Introduction
Colorectal cancer (CRC) is one of the most common
types of malignancy worldwide, with an increasing incidence in
China (1). In addition, it is the
fourth leading cause of cancer-associated mortality, and is
responsible for 529,000 cases of mortality per year worldwide
(2). The long-term prognosis for
patients with CRC remains poor, with a 50–59% 5-year survival rate
(2). Early diagnosis and the use
of molecular targeted therapies are effective at improving the
clinical outcomes for patients with CRC. Therefore, it is important
to disclose the molecular mechanisms underlying the initiation and
development of CRC, which may provide evidence to explore novel
biomarkers and therapeutic targets of CRC.
Forkhead box (FOX) protein A1 (FOXA1) is a pioneer
transcription factor that belongs to the FOX gene superfamily
(3) and exerts fundamental
functions in the process of development and differentiation
(4–8). FOXA1 modulates transcriptional
programs in a tissue-dependent manner by inducing nucleosomal
rearrangement and altering the accessibility of chromatin (3). In addition, FOXA1 is responsible for
various biological processes, including cell proliferation,
apoptosis and differentiation (9).
Due to its critical role in these cellular processes, investigators
have begun to determine its functional significance in human
cancers. Elevated FOXA1 expression has been confirmed in anaplastic
thyroid cancer (10), lung cancer
(11,12) and hepatocellular carcinoma (HCC)
(13), whereas decreased FOXA1
expression has been detected in poorly differentiated pancreatic
cancer tissues (14).
Functionally, FOXA1 is able to promote the proliferation of thyroid
cancer cells (10), potentiate the
metastatic ability of prostate cancer (15), and has been associated with sexual
dimorphism of HCC (16). However,
until now, the expression and biological function of FOXA1 in CRC
have yet to be elucidated.
The present study aimed to determine the difference
in FOXA1 expression between CRC tissues and noncancerous tissues.
The clinical significance of FOXA1 was also explored. Furthermore,
the role of FOXA1 in CRC growth and the potential underlying
mechanisms were also addressed.
Materials and methods
Patients and clinicopathological
data
A total of 90 samples of CRC and matched
tumor-adjacent tissues were collected from the Department of
Pathology, The Second Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China) between January 2007 and January 2009.
The demographic features and clinicopathological parameters are
presented in Table I. All
specimens had a confirmed pathological diagnosis and were
classified according to International Union Against Cancer and
American Joint Committee on Cancer criteria (7th edition) (17). Patients did not receive
preoperative chemotherapy, biotherapy or molecular targeted
therapy. All samples were used after obtaining informed consent
from the patients. The Xi'an Jiaotong University Ethics Committee
approved all protocols, according to the Declaration of Helsinki
(as revised in Tokyo, 2004).
 | Table IClinical association analysis of FOXA1
expression in colorectal cancer. |
Table I
Clinical association analysis of FOXA1
expression in colorectal cancer.
| Feature | Total no. of
patients, n=90 | No. of patients
| P |
|---|
| FOXA1 positive | FOXA1 negative |
|---|
| Age (years) | | | | 0.094 |
| ≤60 | 27 | 12 | 15 | |
| >60 | 63 | 40 | 23 | |
| Gender | | | | 0.223 |
| Male | 54 | 34 | 20 | |
| Female | 36 | 18 | 18 | |
| Tumor grade | | | | 0.005a |
| G1/G2 | 67 | 33 | 34 | |
| G3/G4 | 23 | 19 | 4 | |
| Size (cm) | | | | 0.004a |
| <5 | 32 | 12 | 20 | |
| ≥5 | 58 | 40 | 18 | |
| Tumor invasion | | | | 0.458 |
| T1/T2 | 20 | 13 | 7 | |
| T3/T4 | 70 | 39 | 31 | |
| Lymph node
metastases | | | | 0.040a |
| Absent | 63 | 32 | 31 | |
| Present | 27 | 20 | 7 | |
| Distant
metastasis | | | | 0.609 |
| Absent | 71 | 42 | 29 | |
| Present | 19 | 10 | 9 | |
| TNM stage | | | | 0.023a |
| I/II | 54 | 26 | 28 | |
| III/IV | 36 | 26 | 10 | |
Immunohistochemical staining
The tissue of interest was fixed by immersing it in
10% neutral buffered formalin for 4–24 h at room temperature. The
tissue was subsequently embedded in paraffin and the blocks were
stored at 4°C prior to sectioning. The formalin-fixed and
paraffin-embedded tissues from postoperative patients were cut into
5 µm sections. Sections were deparaffinized in xylene and
rehydrated using a series of graded alcohols. Subsequently, tissue
slides were subjected to antigen retrieval using 0.01 M sodium
citrate (pH 6.0) at a sub-boiling temperature for 10 min and were
blocked with 10% goat serum (ZSGB-Bio, Beijing, China). The samples
were then incubated overnight at 4°C with anti-FOXA1 (1:100; cat.
no. sc-101058; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
antibody. Immunostaining was performed using a SPlink Detection kit
(cat. no. SP-9002; ZSGB-Bio) according to the manufacturer's
protocol. Staining intensity was scored as follows: 0, negative; 1,
weak; or 2, strong; and the extent of staining was based on the
percentage of positive tumor cells: 0, negative; 1, 1–25%; 2,
26–50%; 3, 51–75%; or 4, 76–100% (18). Therefore, each case was ultimately
considered negative if the final score was 0 (negative expression)
or 1–2 (low expression), and positive if the final score was 3–5
(medium expression) or 6–8 (high expression). The final
immunohistochemistry score for each sample (negative or positive)
was calculated by multiplying the intensity score with the
percentage of positive cells.
Cell culture and transfection
Two CRC cell lines, HCT116 and SW480 (The Institute
of Biochemistry and Cell Biology, Chinese Academy of Sciences,
Shanghai, China), were purchased for use in the present study.
Cells were routinely cultured in complete Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
at 37°C in a humidified incubator containing 5% CO2.
The targeted sequences for FOXA1 small interfering
(si)RNA (sense 5′-GCACUGCAAUACUCGCCUU-3′) or a nonspecific duplex
oligonucleotide as a negative control were synthesized by Sangon
Biotech (Shanghai) Co., Ltd. (Shanghai, China). Cells were
transfected with the aforementioned siRNAs using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
Immunoblotting
The tissue was snap-frozen in liquid nitrogen and
diced into 1 mm pieces with a scalpel in a mortar on dry ice. Total
proteins were extracted using radioimmunoprecipitation assay lysis
buffer (Santa Cruz Biotechnology, Inc.) and the protein
concentrations were measured using the bicinchoninic acid assay.
The proteins (20 µg) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and were transferred
onto a polyvinylidene fluoride membrane (Roche Diagnostics,
Indianapolis, IN, USA). The membrane was then blocked with 5%
skimmed milk at room temperature for 15 min and incubated with the
following appropriate primary antibodies at 4°C overnight: FOXA1
(1:1,000), yes-associated protein (YAP; 1:1,000; cat. no.
PA1-46189; Thermo Fisher Scientific, Inc., Rockford, IL, USA),
caspase-3 (1:1,000; cat. no. 9662; Cell Signaling Technology, Inc.,
Danvers, MA, USA) and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH; 1:5,000; cat. no. sc-25778; Santa Cruz Biotechnology,
Inc.). Blots were then incubated with horseradish
peroxidase-conjugated goat anti-mouse/rabbit immunoglobulin G
secondary antibodies (1:5,000; cat. nos. CW0102/103; Cwbiotech,
Shanghai, China) at room temperature for 2 h. Blots were detected
using enhanced chemiluminescence regents (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and semi-quantified by ImageJ software
(1.46; National Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used for total RNA extraction
following tissue homogenization. cDNA synthesis was performed using
PrimeScript RT Reagent kit (Takara Bio, Inc., Otsu, Japan) at 37°C
for 15 min, following treatment with DNase I (Invitrogen; Thermo
Fisher Scientific, Inc.). Amplification and detection of FOXA1 and
GAPDH mRNA were performed using an ABI PRISM 7300 Sequence
Detection system (Applied Biosystems, Inc., Foster City, CA, USA)
and a One Step SYBR® PrimeScript™ RT-PCR kit II (Takara
Bio, Inc.) according to the manufacturers' protocols. The following
primers (Sangon Biotech (Shanghai) Co., Ltd.) were used: FOXA1,
sense primer 5′-AAT CATTGCCATCGTGTG-3′, antisense primer
5′-CGCGGCTTAAAATCTGGTAT-3′; and GAPDH, sense
5′-CAAGCTCATTTCCTGGTATGAC-3′, and antisense
5′-CAGTGAGGGTCTCTCTCTTCCT-3′. The thermocycling conditions were as
follows: 95°C for 60 sec; followed by 40 cycles of 95°C for 5 sec
and 60°C for 34 sec. GAPDH was measured as an internal control for
mRNA. All samples were normalized to internal controls and fold
changes were calculated based on relative quantification using the
2−ΔΔCq method (13).
Proliferation and apoptosis assay
For the proliferation assay, CRC cells were seeded
into 96-well plates at a density of 5,000 cells/well for 24 h and
were assessed using Cell Proliferation enzyme-linked immunosorbent
assay, 5-bromodeoxyuridine (BrdU) (chemiluminescent) (Roche
Diagnostics) according to the manufacturer's protocol. Flow
cytometric analysis was conducted using fluorescence-activated cell
sorting (FACS)Calibur (BD Biosciences, San Jose, CA, USA) and Cell
Quest Pro software (BD Biosciences). An Annexin-V-FLUOS Staining
kit (Roche Diagnostics) was used to analyze the level of apoptosis
according to the manufacturer's protocol. The percentage of
apoptotic cells was calculated using the flow cytometry
software.
Statistical analysis
Results are expressed as the mean ± standard error
of the mean. All experiments were repeated three times. All
statistical analyses were performed using SPSS statistical package
for Windows version 13 (SPSS, Inc., Chicago, IL, USA) or GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). The
quantitative data were compared between groups using the Student's
t-test. Categorical data were analyzed using the Pearson
chi-squared test. The Kaplan-Meier method and log-rank test were
used to compare the cumulative recurrence and survival rates. The
independent factors influencing the survival and recurrence of
patients with CRC were determined using the Cox proportional
hazards model. Correlation analysis was conducted using the
Pearson's correlation coefficient. P<0.05 was considered to
indicate a statistically significant difference.
Results
FOXA1 expression is elevated in CRC
tissues
Immunohistochemical staining was performed to
investigate differences in FOXA1 protein expression between CRC and
adjacent noncancerous tissues. As shown in Fig. 1, FOXA1 staining was negative in the
adjacent noncancerous tissue (Fig.
1A), whereas positive FOXA1 staining with nuclear location was
observed in CRC tissues (Fig.
1B–D). FOXA1 expression was detected in 57.8% (52/90) of the
CRC samples, whereas only 37.8% (34/90) of the noncancerous
specimens exhibited a positive FOXA1 signal (P<0.05; Fig. 2A). Furthermore, 20 randomly
selected cases were subjected to RT-qPCR in order to determine
FOXA1 mRNA expression. The mRNA expression levels of FOXA1 were
significantly increased in CRC tissues compared with in the
adjacent noncancerous tissues (P<0.05; Fig. 2B). There results indicate an
oncogenic role for FOXA1 in CRC.
Positive expression of FOXA1 is
associated with poor clinicopathological features
To elucidate the clinical significance of FOXA1
expression in CRC, the present study investigated the association
between FOXA1 expression and clinicopathological features of
patients with CRC. As shown in Table
I, positive FOXA1 expression in CRC tissues was associated with
poor tumor differentiation (P=0.005), large tumor size (P=0.004),
lymph node metastasis (P=0.040) and advanced tumor-node-metastasis
stage (P=0.023). These results indicate that FOXA1 may promote the
development and progression of CRC.
FOXA1 is a prognostic predictor for
patients with CRC
To further investigate the prognostic value of FOXA1
expression, the overall survival (OS) and recurrence-free survival
(RFS) rates were compared between the FOXA1-positive group (n=52)
and the FOXA1-negative group (n=38). Kaplan-Meier survival curves
indicated that the positive expression of FOXA1 in CRC was
significantly correlated with reduced OS (P=0.0191; Fig. 3) rate and RFS rates (P=0.0155;
Fig. 3). Notably, a multivariate
Cox regression analysis demonstrated that FOXA1 expression was an
independent factor for predicting the OS and RFS of patients with
CRC (P=0.005 and 0.017, respectively; Table II). These data indicate that FOXA1
expression is a promising predictor for patient prognosis in
CRC.
 | Table IIMultivariate Cox regression analysis
of 5-year overall and recurrence-free survival rates of 90 patients
with colorectal cancer. |
Table II
Multivariate Cox regression analysis
of 5-year overall and recurrence-free survival rates of 90 patients
with colorectal cancer.
| Variable | Overall survival
| Recurrence-free
survival
|
|---|
| HR | 95% CI | P | HR | 95% CI | P |
|---|
| Tumor grade (G1/G2
vs. G3/G4) | 1.904 | 0.734–4.982 | 0.190 | 2.343 | 0.950–5.812 | 0.065 |
| Tumor size (cm)
(<5 vs. ≥5) | 1.233 | 0.650–2.023 | 0.342 | 1.371 | 0.864–2.561 | 0.426 |
| Lymph node
metastases (absent vs. present) | 1.403 | 0.703–2.783 | 0.345 | 1.125 | 0.634–2.003 | 0.692 |
| TNM stage (I/II vs.
III/IV) | 2.895 | 1.213–6.695 | 0.034a | 2.197 | 1.148–4.291 | 0.023a |
| FOXA1 expression
(negative vs. positive) | 2.028 | 1.235–3.317 | 0.005a | 2.023 | 1.134–3.603 | 0.017a |
FOXA1 knockdown inhibits CRC cell
proliferation and promotes apoptosis in vitro
To determine the functional role of FOXA1 in CRC,
FOXA1 siRNA was used to inhibit the expression of FOXA1 in SW480
and HCT116 cells, and the proliferation and apoptosis of CRC cells
was subsequently investigated. FOXA1 knockdown was confirmed by
immunoblotting (P<0.05; Fig.
4A). Subsequently, BrdU incorporation assays demonstrated that
the proliferation of SW480 and HCT116 cells was significantly
decreased following FOXA1 knockdown (P<0.05; Fig. 4B). In addition, the percentage of
apoptotic SW480 and HCT116 cells was significantly increased
following suppression of FOXA1 (P<0.05; Fig. 4C). Western blot analysis indicated
that FOXA1 knockdown evidently increased the protein expression of
cleaved caspase-3 in SW480 and HCT116 cells (P<0.05; Fig. 4D).
 | Figure 4Knockdown of forkhead box protein A1
(FOXA1) suppresses colorectal cancer cell proliferation and
promotes apoptosis. (A) SW480 and HCT116 cells were transfected
with FOXA1 small interfering (si)RNA or scrambled siRNA, and were
subjected to western blotting to detect FOXA1 expression. n=3
independent experiments. *P<0.05, as determined by
Student's t-test. (B) FOXA1 knockdown significantly inhibited cell
proliferation as measured by 5-bromodeoxyuridine (BrdU)
incorporation in SW480 and HCT116 cells. n=3 independent
experiments. *P<0.05, as determined by Student's
t-test. (C) Apoptosis of SW480 and HCT116 cells was measured by
flow cytometry, and was promoted by FOXA1 knockdown. n=3
independent repeats with similar results. *P<0.05, as
determined by Student's t-test. (D) Western blot analysis indicated
that FOXA1 knockdown increased the protein expression of cleaved
caspase-3 in SW480 and HCT116 cells. n=3 independent experiments.
*P<0.05, as determined by Student's t-test. GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; RLU, relative light
units; FITC, fluorescein isothiocyanate; PI, propidium iodide. |
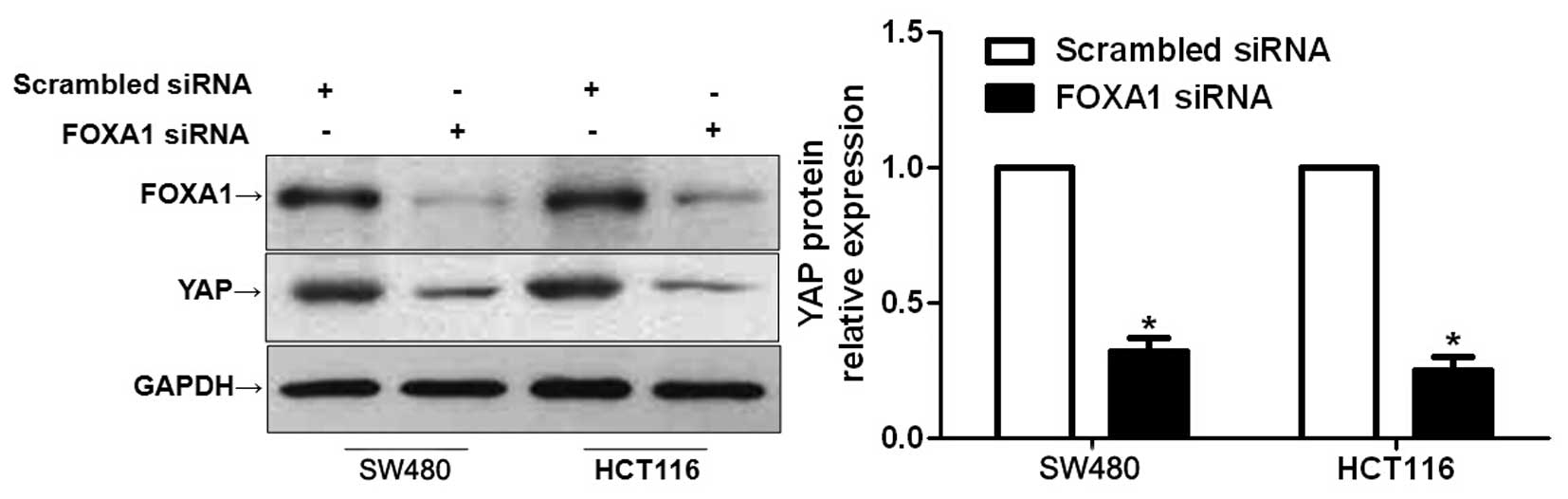
FOXA1 knockdown inhibits YAP expression
in CRC cells
Hippo-YAP signaling has been confirmed to have a
fundamental role in the pathogenesis of CRC (19,20).
Furthermore, inhibiting YAP expression has been reported to lead to
a marked decrease in the proliferation and apoptosis of CRC cells
(21). A recent study reported
that FOXA1 could regulate YAP expression in HCC cells (13). Therefore, the present study
investigated whether the functional effects of FOXA1 in CRC cells
were mediated via the modulation of YAP expression. As expected,
FOXA1 knockdown resulted in a significant decrease in YAP
expression in SW480 and HCT116 cells (P<0.05; Fig. 5). These data indicate that FOXA1
may regulate YAP expression in CRC cells.
Discussion
The FOXA family [also known as the hepatocyte
nuclear factor 3 (HNF3) family] consists of FOXA1 (HNF3α), FOXA2
(HNF3β) and FOXA3 (HNF3γ). It is well established that
dysregulation of FOXA members has a pivotal role in carcinogenesis
(22). FOXA1 is a key
transcription factor of the FOXA family, which exhibits aberrant
expression and functions as a prognostic indicator in various types
of human cancer (13,23,24).
The present study was the first, to the best of our knowledge, to
investigate the expression status of FOXA1 in CRC. Significantly
elevated mRNA and protein expression of FOXA1 was detected in CRC
tissues compared with in the tumor-adjacent tissues. A clinical
association analysis demonstrated that FOXA1 expression was
correlated with poor clinicopathological features and reduced
survival of patients with CRC. In addition, a Cox regression
analysis revealed that the positive expression of FOXA1 may be a
potential factor for predicting a bad patient outcome.
A previous study reported that FOXA1 knockdown
inhibited the effects of ELL-associated factor 2 knockdown, and was
associated with decreased cell proliferation and migration in LNCaP
cells (25). Qiu et al
demonstrated that FOXA1 knockdown reduced the tumor growth of
endometrial cancer in vivo and in vitro (26). Furthermore, FOXA1 has been reported
to promote cell proliferation and inhibit apoptosis in HCC cells
(13). All these studies prompted
the investigation of the biological role of FOXA1 in CRC cells in
the present study. The present in vitro experiments
demonstrated that FOXA1 knockdown inhibited proliferation and
induced apoptosis of CRC cells. These results indicated that FOXA1
may function as an oncogenic factor by promoting proliferation and
suppressing apoptosis of CRC cells. The Hippo pathway has been
revealed to be an important regulator of CRC cell proliferation,
growth and apoptosis (19–21,27).
The majority of genes coding for Hippo pathway proteins have been
shown to function in several types of cancer, either as tumor
suppressors or as oncogenes (28).
The most commonly focused gene, YAP, has been reported to be
involved in tumor development and progression in various
malignancies, including CRC (28).
YAP has been found to be significantly upregulated in CRC tissues,
and affects the proliferation and apoptosis of CRC cells (21,29,30).
In addition, it has been considered a therapeutic target of CRC
(31). Notably, FOXA1 has a
positive effect on YAP expression; opening of compacted chromatin
by FOXA1 around the cAMP response element binding protein (CREB)
binding site within the YAP promoter facilitates CREB-mediated YAP
transcription in HCC cells (32).
In the present study, FOXA1 expression was knocked down following
transfection of CRC cells with a specific siRNA. Western blotting
indicated that the protein expression levels of YAP were
significantly decreased following FOXA1 knockdown. These results
suggested that FOXA1 may regulate cell proliferation and apoptosis
at least in part via the modulation of YAP expression in CRC
cells.
In conclusion, the present study demonstrated that
FOXA1 is overexpressed in CRC tissue specimens. The positive
expression of FOXA1 was associated with poor clinicopathological
features of CRC, thus suggesting that FOXA1 expression may be an
independent indicator of prognosis in patients with CRC.
Furthermore, FOXA1 may facilitate CRC cell proliferation and
inhibit apoptosis by regulating YAP expression. Therefore, FOXA1
may be considered a potential prognostic biomarker and therapeutic
target for CRC.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81171356).
References
|
1
|
Li L and Ma BB: Colorectal cancer in
Chinese patients: Current and emerging treatment options. Onco
Targets Ther. 7:1817–1828. 2014.PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bernardo GM and Keri RA: FOXA1: A
transcription factor with parallel functions in development and
cancer. Biosci Rep. 32:113–130. 2012. View Article : Google Scholar
|
|
4
|
Augello MA, Hickey TE and Knudsen KE:
FOXA1: Master of steroid receptor function in cancer. EMBO J.
30:3885–3894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katoh M, Igarashi M, Fukuda H, Nakagama H
and Katoh M: Cancer genetics and genomics of human FOX family
genes. Cancer Lett. 328:198–206. 2013. View Article : Google Scholar
|
|
6
|
Strazzabosco M: Foxa1 and Foxa2 regulate
bile duct development in mice. J Hepatol. 52:765–767. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Z, Merkurjev D, Yang F, Li W, Oh S,
Friedman MJ, Song X, Zhang F, Ma Q, Ohgi KA, et al: Enhancer
activation requires trans-recruitment of a mega transcription
factor complex. Cell. 159:358–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hurtado A, Holmes KA, Ross-Innes CS,
Schmidt D and Carroll JS: FOXA1 is a key determinant of estrogen
receptor function and endocrine response. Nat Genet. 43:27–33.
2011. View
Article : Google Scholar
|
|
9
|
Zaret KS and Carroll JS: Pioneer
transcription factors: Establishing competence for gene expression.
Genes Dev. 25:2227–2241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nucera C, Eeckhoute J, Finn S, Carroll JS,
Ligon AH, Priolo C, Fadda G, Toner M, Sheils O, Attard M, et al:
FOXA1 is a potential oncogene in anaplastic thyroid carcinoma. Clin
Cancer Res. 15:3680–3689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin L, Miller CT, Contreras JI, Prescott
MS, Dagenais SL, Wu R, Yee J, Orringer MB, Misek DE, Hanash SM, et
al: The hepatocyte nuclear factor 3 alpha gene, HNF3alpha (FOXA1),
on chromosome band 14q13 is amplified and overexpressed in
esophageal and lung adenocarcinomas. Cancer Res. 62:5273–5279.
2002.PubMed/NCBI
|
|
12
|
Deutsch L, Wrage M, Koops S, Glatzel M,
Uzunoglu FG, Kutup A, Hinsch A, Sauter G, Izbicki JR, Pantel K and
Wikman H: Opposite roles of FOXA1 and NKX2-1 in lung cancer
progression. Genes Chromosomes Cancer. 51:618–629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dou C, Wang Y, Li C, Liu Z, Jia Y, Li Q,
Yang W, Yao Y, Liu Q and Tu K: MicroRNA-212 suppresses tumor growth
of human hepatocellular carcinoma by targeting FOXA1. Oncotarget.
6:13216–13228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song Y, Washington MK and Crawford HC:
Loss of FOXA1/2 is essential for the epithelial-to-mesenchymal
transition in pancreatic cancer. Cancer Res. 70:2115–2125. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gerhardt J, Montani M, Wild P, Beer M,
Huber F, Hermanns T, Müntener M and Kristiansen G: FOXA1 promotes
tumor progression in prostate cancer and represents a novel
hallmark of castration-resistant prostate cancer. Am J Pathol.
180:848–861. 2012. View Article : Google Scholar
|
|
16
|
Li Z, Tuteja G, Schug J and Kaestner KH:
Foxa1 and Foxa2 are essential for sexual dimorphism in liver
cancer. Cell. 148:72–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tong LL, Gao P, Wang ZN, Song YX, Xu YY,
Sun Z, Xing CZ and Xu HM: Is the seventh edition of the UICC/AJCC
TNM staging system reasonable for patients with tumor deposits in
colorectal cancer? Ann Surg. 255:208–213. 2012. View Article : Google Scholar
|
|
18
|
Huang W, Chen Z, Shang X, Tian D, Wang D,
Wu K, Fan D and Xia L: Sox12, a direct target of FoxQ1, promotes
hepatocellular carcinoma metastasis through up-regulating Twist1
and FGFBP1. Hepatology. 61:1920–1933. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Avruch J, Zhou D and Bardeesy N: YAP
oncogene overexpression supercharges colon cancer proliferation.
Cell Cycle. 11:1090–1096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barry ER, Morikawa T, Butler BL, Shrestha
K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et
al: Restriction of intestinal stem cell expansion and the
regenerative response by YAP. Nature. 493:106–110. 2013. View Article : Google Scholar :
|
|
21
|
Wang L, Shi S, Guo Z, Zhang X, Han S, Yang
A, Wen W and Zhu Q: Overexpression of YAP and TAZ is an independent
predictor of prognosis in colorectal cancer and related to the
proliferation and metastasis of colon cancer cells. PLoS One.
8:e655392013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao Y and Li Z: Interplay of estrogen
receptors and FOXA factors in the liver cancer. Mol Cell
Endocrinol. 418:334–339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reddy OL, Cates JM, Gellert LL, Crist HS,
Yang Z, Yamashita H, Taylor JA III, Smith JA Jr, Chang SS, Cookson
MS, et al: Loss of FOXA1 drives sexually dimorphic changes in
urothelial differentiation and is an independent predictor of poor
prognosis in bladder cancer. Am J Pathol. 185:1385–1395. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Horimoto Y, Arakawa A, Harada-Shoji N,
Sonoue H, Yoshida Y, Himuro T, Igari F, Tokuda E, Mamat O, Tanabe
M, et al: Low FOXA1 expression predicts good response to
neo-adjuvant chemotherapy resulting in good outcomes for luminal
HER2-negative breast cancer cases. Br J Cancer. 112:345–351. 2015.
View Article : Google Scholar :
|
|
25
|
Guo W, Keener AL, Jing Y, Cai L, Ai J,
Zhang J, Fisher AL, Fu G and Wang Z: FOXA1 modulates EAF2
regulation of AR transcriptional activity, cell proliferation, and
migration in prostate cancer cells. Prostate. 75:976–987. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qiu M, Bao W, Wang J, Yang T, He X, Liao Y
and Wan X: FOXA1 promotes tumor cell proliferation through AR
involving the Notch pathway in endometrial cancer. BMC Cancer.
14:782014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wierzbicki PM and Rybarczyk A: The Hippo
pathway in colorectal cancer. Folia Histochem Cytobiol. 53:105–119.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Konsavage WM Jr, Kyler SL, Rennoll SA, Jin
G and Yochum GS: Wnt/β-catenin signaling regulates Yes-associated
protein (YAP) gene expression in colorectal carcinoma cells. J Biol
Chem. 287:11730–11739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Xie C, Li Q, Xu K and Wang E:
Clinical and prognostic significance of Yes-associated protein in
colorectal cancer. Tumour Biol. 34:2169–2174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liang K, Zhou G, Zhang Q, Li J and Zhang
C: Expression of hippo pathway in colorectal cancer. Saudi J
Gastroenterol. 20:188–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu W, Qiao Y, Tang X, Ma L, Wang Y, Zhang
X, Weng W, Pan Q, Yu Y, Sun F and Wang J: Tumor suppressor long
non-coding RNA, MT1DP is negatively regulated by YAP and Runx2 to
inhibit FoxA1 in liver cancer cells. Cell Signal. 26:2961–2968.
2014. View Article : Google Scholar : PubMed/NCBI
|