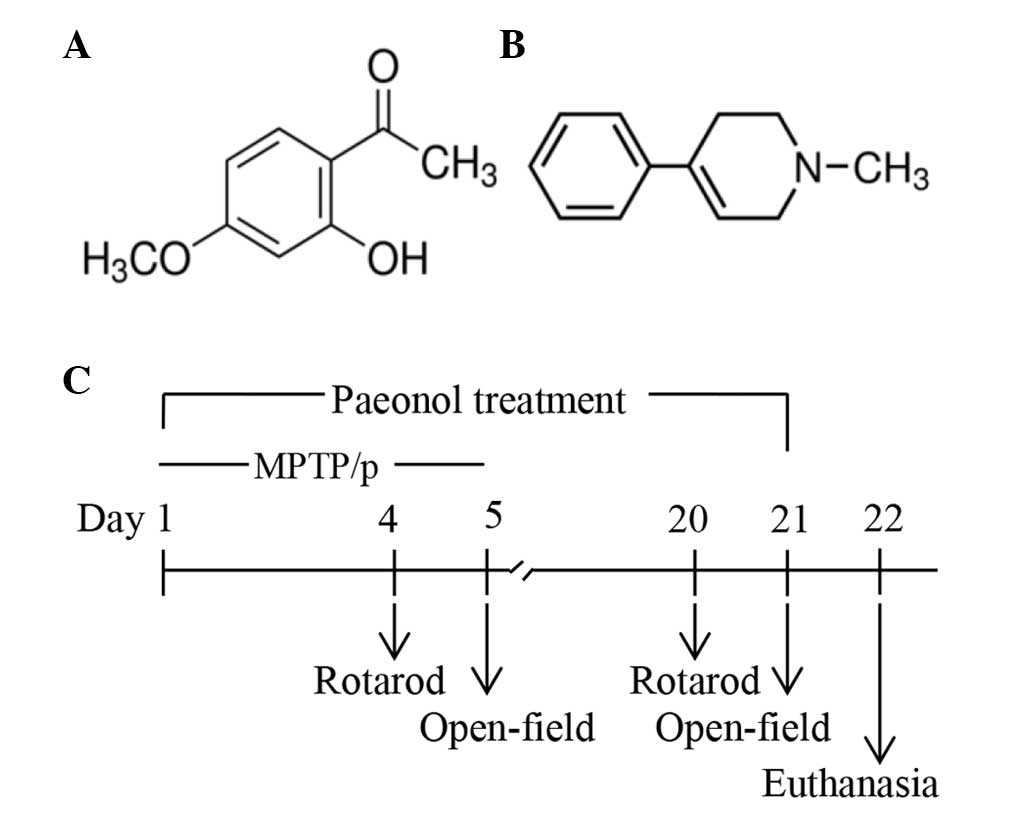
Introduction
Parkinson's disease (PD) is a progressive
neurodegenerative disorder characterized by the selective
degeneration of dopaminergic neurons in the substantia nigra (SN),
a region of the brain that controls movement (1). The primary symptoms of PD include
tremor at rest, muscular rigidity, bradykinesia, postural
abnormalities and instability (2).
Although the precise underlying pathogenic mechanism of PD remains
to be elucidated, previous studies have suggested that oxidative
stress, inflammation, excitotoxicity and mitochondrial dysfunction
may be involved in the progression of PD (3,4).
Post-mortem analysis of patients with PD have indicated that
oxidative stress and inflammatory pathways together cause
dopaminergic neurons to undergo apoptosis, ultimately resulting in
PD (5). These findings suggest
that it may be beneficial to evaluate the therapeutic effects of
antioxidant and anti-inflammatory agents on PD.
Although the symptoms of PD may be relieved by
dopamine (DA) replacement therapy, the efficacy of drug therapy
gradually declines over time (6).
Furthermore, long-term treatment with L-dihydroxyphenylalanine or
DA agonists results in severe adverse effects, which markedly
influence patient quality of life (7,8).
Therapeutic strategies that unequivocally slow or stop the
progression of PD do not currently exist (9). Thus, identifying an agent that
provides effective protection against dopaminergic
neurodegeneration would be a major breakthrough in the treatment of
PD.
Agents that have neurotrophic properties may
potentially promote the survival of neuronal cells and slow the
progression of PD (10). Due to
its neuroprotective and neurotrophic properties,
2′-hydroxy-4′-methoxyacetophenone (paeonol) has been identified as
a potential treatment for PD. Paeonol (Fig. 1A) is a flavonoid derivative that
exerts numerous physiological activities, including antioxidant
(11), anti-inflammatory (12), antidiabetic (13), anticancer, analgesic, and hypnotic
(14) action. An in vitro
study using PC12 cells suggested that paeonol exerts anti-PD
effects, as it prevented oxidative stress and the apoptosis of
dopaminergic neurons (15).
Another in vitro study on microglia demonstrated that
paeonol inhibited the expression of inflammatory mediators, which
suggests that paeonol may have therapeutic properties in
neurodegenerative diseases (16).
However, whether paeonol exerts therapeutic effects against
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid
(MPTP/p)-induced PD in mice remains unknown.
MPTP (Fig. 1B) is a
neurotoxin that induces behavioral, biochemical and
neuropathological changes that are similar to those observed in
patients with idiopathic PD, and it is therefore used to mimic the
pathological process of PD in mice (17,18).
Furthermore, probenecid blocks the excretion of MPTP, which
eventually results in serious neurotoxicity (19). The aim of the present study was to
assess the therapeutic and neurotrophic effects of paeonol in an
MTPT/p-induced mouse model of PD. Previous studies on the
neuroprotective effects of paeonol revealed that doses of 20, 50
and 100 mg/kg body weight significantly improved neuronal damage in
experimental mice (20,21); therefore, paeonol was administered
at a dose of 20 mg/kg in the present study. The effects of paeonol
were determined as follows: i) Evaluating the behavior of mice with
rotarod performance and open-field tests; ii) measuring oxidative
stress levels, specifically the activity levels of superoxide
dismutase (SOD), catalase (CAT) and glutathione (GSH) in the
midbrain; iii) evaluating dopaminergic neurodegeneration in the SN
pars compacta (SNpc) by calculating the number of tyrosine
hydroxylase (TH)-positive cells using immunohistochemical staining;
iv) examining inflammatory mediators, including microglial cells
and interleukin-1β (IL-1β) using immunohistochemical staining; and
v) identifying any neurotrophic properties by measuring the level
of brain-derived neurotrophic factor (BDNF).
Materials and methods
Experimental animals
Adult male C57BL/6 mice (n=40; weight, 18–20 g) were
purchased from the Comparative Medicine Center at Yangzhou
University (Yangzhou, China). The animals were maintained on a 12-h
light/dark cycle at 25±2°C and 60–70% relative humidity with food
and water available ad libitum in the Laboratory Animal
Center of Bengbu Medical College (Bengbu, China). The study was
reviewed and approved by the institutional Animal Ethical Committee
of Bengbu Medical College (Bengbu, China). All surgical procedures
were performed under anesthesia.
Chemicals and reagents
Paeonol (purity, ≥98.0%) and MPTP hydrochloride were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Probenecid was
obtained from Shanghai Jingchun Reagent Co., Ltd. (Shanghai,
China). Rabbit anti-TH (cat. no. ab75875; 1:600), rabbit anti-IL-1β
(cat. no. ab9722; 1:500), rabbit anti-BDNF (cat. no. ab203573;
1:200) and rabbit anti-ionized calcium-binding adapter molecule 1
(Iba1; cat. no. ab15690; 1:200) were purchased from Abcam (Qtar,
Kingdom of Saudi Arabia), and goat-anti-rabbit secondary antibody
(cat. no. K5007; 1:1) was purchased from Dako (Glostrup,
Denmark).
Experimental procedure
The mice were randomly divided into four groups
(n=10 per group): i) Control group, mice were treated with saline;
ii) MPTP/p only group, mice received MPTP via i.p. injection (25
mg/kg in saline) followed by probenecid (250 mg/kg in 0.03 ml
dimethylsulfoxide) once a day for five consecutive days (days 1–5)
to induce the PD mouse model (22); iii) MPTP/p+paeonol group, mice
received paeonol (20 mg/kg in 5% carboxymethylcellulose sodium) by
oral gavage daily for 21 days (days 1–21) and were injected with
MPTP/p (as for the MPTP/p only group); and iv) paeonol only group,
mice received oral paeonol (as for the MPTP/p+ paeonol group) for
21 days. Probenecid was injected 30 min prior to MPTP injection,
and paeonol was administrated orally 1 h prior to MPTP injection.
The rotarod performance and open-field tests were conducted during
the course of MPTP/p administration (days 4 and 5) and during the
final stages of the study (days 20 and 21). Mice were sacrificed by
anesthetic overdose following the behavioral tests, and the brains
were harvested for immunohistochemical and biochemical analyses
(Fig. 1C).
Rotarod performance test
The rotarod performance test is widely used to
assess motor and coordination abilities, particularly bradykinesia,
in mice (23). In the present
study, a rotarod (diameter, 3 cm) at a fixed speed of 30 rpm was
utilized. The duration that each mouse spent on the revolving rod
was measured. The rotating rod automatically stopped at 60 sec. If
the mouse remained on the rod for the duration of the experiment,
the value was recorded as 60 sec. Each mouse was trained in a
separate lane of the rotarod three times a day for two consecutive
days prior to performing the experiment. The rotarod test was
performed on days 4 and 20, and the assessment was repeated three
times for each mouse, with 30-min rest periods between tests. The
mean duration for each mouse was determined and used for comparison
(24).
Open-field test
The open-field test is an efficient technique for
investigating the overall manifestation of motor deficits in animal
models of PD, as it is sensitive to dopaminergic neuronal injury
(25). In the present study, an
open-field composed of a square arena (40×40 cm) and a wall (35-cm
high) was divided into 16 sub-squares (4×4). The mouse was placed
in the center of the arena and the behavior of the mouse was
observed for 5 min; each mouse repeated the assessment three times.
Following each test, the mice were allowed to rest for 30 min and
the open-field was cleaned completely with a 70% ethanol solution.
The number of crossings (a crossing was recorded as each time the
mouse crossed the boundary of a sub-square with at least their two
forepaws), grooming behaviors (rubbing the body with the paws or
mouth and/or rubbing the head with the paws), rearing behaviors
(standing on the hind legs), and the duration of immobility, were
determined. The open-field test was performed on days 5 and 21, and
conducted by an examiner who was blinded to the treatment
groups.
Assessment of SOD, CAT and GSH
activity
The supernatant of the midbrain tissue homogenate
was prepared by centrifugation (Smart R17; Hanil Science Industrial
Co., Ltd., Incheon, South Korea) at 9,184 × g for 10 min at 4°C.
Total SOD activity was measured by spectrophotometry using a Total
Superoxide Dismutase (T-SOD) assay kit (Nanjing Jiancheng
Bioengineering Research Institute, Nanjing, China) according to the
manufacturer's instructions, which contained the experimental steps
and calculation formula. SOD activity is expressed as SOD U/mg
protein, with one SOD unit being the quantity that reduced the
absorbance at 560 nm by 50%, according to a Synergy HT microplate
reader (BioTek Instruments, Inc., Winooski, VT, USA).
CAT activity in the midbrain was measured by
spectrophotometry using a CAT assay kit (Nanjing Jiancheng
Bioengineering Research Institute) according to the manufacturer's
instructions. The absorbance was measured at 405 nm on a microplate
reader, and CAT activity was calculated using the formula provided
by the manufacturer, and expressed as units of CAT activity/mg
protein.
GSH levels were assessed by spectrophotometry using
a reduced GSH assay kit (Nanjing Jiancheng Bioengineering Research
Institute) according to the manufacturer's instructions. The
absorbance was measured at 420 nm and used to calculate the GSH
concentration with the formula provided by the manufacturer, and
the results are expressed as GSH/mg protein.
Immunohistochemical staining
procedure
Mice were anesthetized with 10% chloral hydrate (3
ml/kg; Sigma-Aldrich) following the behavioral test and their
hearts were perfused with saline, followed by 4% paraformaldehyde
for 20 min. The brains were embedded in paraffin and coronally
sectioned through the SNpc region at a thickness of 4 µm.
Sections were fixed on slides, deparaffinized with three changes of
xylene, and then dehydrated through decreasing grades of absolute
alcohol (95, 75 and 50%). Endogenous peroxidases were blocked by
incubating the sections with 3% H2O2 for 10
min. Following antigen retrieval with citrate salt buffer (pH 7.0;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China) in microwave oven for 6 min and rinsing with
phosphate-buffered saline, the sections were incubated at 37°C
overnight with a primary monoclonal antibody (rabbit anti-TH,
rabbit anti-IL-1β, rabbit anti-Iba1 or rabbit anti-BDNF), and then
with a biotinylated goat anti-rabbit secondary antibody for 50 min.
Sections were subsequently washed and developed with a
3,3′-diaminobenzidine staining kit (Dako); the chromogenic reaction
was terminated when brown granules (positive cells) were observed.
Finally, the sections were counterstained with hematoxylin, rinsed
with deionized water, dried and sealed with neutral balsam. The
stained sections were analyzed under an optical microscope (Nikon
Corporation, Tokyo, Japan).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Multiple comparisons analyses were performed using a
one-way analysis of variance followed by Tukey's post-hoc
tests using the Statistical Package for the Social Sciences (SPSS)
software version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of paeonol on the behavior of mice
with MPTP/p-induced PD in the rotarod performance test
The MPTP/p group demonstrated significantly reduced
retention times compared with the control group on days 4 and 20
(P<0.01). The MPTP/p +paeonol group demonstrated significant
improvements in motor performance on day 20 compared with the
MPTP/p only group (P<0.01), although no significant improvement
was observed on day 4. The behavioral performances of the paeonol
only group were similar to those of the control group (Fig. 2).
Effect of paeonol on the behavior of mice
with MPTP/p-induced PD in the open-field test
The results of the open-field test are presented in
Fig. 3. The MPTP/p only group
exhibited significantly reduced locomotor activity (as measured by
lines crossed; Fig. 3A), rearing
(Fig. 3B) and grooming (Fig. 3C), and increased duration of
immobility (Fig. 3D), compared
with the control group on days 5 and 21 (P<0.01). The MPTP/p +
paeonol group demonstrated significantly improved locomotor
activity, rearing and grooming, and decreased immobility time
compared with the MPTP/p only group on day 21 (P<0.01); however,
no significant differences were observed on day 5. Furthermore, no
significant differences were observed between the control and the
paeonol only groups.
 | Figure 3Performances in the open-field test
by control and experimental mice. Parameters measured included (A)
line crossings, (B) rearings, (C) grooming activities, and (D)
immobility time. All types of movement were significantly reduced,
and immobility time was significantly increased by MPTP/p treatment
when compared with the control group on days 5 and 21; however,
this reduction in motor activity was attenuated by treatment with
Pae on day 21. No significant differences were observed between the
control and the Pae only groups. Data are expressed as the mean ±
standard deviation, and statistical analysis was performed using a
one-way analysis of variance. *P<0.01 vs. control;
**P<0.01 vs. MPTP/p only. MPTP,
methyl-4-phenyl-1,2,3,6-tetrahydropyridine; p, probenecid; Pae,
paeonol. |
Paeonol attenuated MPTP/p-induced
oxidative stress
The activity levels of SOD, CAT and GSH in the
midbrain, which reflect the level of oxidative stress, are
presented in Fig. 4. MPTP/p
significantly reduced the levels of SOD, CAT and GSH, and therefore
enhanced the level of oxidative stress in the MPTP/p only group
compared with the control group (P<0.01). Treatment with paeonol
for 21 days significantly restored the activity of SOD, CAT and
GSH, and therefore alleviated oxidative stress in the MPTP/p +
paeonol group compared with the MPTP/p only group (P<0.01). No
significant differences were observed between the control and the
paeonol only groups.
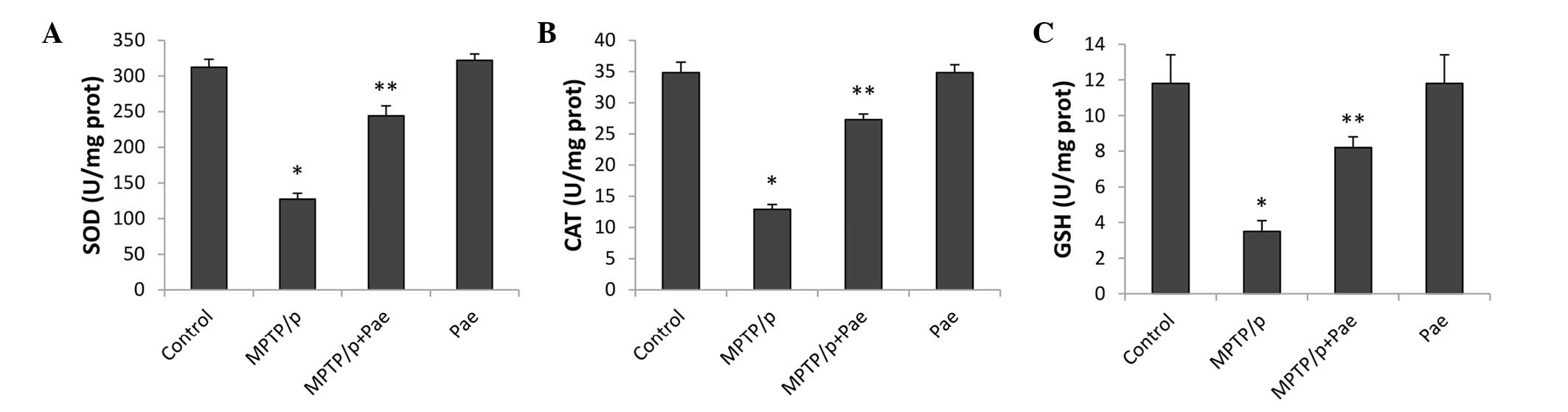 | Figure 4Effects of Pae on the activity levels
of SOD, CAT, and GSH in the midbrain of control and experimental
mice. The levels of (A) SOD, (B) CAT and (C) GSH were significantly
reduced in the MPTP/p only group compared with the control group.
Treatment with Pae significantly restored the activity of SOD, CAT
and GSH in the MPTP/p + Pae group compared with the MPTP/p only
group (P<0.01). No significant differences were observed between
the control and the Pae only groups. Data are expressed as the mean
± standard deviation, and statistical analysis was performed using
a one-way analysis of variance. *P<0.01 vs. control;
**P<0.01 vs. MPTP/p only. SOD, superoxide dismutase;
CAT, catalase; GSH, glutathione; MPTP,
methyl-4-phenyl-1,2,3,6-tet-rahydropyridine; p, probenecid; Pae,
paeonol. |
Paeonol attenuated MPTP/p-induced
neuroinflammation
Microglia were stained with an antibody against Iba1
(a microglial marker). As presented in Fig. 5A, immunohistochemical staining for
Iba1 and IL-1β in the SNpc revealed large brown-colored regions in
the MPTP/p only group. However, the positive cells in the MPTP/p +
paeonol group were not only smaller, but were also lighter in color
than those in the MPTP/p only group. No or little specific staining
of Iba1 or IL-1β was observed in the control and the paeonol only
groups. The numbers of Iba1-positive (51 vs. 11%; P<0.01) and
IL-1β-positive (76 vs. 12%; P<0.01) cells significantly
increased in the MPTP/p only group compared with the control group.
However, the numbers of Iba1-positive (28 vs. 51%; P<0.01) and
IL-1β-positive (39 vs. 76%; P<0.01) cells significantly
decreased in the MPTP/p + paeonol group compared with the MPTP/p
only group. No significant differences were observed between the
paeonol only and control groups.
 | Figure 5Effects of paeonol on Iba1- and
IL-1β-positive cells in the SNpc of control and experimental mice.
(A) Immunohistochemical staining of Iba1 and IL-1β in the SNpc
(magnification, ×200) reveals that the administration of MPTP/p
increased the expression levels of Iba1 and IL-1β. This effect was
alleviated by treatment with paeonol. (B) Quantification of Iba1
and IL-1β staining was performed by counting the number of Iba1-
and IL-1β-positive cells in the SNpc. The mean value was expressed
as the ratio of relative positive cells to the total number of
cells. Data are expressed as the mean ± standard deviation.
*P<0.01 vs. control; **P<0.01 vs.
MPTP/p only. Iba1, ionized calcium-binding adapter molecule 1;
IL-1β, interleukin-1β; SNpc, substantia nigra pars compacta; MPTP,
methyl-4-phenyl-1,2,3,6-tetrahydropyridine; p, probenecid; Pae,
paeonol. |
Paeonol attenuated the MPTP/P-induced
loss of dopaminergic neurons
The results of the immunohistochemical examinations
revealed that the majority of the TH-positive cells (representing
dopaminergic neurons) in the SNpc were fusiform or had an irregular
triangular shape, and dense neurons with dark-stained cytoplasms
were observed in the control and paeonol only groups (Fig. 6A). MPTP administration induced
clear fragmentation of the dopaminergic neurons, causing the
cellular outline to be ambiguous and the cytoplasmic staining to be
faint (Fig. 6B); thus, only 19% of
the TH-positive cells were observed in the SN of the MPTP/p only
group when compared with 100% of the TH-positive cells in the
control group (P<0.01; Fig.
6B). However, the MPTP/p + paeonol group demonstrated
protection of TH-positive cells when compared with the MPTP/p only
group (51 vs. 19%; P<0.01). No significant difference was
observed between the paeonol only and the control groups.
 | Figure 6Effects of paeonol on the expression
level of TH in the SNpc of control and experimental mice. (A)
Immunohistochemical staining of TH (magnification, ×40) reveals
that the administration of MPTP/p reduced the expression of TH,
while co-administration of paeonol and MPTP/p attenuated the loss
of TH. (B) Quantification of TH staining was performed by counting
the number of TH-positive cells in the SNpc. The mean value for
TH-positive cells was expressed as a percentage of that in the
matched control mice. Data are expressed as the mean ± standard
deviation. *P<0.01 vs. control;
**P<0.01 vs. MPTP/p only. TH, tyrosine hydroxylase;
SNpc, substantia nigra pars compacta; MPTP,
methyl-4-phenyl-1,2,3,6-tetrahydropyridine; p, probenecid; Pae,
paeonol. |
Paeonol attenuated the MPTP/p-induced
loss of BDNF
MPTP/p administration significantly reduced the
level of BDNF in the SN of the MPTP/p only group compared with the
level in the control group (19 vs. 49%; P<0.01; Fig. 7). Treatment with paeonol for 21
days significantly restored the level of BDNF in the SN of mice
with MPTP/p-induced PD compared with the level in the MPTP/p only
group (36 vs. 19%; P<0.01). The level of BDNF in the paeonol
only group was slightly increased compared with the level in the
control group; however, the difference was not statistically
significant.
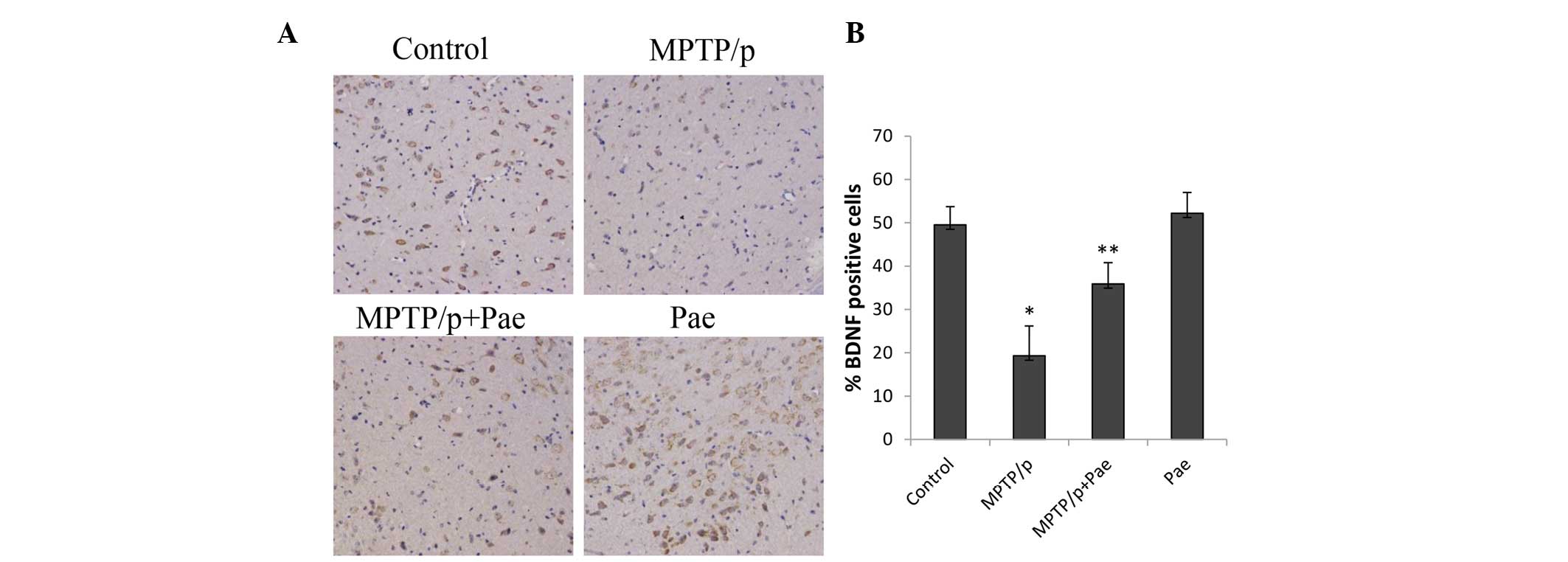 | Figure 7Effects of paeonol on BDNF-positive
cells in the SNpc of control and experimental mice. (A)
Immunohistochemical staining of BDNF (magnification, ×200) in the
SNpc reveals that the administration of MPTP/p reduced the BDNF
expression, and co-administration of paeonol and MPTP/p attenuated
the loss of BDNF. (B) Quantification of BDNF staining was performed
by counting the number of BDNF-positive cells in the SNpc. The mean
value for BDNF-positive cells was expressed as the ratio of
relative positive cells to the number of total cells. Data are
expressed as the mean ± standard deviation. *P<0.01
vs. control; **P<0.01 vs. MPTP/p only. BDNF,
brain-derived neurotrophic factor; SNpc, substantia nigra pars
compacta; MPTP, methyl-4-phenyl-1,2,3,6-tetrahydropyri-dine; p,
probenecid; Pae, paeonol. |
Discussion
The discovery of neuroprotective compounds that slow
or stop the progression of neurodegeneration is essential for
treating various neurodegenerative diseases, including PD (26,27).
The results of the present study demonstrate for the first time, to
the best of our knowledge, that long-term paeonol treatment
exhibits therapeutic effects in mice with MPTP/p-induced PD. Mice
experienced improvements in motor dysfunction, suppression of
oxidative stress and the inflammatory cascade, and protection of
TH-positive neurons. In addition, it was observed that BDNF levels
increased following paeonol treatment, suggesting that paeonol
exerts neurotrophic effects on PD.
In the present study, the mouse model of PD was
induced by co-administration of MPTP and probenecid, which was more
suitable for investigating the therapeutic effects of long-term
drug treatment than mouse models of PD induced with MPTP alone
(28). MPTP effectively crosses
the blood-brain barrier and accumulated MPTP is metabolized into
1-methyl-4-phenylpyridinium (MPP+) by monoamine oxidase-B. MPP+,
which inhibits complex I of the mitochondrial respiratory chain,
enters dopaminergic neurons via the dopamine transporter,
ultimately inducing their degeneration (29,30).
There is increasing evidence that the motor
dysfunctions of MPTP/p-treated mice may be observed using
behavioral tests, including the rotarod performance and open-field
tests (31). In the present study,
the performances of mice with PD significantly improved on the
rotarod performance and open-field tests following long-term
treatment with paeonol, although no apparent improvement was
observed on day 4. This is consistent with the results of previous
studies (28,32). The results of the behavioral tests
suggested that long-term paeonol treatment has a therapeutic effect
on mice with MPTP/p-induced PD.
Oxidative stress is widely considered to be
important in the development of PD (33,34).
Extensive post-mortem studies have indicated that oxidative stress
induces the downregulation of antioxidant protective systems,
including SOD, GSH and CAT, and damages lipids, proteins and DNA,
ultimately resulting in damage to dopaminergic neurons in the SNpc
(34–36). SOD, CAT and GSH effectively remove
oxygen free radicals and lipid peroxides, which may be used to
measure the level of oxidative stress (37). In the present study, the activity
levels of SOD, CAT and GSH were significantly decreased in the
MPTP/p only group, suggesting that oxidative stress is involved in
the pathogenesis of PD. Following long-term paeonol treatment, the
levels of SOD, CAT, and GSH significantly improved in mice with
MPTP/p-induced PD, which is consistent with the findings of a
previous study (38). The results
of the present study suggest that paeonol exerts a therapeutic
effect on MPTP/p-induced oxidative stress, perhaps due to its
antioxidant activity and ability to reduce the toxicity caused by
oxidative stress.
Excessive microglial activation has been observed in
the SN of human post-mortem tissue from PD patients and animal
models, suggesting that neuroinflammation and activated microglia
are crucial in the pathogenesis of PD (39–41).
Microglia are involved in immune defense and surveillance, and
activate a variety of proinflammatory cytokines, including IL-1β,
which may directly induce apoptosis in dopaminergic neurons
(42,43). Studies have demonstrated that the
overactivation of microglia and the overproduction of
proinflammatory cytokines may enhance oxidative stress, eventually
leading to neuronal death (44,45).
In addition, a previous study has revealed that paeonol may be a
potential neuroprotective agent, possibly through inhibiting
microglia-mediated inflammation and oxidative stress-induced
neuronal damage (46). In the
present study, the levels of microglia and IL-1β were significantly
increased in the SNpc of mice in the MPTP/p only group. However,
long-term paeonol treatment significantly decreased these levels.
These results suggest that paeonol was therapeutic, perhaps by
downregulating the inflammatory cascade.
TH is the limiting enzyme of dopamine synthesis, and
TH immunohistochemical analysis measures the number and function of
dopaminergic neurons and fibers in the SN, as reductions in
dopaminergic neurons may be the primary reason for the onset of PD
(47,48). In the present study,
immunohistochemical analysis of TH revealed a significant loss of
dopaminergic neurons in the SNpc of mice in the MPTP/p only group,
perhaps due to MPTP-induced oxidative stress and inflammation
(49). Long-term paeonol treatment
appeared to protect dopaminergic neurons and fibers in mice with
MPTP/p-induced PD, which suggests that its therapeutic effects may
be associated with its anti-inflammatory and antioxidant
effects.
BDNF is a member of a family of neurotrophic factors
that has attracted widespread attention, as it exhibits strong
nutritional and protective effects, particularly in DA neurons
(50,51). Studies have demonstrated that the
level of BDNF significantly decreases in patients with PD (52,53),
which is consistent with the results of the present study. These
findings suggest that novel treatment strategies for PD should aim
to increase the expression levels of BDNF. In the present study, it
was observed that treatment with paeonol enhanced BDNF expression
levels and the number of TH-positive cells in the SN of mice with
MPTP/p-induced PD, while no apparent improvement was observed in
the paeonol only group. These findings suggest that the therapeutic
effects of paeonol may be associated with its potential
neurotrophic effects on dopaminergic neurons.
In conclusion, the results of the present study
demonstrate that paeonol exerts therapeutic effects against
MPTP/p-induced PD in mice, as revealed by improvements in
behavioral tests and enhanced TH expression compared with mice that
did not receive paeonol. In addition, the therapeutic effects may
be associated with the ability of paeonol to reduce oxidative
stress, increase antioxidant defenses, and inhibit the
overactivation of microglia along with the release of
proinflammatory factors. Furthermore, BDNF levels were improved in
mice with MPTP/p-induced PD following long-term treatment with
paeonol, indicating that paeonol has neurotrophic effects on
dopaminergic neurons. Therefore, the results of the present study
suggest that paeonol has potential as a treatment for PD; however,
the dose of paeonol (20 mg/kg) administered in the present study
may not be directly extrapolated to humans. Future studies are
required to investigate the dose-dependent effect on MPTP/p-induced
PD mice and determine an optimal dose. In addition, further studies
are necessary to establish the exact mechanisms underlying the
effects of paeonol.
Acknowledgments
The present study was supported by the Fund of
Education Department of Anhui (grant no. KJ2014A163) and as a
Postgraduate Research and Innovation Project of Bengbu Medical
College (grant no. Byycx1406).
Abbreviations:
|
BDNF
|
brain-derived neurotrophic factor
|
|
CAT
|
catalase
|
|
DA
|
dopamine
|
|
GSH
|
glutathione
|
|
Iba1
|
ionized calcium-binding adapter
molecule 1
|
|
IL-1β
|
interleukin-1β
|
|
MPTP
|
methyl-4-phenyl-1,2,3,6-tetrahydropyridine
|
|
p
|
probenecid
|
|
PD
|
Parkinson's disease
|
|
SOD
|
superoxide dismutase
|
|
SNpc
|
substantia nigra pars compacta
|
|
TH
|
tyrosine hydroxylase
|
References
|
1
|
Meissner WG, Frasier M, Gasser T, Goetz
CG, Lozano A, Piccini P, Obeso JA, Rascol O, Schapira A, Voon V, et
al: Priorities in Parkinson's disease research. Nat Rev Drug
Discov. 10:377–393. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kowal SL, Dall TM, Chakrabarti R, Storm MV
and Jain A: The current and projected economic burden of
Parkinson's disease in the United States. Mov Disord. 28:311–318.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guillot TS, Richardson JR, Wang MZ, Li YJ,
Taylor TN, Ciliax BJ, Zachrisson O, Mercer A and Miller GW: PACAP38
increases vesicular monoamine transporter 2 (VMAT2) expression and
attenuates methamphetamine toxicity. Neuropeptides. 42:423–434.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klegeris A and McGeer PL: R-(−)-Deprenyl
inhibits monocytic THP-1 cell neurotoxicity independently of
monoamine oxidase inhibition. Exp Neurol. 166:458–464. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartmann A: Postmortem studies in
Parkinson's disease. Dialogues Clin Neurosci. 6:281–293.
2004.PubMed/NCBI
|
|
6
|
Birkmayer W and Hornykiewicz O: The
L-3,4-dioxyphenylalanine (DOPA)-effect in Parkinson-akinesia. Wien
Klin Wochenschr. 73:787–788. 1961.In German. PubMed/NCBI
|
|
7
|
Morin N, Morissette M, Grégoire L, Rajput
A, Rajput AH and Di Paolo T: Contribution of brain serotonin
subtype 1B receptors in levodopa-induced motor complications.
Neuropharmacology. 99:356–368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Winner B, Desplats P, Hagl C, Klucken J,
Aigner R, Ploetz S, Laemke J, Karl A, Aigner L, Masliah E, et al:
Dopamine receptor activation promotes adult neurogenesis in an
acute Parkinson model. Exp Neurol. 219:543–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abdel-Salam OM: Drugs used to treat
Parkinson's disease, present status and future directions. CNS
Neurol Disord Drug Targets. 7:321–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan W, Xue-bin C, Tian Z, Xiao-wu C,
Pei-pei H, Zhi-bin C and Bei-sha T: Effects of simvastatin on the
expression of inducible nitric oxide synthase and brain-derived
neurotrophic factor in a lipopolysaccharide-induced rat model of
Parkinson disease. Int J Neurosci. 126:278–286. 2016. View Article : Google Scholar
|
|
11
|
Chen B, Ning M and Yang G: Effect of
paeonol on antioxidant and immune regulatory activity in
hepatocellular carcinoma rats. Molecules. 17:4672–4683. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu PK, Wu CL, Tsai TH and Hsieh CL:
Anti-inflammatory and anticoagulative effects of paeonol on
LPS-induced acute lung injury in rats. Evid Based Complement
Alternat Med. 2012:8375132012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lau CH, Chan CM, Chan YW, Lau KM, Lau TW,
Lam FC, Law WT, Che CT, Leung PC, Fung KP, et al: Pharmacological
investigations of the anti-diabetic effect of Cortex Moutan and its
active component paeonol. Phytomedicine. 14:778–784. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li M, Tan SY and Wang XF: Paeonol exerts
an anticancer effect on human colorectal cancer cells through
inhibition of PGE2 synthesis and COX-2 expression. Oncol
Rep. 32:2845–2853. 2014.PubMed/NCBI
|
|
15
|
Wang H, Geng ZM, Hu ZW, Wang SY and Zhao
B: Neuroprotective effects of paeonol in a cell model of Parkinson
disease. Zhejiang Da Xue Xue Bao Yi Xue Ban. 44:30–36. 2015.In
Chinese. PubMed/NCBI
|
|
16
|
Lin C, Lin HY, Chen JH, Tseng WP, Ko PY,
Liu YS, Yeh WL and Lu DY: Effects of paeonol on
anti-neuroinflammatory responses in microglial cells. Int J Mol
Sci. 16:8844–8860. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mounsey RB, Mustafa S, Robinson L, Ross
RA, Riedel G, Pertwee RG and Teismann P: Increasing levels of the
endocannabinoid 2-AG is neuroprotective in the
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of
Parkinson's disease. Exp Neurol. 273:36–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He XJ and Nakayama H: Transiently impaired
neurogenesis in MPTP mouse model of Parkinson's disease.
Neurotoxicology. 50:46–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lau YS, Patki G, Das-Panja K, Le WD and
Ahmad SO: Neuroprotective effects and mechanisms of exercise in a
chronic mouse model of Parkinson's disease with moderate
neurodegeneration. Eur J Neurosci. 33:1264–1274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsieh CL, Cheng CY, Tsai TH, Lin IH, Liu
CH, Chiang SY, Lin JG, Lao CJ and Tang NY: Paeonol reduced cerebral
infarction involving the superoxide anion and microglia activation
in ischemia-reperfusion injured rats. J Ethnopharmacol.
106:208–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhong SZ, Ge QH, Qu R, Li Q and Ma SP:
Paeonol attenuates neurotoxicity and ameliorates cognitive
impairment induced by d-galactose in ICR mice. J Neurol Sci.
277:58–64. 2009. View Article : Google Scholar
|
|
22
|
Petroske E, Meredith GE, Callen S,
Totterdell S and Lau YS: Mouse model of Parkinsonism: A comparison
between subacute MPTP and chronic MPTP/probenecid treatment.
Neuroscience. 106:589–601. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tarantini S, Hertelendy P, Tucsek Z,
Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner
R, Deak F, et al: Pharmacologically-induced neurovascular
uncoupling is associated with cognitive impairment in mice. J Cereb
Blood Flow Metab. 35:1871–1881. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carter RJ, Morton J and Dunnett SB: Motor
coordination and balance in rodents. Curr Protoc Neurosci Unit
8.12. 2001. View Article : Google Scholar
|
|
25
|
Sedelis M, Hofele K, Auburger GW, Morgan
S, Huston JP and Schwarting RK: MPTP susceptibility in the mouse:
Behavioral, neurochemical, and histological analysis of gender and
strain differences. Behav Genet. 30:171–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aguirre-Vidal Y, Montes S, Tristan-López
L, Anaya-Ramos L, Teiber J, Ríos C, Baron-Flores V and
Monroy-Noyola A: The neuroprotective effect of lovastatin on
MPP(+)-induced neurotoxicity is not mediated by PON2.
Neurotoxicology. 48:166–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palencia G, Garcia E, Osorio-Rico L,
Trejo-Solís C, Escamilla-Ramírez A and Sotelo J: Neuroprotective
effect of thalidomide on MPTP-induced toxicity. Neurotoxicology.
47:82–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patil SP, Jain PD, Ghumatkar PJ, Tambe R
and Sathaye S: Neuroprotective effect of metformin in MPTP-induced
Parkinson's disease in mice. Neuroscience. 277:747–754. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jackson-Lewis V, Blesa J and Przedborski
S: Animal models of Parkinson's disease. Parkinsonism Relat Disord.
18(Suppl 1): S183–S185. 2012. View Article : Google Scholar
|
|
30
|
Zang LY and Misra HP: Generation of
reactive oxygen species during the monoamine oxidase-catalyzed
oxidation of the neurotoxicant,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Biol Chem.
268:16504–16512. 1993.PubMed/NCBI
|
|
31
|
Patil SP, Jain PD, Sancheti JS, Ghumatkar
PJ, Tambe R and Sathaye S: Neuroprotective and neurotrophic effects
of Apigenin and Luteolin in MPTP induced parkinsonism in mice.
Neuropharmacology. 86:192–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang XL, Xing GH, Hong B, Li XM, Zou Y,
Zhang XJ and Dong MX: Gastrodin prevents motor deficits and
oxidative stress in the MPTP mouse model of Parkinson's disease:
Involvement of ERK1/2-Nrf2 signaling pathway. Life Sci. 114:77–85.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gaki GS and Papavassiliou AG: Oxidative
stress-induced signaling pathways implicated in the pathogenesis of
Parkinson's disease. Neuromolecular Med. 16:217–230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang J, Perry G, Smith MA, Robertson D,
Olson SJ, Graham DG and Montine TJ: Parkinson's disease is
associated with oxidative damage to cytoplasmic DNA and RNA in
substantia nigra neurons. Am J Pathol. 154:1423–1429. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jenner P and Olanow CW: Oxidative stress
and the pathogenesis of Parkinson's disease. Neurology. 47(6 Suppl
3): S161–S170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maj MC, Tkachyova I, Patel P, Addis JB,
Mackay N, Levandovskiy V, Lee J, Lang AE, Cameron JM and Robinson
BH: Oxidative stress alters the regulatory control of p66Shc and
Akt in PINK1 deficient cells. Biochem Biophys Res Commun.
399:331–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weydert CJ and Cullen JJ: Measurement of
superoxide dismutase, catalase and glutathione peroxidase in
cultured cells and tissue. Nat Protoc. 5:51–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Selvakumar GP, Janakiraman U, Essa MM,
Justin Thenmozhi A and Manivasagam T: Escin attenuates behavioral
impairments, oxidative stress and inflammation in a chronic
MPTP/probenecid mouse model of Parkinson's disease. Brain Res.
1585:23–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tansey MG and Goldberg MS:
Neuroinflammation in Parkinson's disease: Its role in neuronal
death and implications for therapeutic intervention. Neurobiol Dis.
37:510–518. 2010. View Article : Google Scholar
|
|
40
|
Hirsch EC, Hunot S, Damier P and Faucheux
B: Glial cells and inflammation in Parkinson's disease: A role in
neurodegeneration? Ann Neurol. 44(3 Suppl 1): S115–S120. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hirsch EC and Hunot S: Neuroinflammation
in Parkinson's disease: A target for neuroprotection? Lancet
Neurol. 8:382–397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rossol M, Heine H, Meusch U, Quandt D,
Klein C, Sweet MJ and Hauschildt S: LPS-induced cytokine production
in human monocytes and macrophages. Crit Rev Immunol. 31:379–446.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sarkar S, Chigurupati S, Raymick J, Mann
D, Bowyer JF, Schmitt T, Beger RD, Hanig JP, Schmued LC and Paule
MG: Neuroprotective effect of the chemical chaperone, trehalose in
a chronic MPTP-induced Parkinson's disease mouse model.
Neurotoxicology. 44:250–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Whitton PS: Inflammation as a causative
factor in the aetiology of Parkinson's disease. Br J Pharmacol.
150:963–976. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Niranjan R: The role of inflammatory and
oxidative stress mechanisms in the pathogenesis of Parkinson's
disease: Focus on astrocytes. Mol Neurobiol. 49:28–38. 2014.
View Article : Google Scholar
|
|
46
|
Tseng YT, Hsu YY, Shih YT and Lo YC:
Paeonol attenuates microglia-mediated inflammation and oxidative
stress-induced neurotoxicity in rat primary microglia and cortical
neurons. Shock. 37:312–318. 2012. View Article : Google Scholar
|
|
47
|
Daubner SC, Le T and Wang S: Tyrosine
hydroxylase and regulation of dopamine synthesis. Arch Biochem
Biophys. 508:1–12. 2011. View Article : Google Scholar :
|
|
48
|
Fukuda T, Takahashi J and Tanaka J:
Tyrosine hydroxylase-immunoreactive neurons are decreased in number
in the cerebral cortex of Parkinson's disease. Neuropathology.
19:10–13. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Episcopo FL, Tirolo C, Testa N, Caniglia
S, Morale MC and Marchetti B: Reactive astrocytes are key players
in nigrostriatal dopaminergic neurorepair in the MPTP mouse model
of Parkinson's disease: Focus on endogenous neurorestoration. Curr
Aging Sci. 6:45–55. 2013. View Article : Google Scholar
|
|
50
|
Weissmiller AM and Wu C: Current advances
in using neurotrophic factors to treat neurodegenerative disorders.
Transl Neurodegener. 1:142012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dai L, Wang D, Meng H, Zhang K, Fu L, Wu Y
and Bai Y: Association between the BDNF G196A and C270T
polymorphisms and Parkinson's disease: A meta-analysis. Int J
Neurosci. 123:675–683. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Scalzo P, Kummer A, Bretas TL, Cardoso F
and Teixeira AL: Serum levels of brain-derived neurotrophic factor
correlate with motor impairment in Parkinson's disease. J Neurol.
257:540–545. 2010. View Article : Google Scholar
|
|
53
|
Salehi Z and Mashayekhi F: Brain-derived
neurotrophic factor concentrations in the cerebrospinal fluid of
patients with Parkinson's disease. J Clin Neurosci. 16:90–93. 2009.
View Article : Google Scholar
|