Introduction
Lung cancer is a common type of malignancy, with the
majority of individuals presenting with advanced disease (1). However, the tumor treatment is a
complex process. In the past few decades, the survival of patients
with lung cancer has improved as surgical techniques have become
more aggressive to achieve optimal cytoreduction and platinum-based
treatment has been introduced (2).
Although 80% of cancers initially respond to chemotherapy, the
majority ultimately reoccur with <15% of patients remaining in
remission (3). Therefore, there is
an urgent requirement for novel drugs for the prevention and
treatment of lung cancer (4).
Thus, research has focused on identifying novel effective antitumor
drugs and determining their mechanisms of action (5). Previous studies have indicated that
the consumption of Evodia rutaecarpa may reduce the
incidence of cancer. Evodiamine is active ingredient that exerts
antitumor activity. Evodiamine, as a naturally occurring alkaloid,
is widely present in E. rutaecarpa (6–8).
Despite its fairly high antitumor activity, the effects of
evodiamine on lung cancer have not been fully elucidated to the
best of our knowledge. The inhibitory effect of evodiamine and its
mechanism of action on the A549 human lung cancer cell line was
investigated by observing the effects of evodiamine on cell
proliferation, apoptosis, the cell cycle, reactive oxygen species
(ROS) production and the relevant signal transduction pathways.
Materials and methods
Cell culture
A549 cells (American Type Culture Collection,
Manassas, VA, USA) were maintained in 90% Dulbecco's modified
Eagle's medium (Corning, Manassas, VA, USA) containing 10% fetal
bovine serum and cultured in an incubator at 37°C and 5%
CO2 with saturated humidity conditions. The cells were
digested with 0.25% trypsin-EDTA for passaging. All experiments
used cells in the logarithmic growth phase.
Determination of the effect of evodiamine
on tumor cell proliferation with an MTS assay
Cells were seeded at 5×103 cells/well in
96-well plates. Evodiamine (Sigma-Aldrich, St. Louis, MO, USA) was
added to obtain final concentrations of 0, 2.5, 5, 10, 25, 50 and
100 µM. The plates were then placed in an incubator for
routine culture under 37°C and 5% CO2 conditions.
Samples were collected at 72 h to determine the optical density
(OD). Inhibition rate = (1 − OD of experimental group / OD of
control group) × 100. The fitting curve was plotted using
logarithmic concentration of evodiamine as abscissa and inhibition
rate as ordinate. The compound concentration corresponding to 50%
inhibition rate was the IC50. Phosphate-buffered saline
(PBS) was used as the negative control.
Determination of apoptosis, cell cycle
and ROS expression with flow cytometry
Tumor cells in the logarithmic growth phase were
seeded in 6-well plates at a density of 5×105 and
cultured for 24 h. PBS or 1 or 2.5 µM evodiamine were
separately added to the test wells and cells were cultured for 24
h. The cells were harvested and incubated with 10 µl Annexin
V-fluorescein isothiocyanate/propidium iodide (PI) away from light
for 15 min prior to flow cytometry using a FACSAria flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA) in order to determine the
effect of evodiamine on the apoptosis of tumor cells. Next, PBS or
1 and 2.5 µM evodiamine were added to the test wells and
cells were cultured for 24 h. The cells were harvested and
incubated with 25 µl PI away from light for 15 min. Flow
cytometry was used to determine the effect of evodiamine on the
cell cycle of tumor cells. PBS or 1 or 2.5 µM evodiamine
were added to the test wells to continue the culture for 24 h. The
cells were harvested and incubated with 20 µl
dihydroethidium away from light for 15 min prior to the use of flow
cytometry to determine the effect of evodiamine on ROS expression
in tumor cells. N-acetyl-L-cysteine (NAC; 20 mM; Sigma-Aldrich),
which protects against oxidation, was added to determine whether
evodiamine affects oxidative stress. DIVA 6.1.3 software (BD
Biosciences) was used to analyze flow cytometry results.
Determination of caspase-3/8 activity in
tumor cells with a microplate reader
Tumor cells in logarithmic growth phase were seeded
in 6-well plates at a density of 5×105 and cultured for
24 h. PBS or 1 or 2.5 µM evodiamine were separately added to
the test wells and were cultured for 24 h. The cells were harvested
and lysed to extract the proteins by protein extraction kit
(Beyotime Institute of Biotechnology, Shanghai, China). The protein
content in cell lysate was determined with a bicinchoninic acid
assay. Then, caspase-3 and caspase-8 activity test reagent was
added in accordance with the kit (Promega Corporation, Madison, WI,
USA) instructions to incubate at room temperature for 30 min. The
caspase-3/8 activity in these cells was determined with a
microplate reader by luminescent signal.
Determination of mRNA expression of
proliferation-associated genes in tumor cells with reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Tumor cells (5×105) in logarithmic growth
phase were seeded in 6-well plates and cultured for 24 h. PBS or 1
or 2.5 µM evodiamine were separately added to the test wells
and cultured for 24 h. Once the total RNA was extracted with TRIzol
(Invitrogen, Thermo Fisher Scientific, Inc.) from each group, the
real-time PCR kit [Takara Biotechnology (Dalian) Co., Ltd., Dalian,
China] was used for reverse transcription to obtain the cDNA (2
µl). The cDNA was then amplified by specific primers using
the ABI7500 system (Applied Biosystems, Waltham, MA, USA). The
primer pairs were as follows: Survivin, forward (F)
5′-CGAGGCTGGCTTCATCCACT-3′ and reverse (R)
5′-ACGGCGCACTTTCTTCGCA-3′; Bcl-2, F 5′-GGCTGGGATGCCTTTGTG-3′ and R
5′-GCCAGGAGAAATCAAACAGAGG-3′; cyclin B1, F
5′-TCTGGATAATGGTGAATGGACA-3′ and R 5′-CGATGTGGCATACTTGTTCTTG-3′;
Sonic hedgehog (SHH), F 5′-GTGGCCGAGAAGACCCTA-3′ and R
5′-CAAAGCGTTCAACTTGTCCTTA-3′; GLI family zinc finger 1 (GLI1), F
5′-AGCGTGAGCCTGAATCTGTG-3′ and R 5′-CAGCATGTACTGGGCTTTGAA-3′;
β-actin, F 5′-CTCGCTGTCCACCTTCCA-3′ and R
5′-GCTGTCACCTTCACCGTTC-3′. The PCR conditions were 95°C for 5 min,
and then 40 cycles at 95°C for 35 sec, 60°C for 35 sec, and 72°C
for 65 sec, and extension step at 72°C for 10 min. The
2−ΔΔCq method was used for relative quantifications
(9).
Determination of protein expression of
proliferation-associated genes in tumor cells using western
blotting
Tumor cells in logarithmic growth phase
(5×105) were seeded in 6-well plates and cultured for 24
h. PBS or 1 or 2.5 µM evodiamine were separately added to
the test wells and cultured for 24 h. The cells were harvested and
lysed to extract the proteins by protein extraction kit (Beyotime
Institute of Biotechnology). Next, 100 µl total protein was
applied in 12% sodium dodecyl sulfate polyacrylamide gel
electrophoresis for separation by electrophoresis. The separated
proteins were transferred onto a polyvinylidene difluoride
membrane. The membrane was blocked in blocking solution containing
5% skimmed milk powder for 2 h. The membranes were then incubated
with monoclonal antibodies against Survivin (cat. no. sc-8807;
1:1,500); Bcl-2 (cat. no. sc-492; 1:1,500); cyclin B1, (cat. no.
sc-752; 1:1,500); AKT (cat. no. sc-5298; 1:1,000); p-AKT (cat. no.
sc-135650; 1:1,000); nuclear factor (NF)-κB p65 (cat. no. sc-372;
1:1,000); p-p65, (cat. no. sc-293111; 1:1,000); SHH (cat. no.
sc-373779; 1:1,000) and GLI1 (cat. no. sc-20687; 1:1,000) (all from
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight.
The membrane was washed 3 times with tris-buffered saline and
Tween-20 (TBST) for 15 min each time. Horseradish
peroxidase-labeled goat anti-rabbit secondary antibody (cat. no.
sc-516087; 1:4,000) was added and incubated at room temperature for
1.5 h. The membrane was washed again 3 times with TBST for 15 min
per wash. Finally, enhanced chemiluminescence developing agent was
used for coloration and fixation.
Reduced glutathione (GSH) assay
Tumor cells (5×105) in logarithmic growth
phase were seeded in 6-well plates and cultured for 24 h. PBS or 1
or 2.5 µM evodiamine were separately added to the test wells
and cultured for 24 h. Reduced GSH in the cells was quantified
using a commercial GSH determination kit (Jiancheng Institute of
Biotechnology, Nanjing, China), following the manufacturer's
protocol. The result was detected by spectrophotometry at 420
nm.
Telomerase activity assay
Tumor cells (5×105) in logarithmic growth
phase were seeded in 6-well plates and cultured for 24 h. PBS or 1
or 2.5 µM evodiamine were separately added to the test wells
and cells were cultured for 24 h. Cell pellets were washed twice
with cold PBS, and the cells were then lysed with an appropriate
volume of the provided lysis buffer from the protein extraction kit
(Beyotime Institute of Biotechnology). After 30 min of incubation
on ice, the suspension was centrifuged for 30 min at 4°C and 12,000
× g. The supernatant (2 µl) was then used to to assess the
telomerase activity. The telomerase activity was determined by SYBR
Green real-time PCR.
Statistical analysis
The data are presented as the mean ± standard
deviation and analyzed with SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA). Data were compared with one-way analysis of
variance followed by the Bonferroni test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Evodiamine significantly inhibits in
vitro proliferation of A549 cells
An MTS assay was used to investigate the effect of
evodiamine treatment for 72 h on in vitro proliferation of
A549 cells. The results indicated that evodiamine significantly
inhibited the in vitro proliferation of A549 cells (Fig. 1).
Evodiamine induces apoptosis and ROS
expression, and arrests the A549 cell cycle
When treated with 1 and 2.5 µM evodiamine,
the distribution of cells in different phases of the cell cycle
changed significantly compared with control, with the majority of
cells arrested at the G2/M phase (Fig.
2A). Evodiamine at 1 and 2.5 µM was used to determine
its effect on apoptosis. Incubation with 1 and 2.5 µM
evodiamine for 24 h significantly induced apoptosis (P<0.01;
Fig. 2B). Following the incubation
of the tumor cells with 1 and 2.5 µM evodiamine for 24 h,
the ROS level was 217.3% and 354.2% compared with the control group
(P<0.05; Fig. 2C). However, no
significant difference was identified in ROS production in the NAC
group (data not shown); therefore, evodiamine-induced tumor cell
apoptosis may be associated with oxidative injury in these
cells.
Evodiamine regulates
proliferation-associated gene expression in A549 cells
To further investigate the inhibitory effect of
evodiamine and its mechanism of action on tumor cell proliferation,
the changes in proliferation-associated gene expression were
examined. The results indicated that the protein and mRNA
expression levels of survivin, Bcl-2, cyclin B1, p-Src, SHH and
GLI1 proteins were downregulated following incubated with 1 and 2.5
µM evodiamine for 24 h (Fig.
3).
Evodiamine reduces telomerase activity in
A549 cells
In order to evaluate the role of evodiamine in the
regulation of telomerase activity in A549 cells, the cells were
cultured with 1 µM evodiamine for increasing time periods.
Addition of evodiamine to A549 cells substantially reduced the
telomerase activity (P<0.01; Fig.
4).
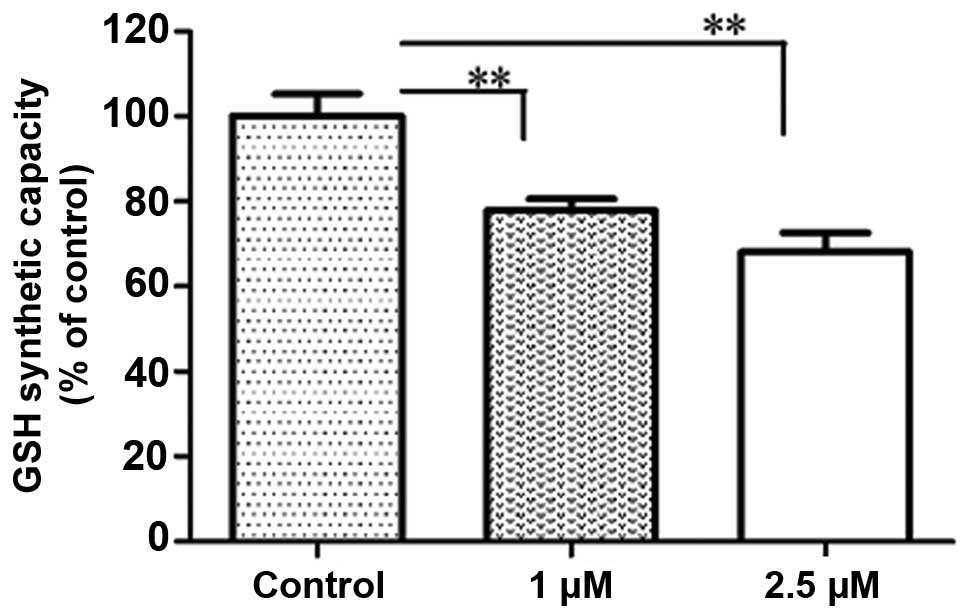
Evodiamine affects the cellular GSH
content in A549 cells
GSH is important for the antioxidant defense of
cells. Under oxidative stress conditions, ROS are reduced by GSH by
the concomitant formation of glutathione disulfide (GSSG). The
intracellular concentration of GSH for detoxifying ROS
significantly decreased following treatment with 1 and 2.5
µM evodiamine for 24 h (P<0.01; Fig. 5). No significant difference in ROS
production with 1 and 2.5 µM evodiamine by NAC treatment
when compared with the control.
Effect of evodiamine on cell
proliferation through SHH/GLI1 signaling in A549 cells
SHH is a member of the family of hedgehog proteins.
It is critical for oligodendrocyte development, including
induction, survival, proliferation and migration of
oligodendrocytes and control of axon growth (10,11).
SHH binds to the transmembrane receptor protein, patched, to
activate the transmembrane receptor, smoothened, and induces a
complex series of intracellular reactions that target the GLI
family of transcription factors. GLI1 is the principal effector of
SHH signaling in neural progenitor cells (12,13).
To assess the effect of evodiamine on the SHH/GLI1 signaling
pathway, A549 cells were treated with 1 and 2.5 µM
evodiamine. The protein and mRNA expression levels of GLI1 and SHH
were reduced following evodiamine treatment (Fig. 6A and B). These findings suggest
that evodiamine decreases the cellular levels of SHH and GLI1.
Evodiamine modulates SHH/GLI1 levels
through inhibition of the AKT/NF-κB pathway
Recent studies have demonstrated that
phosphoinositide 3-kinase (PI3K)/AKT induction of cell survival
signals is mediated, in part, through the activation of the NF-κB
transcription factor (14–16). NF-κB is important for tumorigenesis
and tumor progression. It is associated with various signal
transduction pathways and transcription activation events that
mediate cell proliferation, cell migration and angiogenesis.
Aberrant or constitutively activated NF-κB has been detected in
human cancer (17,18). To investigate the contribution of
the AKT/NF-κB signaling pathway to the inhibition of the SHH/GLI1
pathway, AKT and NF-κB phosphorylation was analyzed by
immunoblotting. AKT and NF-κB phosphorylation levels were decreased
following treatment with evodiamine (Fig. 6C). These results suggested that
evodiamine inhibited cell proliferation via inhibition of
SHH/GLI1/AKT/NF-κB signaling in A549 cells.
Discussion
Lung cancer is a common type of human malignancy,
and its incidence in Asia is increasing. There is a low 5-year
survival rate despite routine surgery and chemotherapy, which is
due to distant metastases (19).
Growing evidence highlights natural products with antitumor
activity (20). It was reported
that >20 alkaloids have been extracted and separated from E.
rutaecarpa, and were shown to be the main active components
(21,22). Futhermore, evodiamine, an indole
alkaloid, is considered to be the main active ingredient in the
total alkaloids. The experimental study on pharmacological activity
determined that evodiamine had clear antitumor activity (23,24);
however, the effect of evodiamine on lung cancer has not been
adequately determined, to the best of our knowledge.
The present study demonstrated that evodiamine was
able to significantly inhibit the in vitro proliferation of
A549 cells. Under 1 and 2.5 µM concentrations, evodiamine
significantly enhanced apoptosis. At 1 and 2.5 µM
concentrations, evodiamine arrested the tumor cell cycle at the
G2/M phase; therefore, the 1 and 2.5 µM concentration was
used in the remaining experiments to prevent interference of tumor
cell death. The molecular mechanism of evodiamine-induced
inhibition of tumors was further investigated. It was determined
that evodiamine induced tumor cells to produce ROS capable of
initiating apoptosis of tumor cells. GSH is important for the
antioxidant defense of cells (25). The intracellular concentration of
GSH reflects a dynamic balance between the synthesis and
consumption of GSH within the cell and loss through efflux. Under
oxidative stress conditions, ROS are reduced by GSH with
concomitant formation of the GSSG (26). The present study determined that
the GSH level was also reduced following evodiamine treatment.
Therefore, it is possible that evodiamine increased ROS levels via
inhibition of GSH activity.
Previous studies have indicated that evodiamine may
stimulate the production of ROS to induce apoptosis by causing
mitochondrial damage (27,28). The present study found that
following treatment with evodiamine, the anti-apoptotic genes
Survivin and Bcl-2 in the mitochondria-associated apoptosis pathway
were significantly downregulated, while the downstream caspase-3
and caspase-8 activity was significantly upregulated, indicating
that the mitochondrial pathway is important for evodiamine-induced
apoptosis. In addition, corresponding changes in the expression of
cell cycle regulatory gene cyclin B1 was also observed.
Telomerase is a ribonucleoprotein complex, which is
active in the majority tumors (29) and is important in tumor
proliferation and metastasis. Previous studies have demonstrated,
that telomerase activity is required for the malignant properties
of cancer cells and may be a good target for the development of
anticancer therapeutic agents (30,31).
Additionally, mortalin overexpression cooperates with telomerase to
extend the in vitro lifespan of normal human fibroblasts
(32). When this is considered
with the present data, it is possible that nuclear mortalin
interacts with telomerase and activates its function contributing
to proliferation and the malignant characteristics of cancer cells.
Telomerase may be associated with the inhibition of apoptotic
signaling and may be involved in the regulated process of
apoptosis, which to the best of our knowledge, has not been fully
elucidated. The present study determined that evodiamine may
inhibit telomerase activity, suggesting that the downregulation of
Bcl-2 and telomerase may be important for evodiamine-induced
apoptosis.
The mechanism of evodiamine-induced inhibition of
tumors was investigated using signal transduction molecules. The
SHH/GLI1 signaling pathway is an important signal transduction
pathway, which is abnormally activated in lung cancer cells and is
important for oncogenesis and tumor development. It primarily
consists of the signaling molecule SHH and the downstream
transcription factor GLI1, hence it is termed the SHH/GLI1
signaling pathway. Additionally, it is involved in the growth,
invasion, metastasis, epithelial-mesenchymal transition, apoptosis,
angiogenesis and multiple related aspects of tumor cells. The
present study detected the changes in the SHH/GLI1 signaling
pathway when A549 cell proliferation was inhibited by
evodiamine.
The activation of the PI3K/AKT signaling pathway is
involved in cell proliferation, survival, apoptosis and malignant
transformation, by regulating NF-κB activation in several cell
lines (33). The NF-κB family of
transcription factors controls the expression of genes involved in
immune and inflammatory responses, cell proliferation, oncogenesis,
angiogenesis and Bcl-2 family members (34). NF-κB is important for the
resistance of cancer cells to anticancer therapies by protecting
them from apoptosis (35). The
present study determined that evodiamine inhibited the activation
of AKT and NF-κB. In addition, NF-κB has been shown to contribute
to SHH signaling activation through SHH ligand induction in
pancreatic cells (36). The
inhibitory effect of evodiamine on SHH and GLI1 signaling by NF-κB
observed in the present study suggested that NF-κB stimulates SHH
signaling. In conclusion, these results suggest that evodiamine may
be a potential anticancer agent for the treatment of lung
cancer.
References
|
1
|
Wang N, Liang H, Zhou Y, Wang C, Zhang S,
Pan Y, Wang Y, Yan X, Zhang J, Zhang CY, et al: miR-203 suppresses
the proliferation and migration and promotes the apoptosis of lung
cancer cells by targeting SRC. PLoS One. 9:e1055702014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao L, Li C, Zhang Y, Wen Q and Ren D:
Phytochemical and biological activities of an anticancer plant
medicine: Brucea javanica. Anticancer Agents Med Chem. 14:440–458.
2014. View Article : Google Scholar
|
|
3
|
De Marchi T, Foekens JA, Umar A and
Martens JW: Endocrine therapy resistance in estrogen receptor
(ER)-positive breast cancer. Drug Discov Today. 7:1181–1188. 2016.
View Article : Google Scholar
|
|
4
|
Camidge DR: Targeted therapy vs
chemotherapy: Which has had more impact on survival in lung cancer?
Does targeted therapy make patients live longer? Hard to prove, but
impossible to ignore. Clin Adv Hematol Oncol. 12:763–766. 2014.
|
|
5
|
Wahl O, Oswald M, Tretzel L, Herres E,
Arend J and Efferth T: Inhibition of tumor angiogenesis by
antibodies, synthetic small molecules and natural products. Curr
Med Chem. 18:3136–3155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruden M and Puri N: Novel anticancer
therapeutics targeting telomerase. Cancer Treat Rev. 39:444–456.
2013. View Article : Google Scholar
|
|
7
|
Liao CH, Pan SL, Guh JH, Chang YL, Pai HC,
Lin CH and Teng CM: Antitumor mechanism of evodiamine, a
constituent from Chinese herb Evodiae fructus, in human
multiple-drug resistant breast cancer NCI/ADR-RES cells in vitro
and in vivo. Carcinogenesis. 26:968–975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen MC, Yu CH, Wang SW, Pu HF, Kan SF,
Lin LC, Chi CW, Ho LL, Lee CH and Wang PS: Anti-proliferative
effects of evodiamine on human thyroid cancer cell line ARO. J Cell
Biochem. 110:1495–1503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
10
|
Merchán P, Bribián A, Sánchez-Camacho C,
Lezameta M, Bovolenta P and de Castro F: Sonic hedgehog promotes
the migration and proliferation of optic nerve oligodendrocyte
precursors. Mol Cell Neurosci. 36:355–368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seifert T, Bauer J, Weissert R, Fazekas F
and Storch MK: Differential expression of sonic hedgehog
immunoreactivity during lesion evolution in autoimmune
encephalomyelitis. J Neuropathol Exp Neurol. 64:404–411. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wetmore C: Sonic hedgehog in normal and
neoplastic proliferation: Insight gained from human tumors and
animal models. Curr Opin Genet Dev. 13:34–42. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gomes DC, Leal LF, Mermejo LM, Scrideli
CA, Martinelli CE Jr, Fragoso MC, Latronico AC, Tone LG, Tucci S,
Yunes JA, et al: Sonic hedgehog signaling is active in human
adrenal cortex development and deregulated in adrenocortical
tumors. J Clin Endocrinol Metab. 99:E1209–E1216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen KC, Chen CY, Lin CR, Yang TY, Chen
TH, Wu LC and Wu CC: Luteolin attenuates TGF-β1-induced
epithelial-mesenchymal transition of lung cancer cells by
interfering in the PI3K/Akt-NF-κB-Snail pathway. Life Sci.
93:924–933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng Y, Huang S, Wu Y, Cheng B, Nie X, Liu
H, Ma K, Zhou J, Gao D, Feng C, et al: Platelet rich plasma clot
releasate preconditioning induced PI3K/AKT/NFκB signaling enhances
survival and regenerative function of rat bone marrow mesenchymal
stem cells in hostile microenvironments. Stem Cells Dev.
22:3236–3251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rasul A, Ding C, Li X, Khan M, Yi F, Ali M
and Ma T: Dracorhodin perchlorate inhibits PI3K/Akt and NF-κB
activation, up-regulates the expression of p53, and enhances
apoptosis. Apoptosis. 17:1104–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J and Richmond AJ: Monitoring
NF-kappaB mediated chemokine transcription in tumorigenesis.
Methods Enzymol. 460:347–355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L, Yue GG, Pu JX, Sun HD, Fung KP,
Leung PC, Han QB, Lau CB and Leung PS: Eriocalyxin B-induced
apoptosis in pancreatic adenocarcinoma cells through
thiol-containing antioxidant systems and downstream signalling
pathways. Curr Mol Med. 14:673–689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi Z, Yang DY and Cao J: Increased
micro-RNA 17, 21, and 192 gene expressions improve early diagnosis
in non-small cell lung cancer. Med Oncol. 31:1952014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robles-Fernández I, Rodríguez-Serrano F,
Álvarez PJ, Ortiz R, Rama AR, Prados J, Melguizo C,
Álvarez-Manzaneda E and Aránega A: Antitumor properties of natural
compounds and related molecules. Recent Pat Anticancer Drug Discov.
8:203–215. 2013. View Article : Google Scholar
|
|
21
|
Wang XX, Zan K, Shi SP, Zeng KW, Jiang Y,
Guan Y, Xiao CL, Gao HY, Wu LJ and Tu PF: Quinolone alkaloids with
antibacterial and cytotoxic activities from the fruits of Evodia
rutaecarpa. Fitoterapia. 89:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ko JS, Rho MC, Chung MY, Song HY, Kang JS,
Kim K, Lee HS and Kim YK: Quinolone alkaloids, diacylglycerol
acyltransferase inhibitors from the fruits of Evodia rutaecarpa.
Planta Med. 68:1131–1133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu AJ, Wang SH, Chen KC, Kuei HP, Shih
YL, Hou SY, Chiu WT, Hsiao SH and Shih CM: Evodiamine, a plant
alkaloid, induces calcium/JNK-mediated autophagy and
calcium/mitochondria-mediated apoptosis in human glioblastoma
cells. Chem Biol Interact. 205:20–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan X, Hartley JM, Hartley JA, White KN,
Wang Z and Bligh SW: Evodiamine, a dual catalytic inhibitor of type
I and II topoisomerases, exhibits enhanced inhibition against
camptothecin resistant cells. Phytomedicine. 19:618–624. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma T, Airao V, Panara N, Vaishnav D,
Ranpariya V, Sheth N and Parmar S: Solasodine protects rat brain
against ischemia/reperfusion injury through its antioxidant
activity. Eur J Pharmacol. 725:40–46. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tobwala S, Fan W, Hines CJ, Folk WR and
Ercal N: Antioxidant potential of Sutherlandia frutescens and its
protective effects against oxidative stress in various cell
cultures. BMC Complement Altern Med. 14:2712014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue S, Chen YX, Qin SK, Yang AZ, Wang L,
Xu HJ and Geng HY: Raltitrexed induces mitochondrial-mediated
apoptosis in SGC7901 human gastric cancer cells. Mol Med Rep.
10:1927–1934. 2014.PubMed/NCBI
|
|
28
|
Ma L, Li X, Wang Y, Zheng W and Chen T:
Cu(II) inhibits hIAPP fibrillation and promotes hIAPP-induced beta
cell apoptosis through induction of ROS-mediated mitochondrial
dysfunction. J Inorg Biochem. 140:143–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murnane JP: Telomere dysfunction and
chromosome instability. Mutat Res. 730:28–36. 2012. View Article : Google Scholar
|
|
30
|
Saretzki G: Extra-telomeric functions of
human telomerase: cancer, mitochondria and oxidative stress. Curr
Pharm Des. 41:6386–6403. 2014. View Article : Google Scholar
|
|
31
|
Rasul A, Yu B, Zhong L, Khan M, Yang H and
Ma T: Cytotoxic effect of evodiamine in SGC-7901 human gastric
adenocarcinoma cells via simultaneous induction of apoptosis and
autophagy. Oncol Rep. 27:1481–1487. 2012.PubMed/NCBI
|
|
32
|
Kaul SC, Yaguchi T, Taira K, Reddel RR and
Wadhwa R: Overexpressed mortalin (mot-2)/mthsp70/GRP75 and hTERT
cooperate to extend the in vitro lifespan of human fibroblasts. Exp
Cell Res. 286:96–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shao N, Lu Z, Zhang Y, Wang M, Li W, Hu Z,
Wang S and Lin Y: Interleukin-8 upregulates integrin β3 expression
and promotes estrogen receptor-negative breast cancer cell invasion
by activating the PI3K/Akt/NF-κB pathway. Cancer Lett. 364:165–172.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Zhu P, Wang Y, Wei Z, Tao L, Zhu Z,
Sheng X, Wang S, Ruan J, Liu Z, et al: Antimetastatic therapies of
the Polysulfide Diallyl Trisulfide against triple-negative breast
cancer (TNBC) via suppressing MMP2/9 by blocking NF-κB and ERK/MAPK
signaling pathways. PLoS One. 10:e01237812015. View Article : Google Scholar
|
|
36
|
Cardenas-Rodriguez M and Badano JL:
Ciliary biology: Understanding the cellular and genetic basis of
human ciliopathies. Am J Med Genet C Semin Med Genet. 151C:263–280.
2009. View Article : Google Scholar : PubMed/NCBI
|