline. On day 18, the
blood pressure was detected and urinary protein was measured using
a urine analyzer (Hitachi, Ltd., Tokyo, Japan). The increased blood
pressure and level of urinary proteins indicated successful
generation of preeclamptic models (15). On day 21, the blood was collected
via abdominal aorta. The rats were anesthetized using diethyl ether
(Sinopharm Chemical Reagent, Co., Ltd., Beijing, China) and
sacrificed by blooding via the femoral artery. The placental
tissues were collected, fixed in 4% paraformaldehyde for 24 h and
embedded in paraffin.
Measurement of serum oxidative
parameters
The blood was centrifuged at 1,000 × g for 10 min at
4°C to collect serum. The activity of serum malondialdehyde (MDA),
superoxide dismutase (SOD) and nitric oxide synthase (NOS), and the
levels of serum NO and endothelin were measured by kits (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) according to
the manufacturer's instructions.
Immunohistochemistry
The placental sections (3–4 µm) were
deparaffinized and rehydrated in a graded series of ethanol. The
sections were boiled in 0.01 M sodium citrate buffer (pH 6.0) for
15 min and treated with 3% H2O2 in methanol
for 10 min to quench endogenous peroxidase. Then the sections were
blocked in horse serum for 30 min and incubated with various
anti-mouse monoclonal antibodies: Anti-nuclear factor (NF)-κB
(Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA; 1:100; cat.
no. sc-8008), anti-Rho-associated protein kinase II (ROCK II; Santa
Cruz Biotechnology Inc.; 1:200; cat. no. sc-398519), anti-Caspase 3
(Santa Cruz Biotechnology Inc.; 1:100; cat. no. sc-271759) and goat
anti-mouse polyclonal heme oxygenase-1 (HO-1) antibody (Bioss Inc.,
Woburn, MA, USA; 1:200; cat. no. bs-2075R) overnight at 4°C. The
control sections were omitted for primary antibody. The sections
were washed with PBS and incubated with biotinylated goat
anti-mouse (cat. no. sc-2005) and donkey anti-goat (cat. no.
sc-2020) secondary antibodies (Santa Cruz Biotechnology Inc.;
1:200) for 2 h at room temperature, followed by the incubation with
the streptavidin-biotin-peroxidase complex. The sections were
stained with a DAB (3,3′-diaminobenzidine) kit (Vector
Laboratories, Inc., Burlingame, CA, USA) and counterstained with
hematoxylin, dehydrated with ethanol, cleared with xylene and
mounted in synthetic resin. Positive staining, which appeared as a
brown color, was visualized under a light microscope. The
expression of NF-κB, HO-1, Caspase 3 and ROCK II was quantified
using by comparing positive-labeled areas and total areas using
Image-Pro Plus 7.0 software (Media Cybernetics, Inc., Rockville,
MD, USA).
Statistical analysis
The results are expressed as the mean ± standard
error of mean. Statistical analyses were performed using the SPSS
software, version 16.0 (SPSS Inc., Chicago, IL, USA). Differences
among groups were analyzed by using one-way analysis of variance
and Duncan's test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of astaxanthin on cell viability
in H2O2-treated HUVECs
HUVECs were treated with two different
concentrations of H2O2 and cell viability was
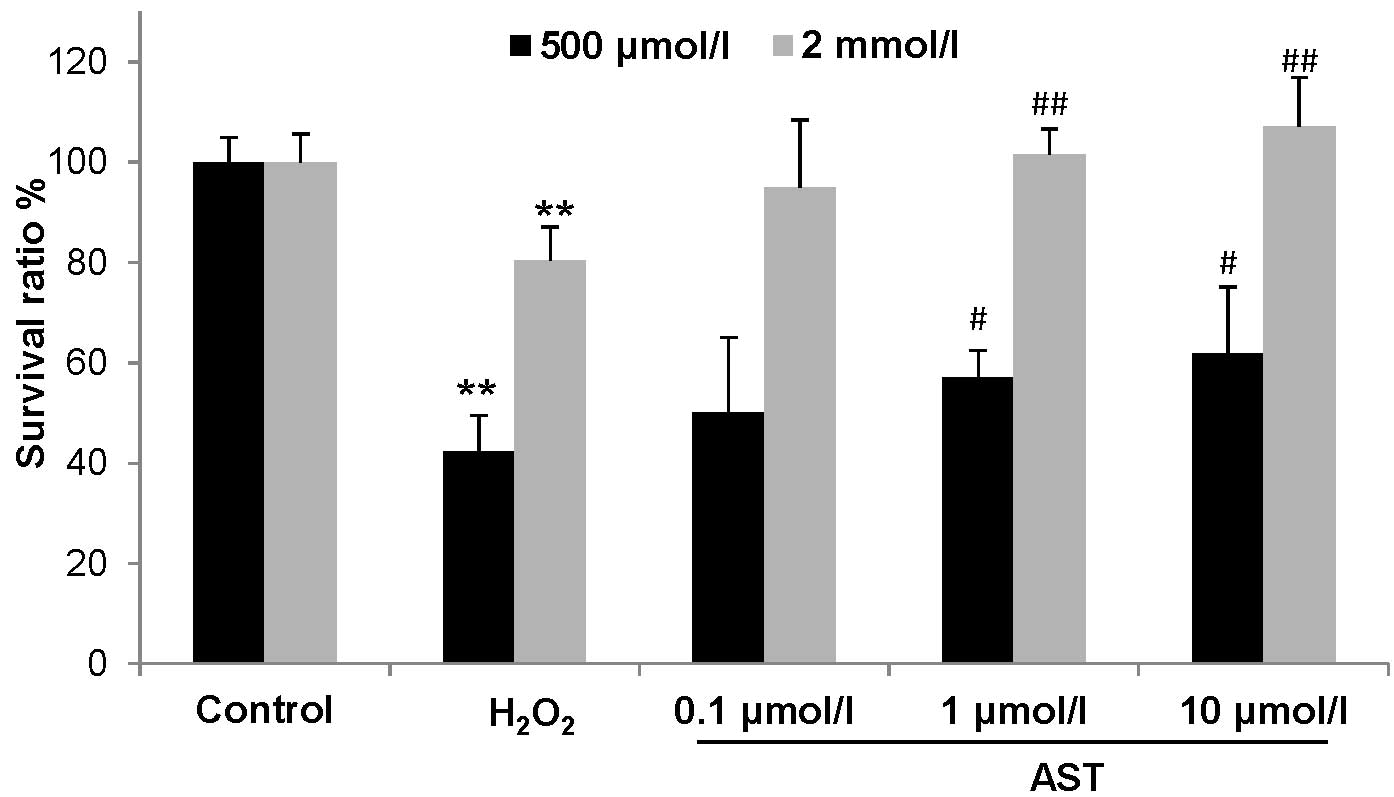
examined by an MTT assay. As shown in Fig. 1, compared with control cells, cell
viability was reduced to 80.47% after treatment with 500
µmol/l H2O2 for 24 h, and to 42.22%
after treatment with 2.0 mmol/l H2O2 for 1 h
(P<0.01). However, astaxanthin treatment at 1.0 µmol/l
and 10 µmol/l significantly improved cell viability in two
concentrations of H2O2-treated HUVECs, in
which cell viability in 500 µmol/l but not 2.0 mmol/l
H2O2-treated HUVECs was completed rescued by
1.0 and 10 µmol/l astaxanthin (Fig. 1). Thus, 2.0 mmol/l
H2O2 was used to examine the effect of
astaxanthin on ROS.
Effect of astaxanthin on ROS and MMP in
H2O2-treated HUVECs
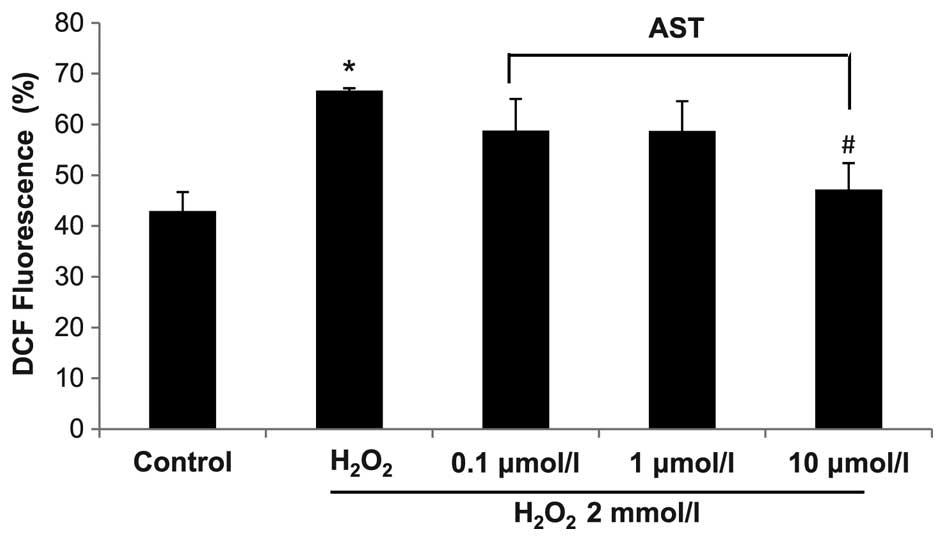
Compared with control, H2O2
treatment significantly increased the level of ROS in HUVECs
(P<0.05) (Fig. 2). Astaxanthin
treatment at 0.1 and 1.0 µmol/l markedly reduced ROS level
in H2O2-treated HUVECs (P>0.05), and 10
µmol/l astaxanthin significantly reduced the ROS level in
H2O2-treated HUVECs (P<0.05). These data
indicate that high level of astaxanthin can reduce the level of ROS
in HUVECs. The effect of astaxanthin on MMP was also examined in
H2O2-treated HUVECs.
Mitochondria are considered the predominant
intracellular source of reactive oxygen species. The decrease in
MMP is associated with reduced mitochondrial function. When MMP is
high, the fluorescence probe JC-1 gathers in the mitochondrial
matrix to form aggregates that fluoresce red, while at low MMP JC-1
monomers fluoresce green. In the present study, the green
fluorescence was strong following H2O2
treatment, suggesting low levels of MMP (Fig. 3). However, astaxanthin treatment
gradually reduced green fluorescence but increased red fluorescence
in H2O2-treated HUVECs (Fig. 3). These data suggest that
astaxanthin treatment can effectively protect MMP in a
concentration-dependent manner.
Effect of astaxanthin on blood pressure
and urinary protein in preeclamptic rats
L-NAME is the conventional agent used to establish
animal models of hypertension. After injection with L-NAME, the
systolic and diastolic blood pressures were increased by 25.75
(P<0.05) and 23.00%, respectively. Moreover, the urinary protein
was increased. After treatment with different concentrations of
astaxanthin, blood pressure and urinary protein were reduced in a
concentration-dependent manner. Notably, compared with the L-NAME
group, 25 mg/kg bw/day astaxanthin reduced systolic and diastolic
blood pressure by 38.09 and 57.80% (P<0.01), respectively, which
was even significantly lower than that of the blank group
(P<0.05, Table I). Urinary
protein was also reduced by 25 mg/kg bw/day astaxanthin.
 | Table IEffect of astaxanthin on blood
pressure and proteinuria in rats. |
Table I
Effect of astaxanthin on blood
pressure and proteinuria in rats.
| Group | Systolic blood
pressure (mmHg) | Diastolic blood
pressure (mmHg) | Urinary protein
(pg/ml) |
|---|
| Blank | 83.50±9.11 | 56.50±10.60 | 10.51±1.56 |
| L-NAME |
105.00±11.80a | 69.50±12.34 | 11.02±0.79 |
| Astaxanthin (5
mg/kg bw/day) | 98.30±5.72 | 61.05±3.35 | 10.84±1.54 |
| Astaxanthin (15
mg/kg bw/day) | 85.00±3.88b | 45.26±2.87c,d | 10.69±1.37 |
| Astaxanthin (25
mg/kg bw/day) | 65.00±4.58c,d | 29.33±1.53c | 10.24±1.10 |
Effect of astaxanthin on serum parameters
in preeclamptic rats
In L-NAME-treated preeclamptic rats, serum NO and
SOD were significantly reduced (P<0.05), while serum NOS and MDA
was markedly changed (P>0.05, Table II). However, 15 mg/kg bw/day and
higher levels of astaxanthin treatment significantly decreased
serum MDA but increased SOD in preeclamptic rats. Moreover,
following treatment with 25 mg/kg bw/day astaxanthin, serum MDA was
decreased while serum SOD level was increased compared with that in
the blank group (P<0.05), while serum NO and NOS were not
identified to be significantly changed (P>0.05).
 | Table IIEffect of astaxanthin on the content
of NO and MDA, and the activity of NOS and SOD in rat serum. |
Table II
Effect of astaxanthin on the content
of NO and MDA, and the activity of NOS and SOD in rat serum.
| Group | NO (pg/ml) | NOS (U/ml) | MDA (nmol/ml) | SOD (U/ml) |
|---|
| Blank | 6.29±3.57 | 37.08±4.27 | 1.81±1.59 | 25.45±4.64 |
| L-NAME | 4.74±2.77a | 35.87±4.97 | 1.96±1.80 | 16.52±2.77a |
| Astaxanthin (5
mg/kg bw/day) | 4.76±2.53 | 36.05±3.26 | 1.84±0.42 | 20.58±6.72 |
| Astaxanthin (15
mg/kg bw/day) | 4.85±1.36 | 36.78±5.15 | 1.28±0.70b | 25.57±3.83b |
| Astaxanthin (25
mg/kg bw/day) | 4.90±1.93 | 37.35±4.06 | 0.84±0.56a,b | 33.34±8.16a,b |
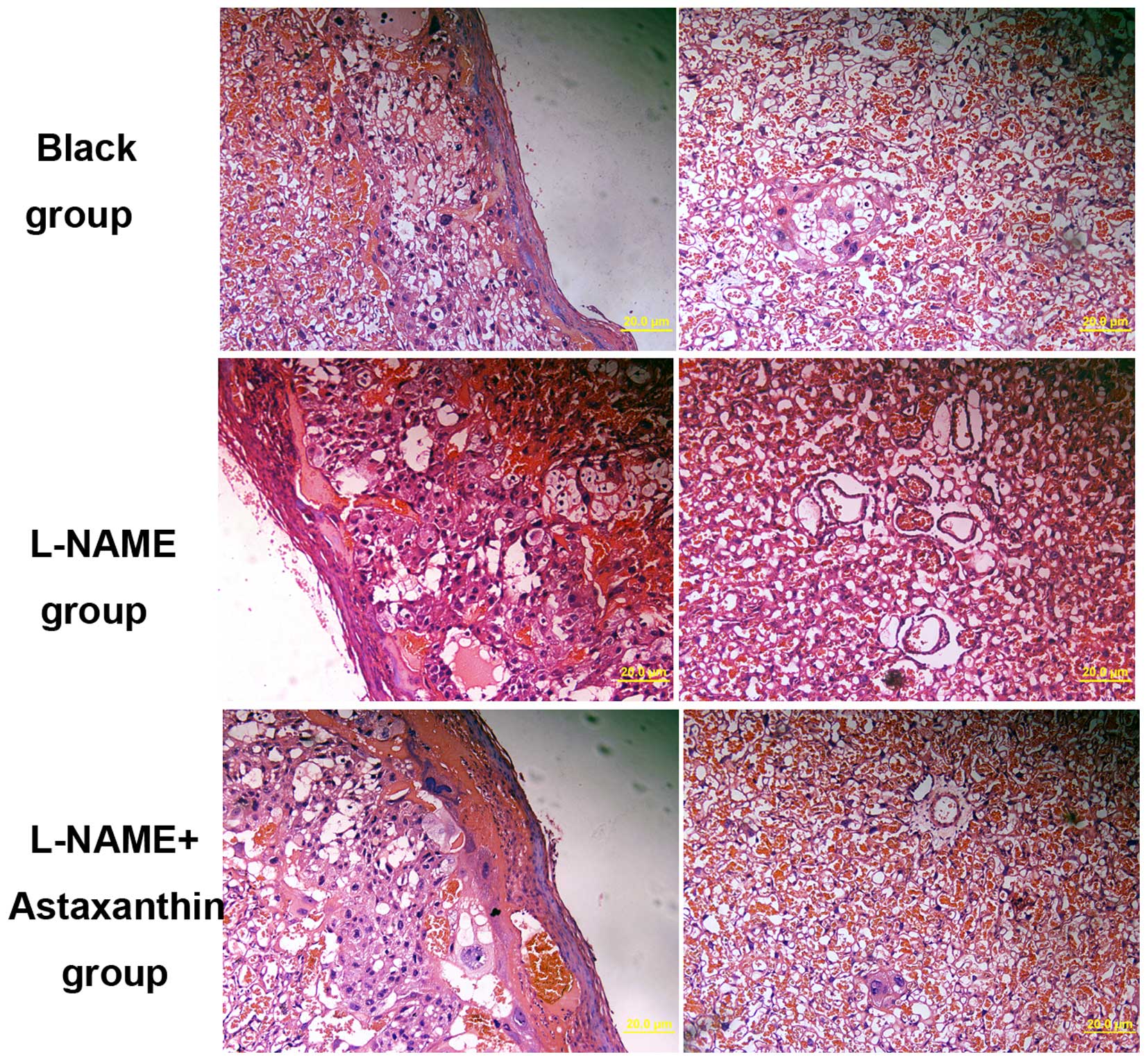
Effect of astaxanthin on histological
changes in preeclamptic rats
The histological changes in placental tissues were
also determined following treatment with 25 mg/kg bw/day
astaxanthin in preeclamptic rats. In preeclamptic placental
tissues, a few spiral arteries with large lumen and thin vessel
walls were observed (Fig. 4). The
basement membrane of placental tissue was irregularly thickened
with increased cell nodules (Fig.
4). Following treatment with astaxanthin, the number of spiral
arteries was decreased compared with the control group, and a few
trophoblasts were identified around spiral arteries (Fig. 4). Thus, astaxanthin treatment can
significantly improve the pathological changes in preeclamptic
rats.
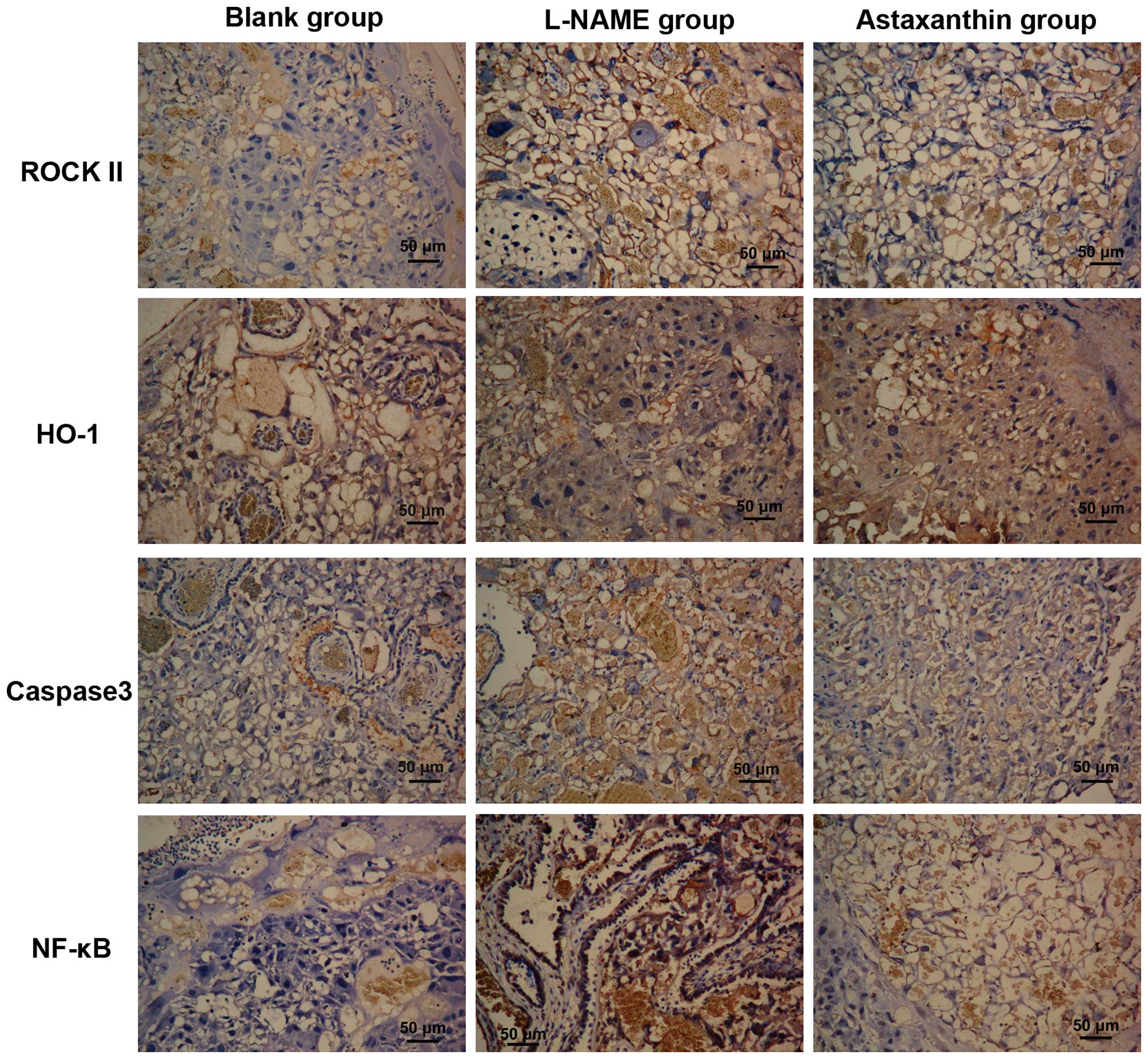
Effect of astaxanthin on the expression
of certain proteins in preeclamptic rats
The expression of PE-associated proteins in
placental tissues by immunohistochemistry. In the control and
astaxanthin groups, ROCK II protein was expressed in the cytoplasm
of cytotrophoblasts, syncytiotrophoblasts, endothelial cells and
stromal cells, with high expression in the cytoplasm of
trophoblasts (Fig. 5). Moreover,
after quantitative analysis, ROCK II expression in the control and
astaxanthin groups was significantly higher than that in the blank
group (P<0.01), while its expression in the astaxanthin group
was significantly lower than that in the control group (P<0.05,
Table III).
 | Table IIIEffect of astaxanthin on the
expression of preeclampsia-associated proteins in the placenta. |
Table III
Effect of astaxanthin on the
expression of preeclampsia-associated proteins in the placenta.
| Group | ROCK II | HO-1 | Caspase 3 | NF-κB |
|---|
| Blank | 260.69±60.50 | 6484.38±638.70 | 1601.96±388.09 | 1748.65±592.34 |
| L-NAME |
1002.44±141.75a |
1893.16±392.74a |
4253.50±611.60a |
5110.65±276.43a |
| Astaxanthin (25
mg/kg bw/day) |
678.37±110.03a,b |
4875.39±638.70c |
3025.32±366.26a |
3607.98±859.52a,b |
HO-1 was expressed in the cytoplasm of villous
syncytiotrophoblasts and endothelial cells, with low expression in
the stromal cells (Fig. 5). Based
on the quantitative results, HO-1 expression in the control group
was significantly lower than that in the blank group (P<0.01),
astaxanthin treatment significantly increased HO-1 expression in
placental tissue compared with the L-NAME group (P<0.01),
however, its expression still lower than that in the blank
group.
Caspase 3 was expressed in the membrane and
cytoplasm of trophoblasts (Fig.
5). Its expression was significantly increased in preeclamptic
placentas compared with the blank group (P<0.01), while
astaxanthin treatment significantly decreased Caspase 3 expression
in the placental tissues (P<0.05).
NF-κB was predominantly expressed in the
cytotrophoblasts, with low expression in villous stromal cells. In
the control group, high NF-κB expression was observed in the
trophoblasts, with a degree of nucleus staining (Fig. 5). Compared with the blank group,
NF-κB expression was significantly increased in the control group
(P<0.01), while its expression in the astaxanthin group was
significantly lower than that in the control group (P<0.05) but
significantly higher than that in the blank group (P<0.01).
Discussion
PE is a specific disorder associated with
hypertension during gestation. The pathological mechanisms for PE
are complex; however, in recent years, PE has been shown to be
associated with the damage of endothelial cells, oxidative stress
and inflammation in the placenta (2–6).
Abnormal oxidative stress to pregnant women is a key factor
involved in the induction of PE. If placental lesions (such as less
placenta blood infusion) affect maternal lipid metabolism to result
in uncontrolled lipid peroxidation, they will provide excessive
active oxygen and induce oxidative stress, and result in the damage
to vessel endothelial cells in the placenta, which will cause
pathological damage to placenta and induce PE (16,17).
Excessive inflammation has been observed in the early stages of PE
and the production of inflammation-related chemokines and cytokines
is markedly increased (18).
Moreover, a previous study suggested that the development and
progression of PE is the consequence of inflammation and oxidative
stress to vessel endothelial cells (19). Thus, antioxidative therapy has been
applied in PE treatment (20).
Serdar et al (21) found
that the level of vitamin E and carotene in patients with severe PE
patients is markedly lower than that in pregnant women, suggesting
that antioxidant supplements at the early stages of gestation is
key in the prevention of PE. Poston et al (22) reported that the incidence of PE in
pregnant women taking vitamin E and C supplements is lower than
that in control and placebo groups. Antioxidative therapy to
prevent PE has been performed in the United States, Canada, Mexico,
England and other countries, may be particularly efficacious for PE
treatment (23).
Astaxanthin is an antioxidant and the strongest
known singlet-oxygen quencher (11). Moreover, astaxanthin has an
anti-inflammatory effect through inhibiting the expression of
inflammation-associated genes and changing the ratio of Th1/Th2
cells (12,24). Additionally, it was reported that
astaxanthin can decrease blood pressure in hypertensive mice and
increase the utility of NO (13,25).
Based on the effects of astaxanthin, it is reasonable to assume
that astaxanthin may have therapeutic efficacy in PE, although its
application for PE therapy has never been reported.
The polyene structure of astaxanthin leads to low
polarity, and it predominantly exists in a crystal form. This
results in low permeabilization into cells and thus few studies
have been conducted on its effects. However, Chew et al
(26) found that astaxanthin does
slowly permeate into cells, with a peak at 24–48 h, and thus may be
a treatment candidate. In this study, H2O2
was used to induce oxidative damage and astaxanthin treatment for
48 h was shown to effectively relieve
H2O2-induced endothelial cell death, reduce
the production of active oxygen, and protect the MMP. Astaxanthin
treatment significantly increased MMP in
H2O2-treated HUVECs, suggesting that it can
effectively decrease the production of ROS, rescue active
oxygen-induced oxidative damage to the membrane, and thus protect
the function of mitochondria.
Consistent with a previous study (13), astaxanthin treatment significantly
decreased blood pressure and urinary protein in L-NAME-induced
preeclamptic rats. The blood pressure in astaxanthin-treated rats
was even lower than that in healthy pregnant rats, thus it requires
further assessment whether astaxanthin treatment induces low blood
pressure. According to the histological data, astaxanthin treatment
significantly decreased the number of spiral arteries, villous
microvessels and cell nodules in syncytiotrophoblasts, and reversed
irregular thickening of the base membrane of trophoblasts. These
data indicate astaxanthin treatment can effectively reduce the
symptoms of PE in rats.
Astaxanthin treatment was shown to reduce MDA and
increase SOD activity, which supports the major role of astaxanthin
treatment in antioxidation. Additionally, NO is a strong
vasodilator, which is involved in regulating vascular tone and
maintaining hemodynamic equilibrium during gestation. In
preeclamptic conditions, the dysfunction of endothelial cells
decreases the activity of NOS and the production of NO, and thus
causes hypertension (13). In
L-NAME-induced preeclamptic rats, serum NOS and NO were markedly
decreased; however, astaxanthin treatment only marginally increased
serum NOS and NO. Thus, the role of astaxanthin treatment in
mediating serum NOS and NO requires further investigation.
It has been demonstrated that NF-κB is involved in
the regulation of immunity and inflammation (27). In patients with severe PE,
increased NF-κB expression in placenta tissue affects the invasive
capacity of trophoblasts, causes the damage to endothelial cells
and induces the production of inflammatory cytokines (28). It was demonstrated that astaxanthin
treatment significantly suppressed overexpression of NF-κB
L-NAME-induced preeclamptic rats. These results indicate that
astaxanthin treatment may decrease the production of inflammatory
cytokines and the damage to endothelial cells. The Rho/ROCK
signaling pathway is important in the regulation of trophoblast
proliferation, apoptosis and invasion (29), and ROCK II is regarded as an
important effector downstream of the Rho signaling pathway. ROCK II
expression is significantly elevated in patients with severe PE,
suggesting it may be involved in the development of PE (30). The present study also observed high
ROCK II expression in L-NAME-treated rats, but astaxanthin
treatment significantly neutralized L-NAME-induced ROCK II
expression. These data suggest that astaxanthin may mediate the
invasive capacity of trophoblasts through inhibiting ROCK II
expression, and inhibiting the progression of PE. HO-1 is a
rate-limiting enzyme that catalyzes oxidative degradation of
cellular heme to liberate free iron, carbon monoxide (CO) and
biliverdin in mammalian cells (31). In addition, its role in heme
catabolism, HO-1 exhibits anti-oxidative and anti-inflammatory
functions via the actions of biliverdin and CO, respectively
(31). Compared with healthy
pregnant women, HO-1 expression is significantly reduced in
patients with pregnancy-induced hypertension. The present study
demonstrated that L-NAME treatment significantly reduced HO-1
expression, while astaxanthin treatment significantly increased
HO-1 expression in L-NAME-induced preeclamptic rats. These results
suggest that astaxanthin treatment may degrade aging hemoglobin,
inhibit antioxidative damage and promote CO production. It has been
demonstrated that oxidative stress and inflammation can cause the
apoptosis of trophoblasts and endothelial cells (32). In the present study, it was
demonstrated that L-NAME treatment significantly increased caspase
3 expression, while astaxanthin treatment could decrease caspase 3
expression in preeclamptic rats, suggesting that astaxanthin can
alleviate apoptosis in PE.
In conclusion, astaxanthin has an antioxidative
effect in endothelial cells. Early application of astaxanthin can
effectively decrease L-NAME-induced high blood pressure, urinary
protein, oxidative stress, inflammation and apoptosis in the
placenta. Thus, astaxanthin can reduce the damage to endothelial
cells and improve the symptoms of PE; however, its safety in
pregnant women requires further investigation.
Acknowledgments
This study was financed by grants from the Zhejiang
Medical Technology Project (grant no.2013KYB238) and the K.C. Wong
Magna Fund of Ningbo University, Zhejiang 151 Talents Project.
Abbreviations:
|
L-NAME
|
Nω-nitro-L-arginine methyl ester
|
|
ROS
|
reactive oxygen species
|
|
MMP
|
mitochondrial membrane potential
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
SD
|
sprague-dawley
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
NOS
|
nitric oxide synthase
|
|
ROCK II
|
Rho-associated protein kinase II
|
|
HO-1
|
heme oxygenase-1
|
|
PE
|
preeclampsia
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)
2,5-diphenyltetrazolium bromide
|
References
|
1
|
Sibai B, Dekker G and KuPerminc M:
Pre-eclampsia. Lancet. 365:785–799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Redman CW and Sargent IL: Latest advances
in understanding preeclampsia. Science. 308:1592–1594. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vanderlelie J, Gude N and Perkins AV:
Antioxidant gene expression in preeclamptic placentae: A
preliminary investigation. Placenta. 29:519–522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Biondi C, Pavan B, Lunghi L, Fiorini S and
Vesce F: The role and modulation of the oxidative balance in
pregnancy. Curr Pharm Des. 11:2075–2089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dekker GA and Sibai BM: Etiology and
pathogenesis of preeclampsia: Current concepts. Am J Obstet
Gynecol. 179:1359–1375. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Var A, Yildirim Y, Onur E, Kuscu NK,
Uyanik BS, Goktalay K and Guvenc Y: Endothelial dysfunction in
preeclampsia. Increased homocysteine and decreased nitric oxide
levels. Gynecol Obstet Invest. 56:221–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Germain SJ, Sacks GP, Sooranna SR, Sargent
IL and Redman CW: Systemic inflammatory priming in normal pregnancy
and preeclampsia: The role of circulating syncytiotrophoblast
microparticles. J Immunol. 178:5949–5956. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Magee LA, Miremadi S, Li J, Cheng C, Ensom
MH, Carleton B, Côté AM and von Dadelszen P: Therapy with both
magnesium sulfate and nifedipine does not increase the risk of
serious magnesium-related maternal side effects in women with
preeclampsia. Am J Obstet Gynecol. 193:153–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harma M, Harma M and Erel O: Oxidative
stress in women with preeclampsia. Am J Obstet Gynecol.
192:656–657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raijmakers MT, Dechend R and Poston L:
Oxidative stress and preeclampsia: Rationale for antioxidant
clinical trials. Hypertension. 44:374–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guerin M, Huntley ME and Olaizola M:
Haematococcus astaxanthin: Applications for human health and
nutrition. Trends Biotechnol. 21:210–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pashkow FJ, Watumull DG and Campbell CL:
Astaxanthin: A novel potential treatment for oxidative stress and
inflammation in cardiovascular disease. Am J Cardiol. 101:58D–68D.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hussein G, Goto H, Oda S, Sankawa U,
Matsumoto K and Watanabe H: Antihypertensive potential and
mechanism of action of astaxanthin: III. Antioxidant and
histopathological effects in spontaneously hypertensive rats. Biol
Pharm Bull. 29:684–688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohgami K, Shiratori K, Kotake S, Nishida
T, Mizuki N, Yazawa K and Ohno S: Effects of astaxanthin on
lipopolysaccharide-induced inflammation in vitro and in vivo.
Invest Ophthalmol Vis Sci. 44:2694–2701. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takei H, Nakai Y, Hattori N, Yamonoto M,
Kurauchi K, Sasaki H and Aburada M: The herbal medicine
Toki-shakuyaku-san improves the hypertension and intrauterine
growth retardation in preeclampsia rats induced by
Nomega-nitro-L-arginine methyl ester. Phytomedicine. 11:43–50.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bowen RS, Moodley J, Dutton MF and Theron
AJ: Oxidative stress in pre-eclampsia. Acta Obstet Gynecol Scan.
80:719–725. 2001. View Article : Google Scholar
|
|
17
|
Tsukimori K, Fukushima K, Tsushima A and
Nakano H: Generation of reactive oxygen species by neutrophils and
endothelial cell injury in normal and preeclamptic pregnancies.
Hypertension. 46:696–700. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Redman CW and Sargent IL: Preeclampsia and
the systemic inflammatory response. Semin Nephrol. 24:565–570.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bernardi F, Guolo F, Bortolin T,
Petronilho F and Dal-Pizzol F: Oxidative stress and inflammatory
markers in normal pregnancy and preeclampsia. J Obstet Gynaecol
Res. 34:948–951. 2008.PubMed/NCBI
|
|
20
|
Rumbold A, Duley L, Crowther C and Haslam
R: Antioxidants for preventing pr-eclampsia. Cochrane Database Syst
Rev. CD0042272005.
|
|
21
|
Serdar Z, Gür E, Colakoethullarý M,
Develioethlu O and Sarandöl E: Lipid and protein oxidation and
antioxidant function in women with mild and severe preeclampsia.
Arch Gynecol Obstet. 268:19–25. 2003.PubMed/NCBI
|
|
22
|
Poston L, Briley AL, Seed PT, Kelly FJ and
Shennan AH; Vitamins in Pre-eclampsia (VIP) Trial Consortium:
Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia
(VIP trial): Randomised placebo-controlled trial. Lancet.
367:1145–1154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sibai BM: Preeclampsia: An inflammatory
syndrome? Am J Obstet Gynecol. 191:1061–1062. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kidd P: Astaxanthin, cell membrane
nutrient with diverse clinical benefits and anti-aging potential.
Altern Med Rev. 16:355–364. 2011.
|
|
25
|
Mortensen A, Skibsted LH, Sampson J,
Rice-Evans CR and Everett SA: Comparative mechanisms and rates of
free radical scaveging by carotenoid antioxidants. FEBS Lett.
418:91–97. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chew W, Mathison BD, Kimble LL, Mixter PF
and Chew BP: Astaxanthin decreases inflammatory biomarkers
associated with cardiovascular disease in human umbilical vein
endothelial cells. Am J Adv Food Sci Technol. 1:1–175. 2013.
|
|
27
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shah TJ and Walsh SW: Activation of
NF-kappaB and expression of COX-2 in association with neutrophil
infiltration in systemic vascular tissue of women with
preeclampsia. Am J Obstet Gynecol. 196:48.e1–e8. 2007. View Article : Google Scholar
|
|
29
|
Pollheimer J and Knöfler M: Signaling
pathways regulating the invasive differentiation of human
trophoblasts: A review. Placenta. 26(Suppl A): S21–S30. 2005.
View Article : Google Scholar
|
|
30
|
Ark M, Yilmaz N, Yazici G, Kubat H and
Aktaş S: Rho-associated protein kinase II (rock II) expression in
normal and preeclamptic human placentas. Placenta. 26:81–84. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Appleton SD, Lash GE, Marks GS, Nakatsu K,
Brien JF, Smith GN and Graham CH: Effect of glucose and oxygenase
depriation on heme oxygenase expression in human chorionic villi
explants and immortalized trophoblast cells. Am J Physiol Regul
Integr Comp Physiol. 285:R1453–R1460. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Myatt L and Cui X: Oxidative stress in the
placenta. Histochem Cell Biol. 122:369–382. 2004. View Article : Google Scholar : PubMed/NCBI
|