Introduction
Increasing evidence indicates that the incidence of
cholangiocarcinoma has risen in previous decades (1,2). Due
to difficulties in early diagnosis and relative resistance of the
tumors to chemotherapy, the prognosis of patients with
cholangiocarcinoma remains poor (3,4).
Although palliative surgical resection has been considered to be
the mainstay treatment for cholangiocarcinoma, the high mortality
rate and low 5-year survival rate warrants the investigation of
novel approaches to overcome the issue (5).
Macroautophagy, hereafter referred to as autophagy,
is an evolutionarily conserved catabolic mechanism to suppress
carcinogenesis by eliminating oncogenic molecules and damaged
organelles (6). Autophagy fuels
cancer cell metabolism (6), and is
involved in the abnormal proliferation and invasion of cells, and
in resistance to chemotherapy and radiation therapy. The inhibition
of autophagy can result in mitochondrial dysfunction (MtD), which
leads to cell death (7,8), and may be a promising strategy for
the treatment of cancer.
Natural products and their synthetic derivatives
have been a continuous source of novel compounds for the treatment
of cancer (9,10). Oblongifolin C (OC), a natural small
molecule compound extracted and purified from Garcinia
yunnanensis Hu, can activate a mitochondrial apoptotic pathway
in human cervical cancer cells (11). In addition, Lao et al
(12) demonstrated that OC is a
novel autophagic flux inhibitor by inhibiting
autophagosome-lysosome fusion and autophagic degradation.
Therefore, the primary aim of the present study was
to determine whether the hypothesis that OC directly induces
apoptosis via inhibiting autophagy and MtD in the human
cholangiocarcinoma QBC939 cell line, is correct.
Materials and methods
Materials
The established QBC939 cell line was obtained from
the Cell Bank of Wuhan University (Wuhan, China). OC,
3-methyladenine (3-MA) and rapamycin (RP) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). β-actin, microtubule-associated
protein light chain 3 (LC3) and p62 antibodies were purchased from
Cell Signaling Technology, Inc. (Beverly, MA, USA). All other
chemicals were of analytical grade.
Cell culture
The cells were maintained in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS;
Sigma-Aldrich) at 37°C in an atmosphere containing 5%
CO2. When the cells reached 80–90% confluence, the cells
were added to the DMEM (10% FCS in media) with different
concentrations (5, 10, 20 and 40 μM) of OC for another 48
h.
Methylthiazole tetrazolium (MTT)
assay
An MTT reduction assay was used as a qualitative
index of cell viability. The effect of OC on cell viability was
assessed as the percentage cell viability, compared with that of
untreated control cells, which were arbitrarily assigned a
viability of 100%.
Assessment of cell apoptosis
The cytosolic DNA-histone complexes generated during
apoptotic DNA fragmentation in the treated QBC939 cells were
evaluated using a cell death detection enzyme-linked immunosorbent
assay kit (Cell Death Detection ELISA PLUS; Roche Applied Science,
Indianapolis, IN, USA), according to the manufacturer's
protocol.
Mitochondrial membrane potential
(MMP)
The MMP of the QBC939 cells was monitored using
JC-1, an MMP-sensitive fluorescent dye, as described previously
(13). Briefly, the dissociated
90% confluent QBC939 cells were washed twice with Hank's balanced
salt solution (Sigma-Aldrich) and incubated in the dark with JC-1
(7.5 mmol/l; Sigma-Aldrich) for 30 min at 37°C. The cells were then
washed with JC-1 washing buffer, and fluorescence was detected by
fluorescence-assisted cell sorting of the QBC939 cells. The
relative MMP was calculated using the ratio of J-aggregate/monomer
(590/520 nm).
Measurement of adenosine triphosphate
(ATP) content
The ATP levels in 90% confluent QBC939 cells were
determined using a luciferase-based bioluminescence assay kit
(Sigma-Aldrich) in a FLUOstar Optima reader (BMG Labtech GmbH,
Ortenberg, Germany), according to the manufacturer's protocol. The
total ATP levels were determined as the respective luminescence
normalized by the protein concentration.
Western blot analysis
The total cellular proteins from the cells of the
respective groups were extracted by lysis of the cells with buffer
containing 150 mM NaCl, 0.1% Triton X-100, 0.5% deoxycholate, 0.1%
sodium dodecyl sulfate (SDS), 50 mM Tris-HCl (pH 7.0) and 1 mM
ethylenediaminetetraacetic acid. Protein concentrations were
determined using the bicinchoninic acid method (Beyotime Institute
of Biotechnology, Haimen, China) Equal amounts of protein (50
μg) were subjected to sodium dodecyl sulfate-polyacrylamide
gel electrophoresis on 10 % gels and transferred to a
polyvinylidene difluoride membrane. The membrane was blocked for 1
h with blocking buffer containing 5% non-fat dry milk and 0.05%
Tween-20 in Tris-buffered saline, followed by overnight incubation
at 4°C with the primary antibodies as follows: rabbit polyclonal
anti-LC3 (1:1,000; cat. no. ab48394), mouse monoclonal anti-p62
(1:2,000; ab56416) and mouse monoclonal anti-β-actin (1:1,000;
ab8226) obtained from Abcam (Cambridge, UK). Following further
incubation with the corresponding secondary antibody (1:2,500; cat.
no. A27041; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 2
h at room temperature, immune complexes were detected using
enhanced chemiluminescence western blotting reagents (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China). The relative
intensity of each respective band was normalized to β-actin.
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation. Statistical significance was determined with
one-way analysis of variance and Dunnett's post-test using GraphPad
Prism software (version 6.0; GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
OC decreases cell viability and induces
apoptosis in human cholangiocarcinoma QBC939 cells
The chemical structure of OC is shown in Fig. 1A. The results of the MTT assay
showed that, compared with the normal group, the addition of OC for
48 h inhibited human cholangiocarcinoma cell viability in a
dose-dependent manner at concentrations between 10 and 40 μM
(P<0.05; Fig. 1B). As shown in
Fig. 1C, when compared with the
control cholangiocarcinoma cells, without OC, treatment of the
QBC939 cells with OC for 48 h resulted in a 16–23% (P<0.05)
increase in cell apoptosis at 10 μM, a 21–39% (P<0.01)
increase in cell death at 20 μM and a 38–52% (P<0.01)
increase in cell death at 40 μM.
OC inhibits autophagy in QBC939
cells
To monitor autophagic fluctuation, the levels of
LC3II/LC3I and p62, a selective substrate of autophagy, were
measured in the QBC939 cells. Following incubation with different
concentrations (5, 10, 20 and 40 μM) of OC for 48 h, the
levels of LC3II/LC3I and p62 were increased, compared with the
control cells (Fig. 2A and B).
These results suggested that OC treatment inhibited cellular
autophagic flux in the QBC939 cells.
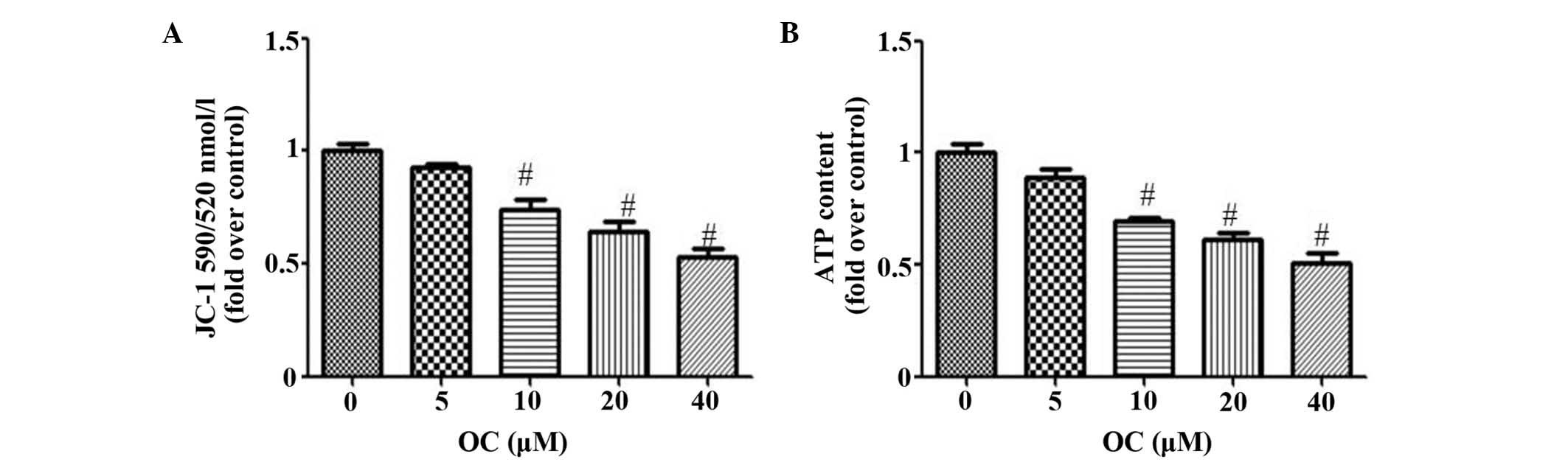
OC inhibits MtD in QBC939 cells
To evaluate the MtD, the present study used two
independent parameters: Levels of MMP and ATP. MMP collapse has
been shown to be important in the mediation of apoptosis, in which
it allows the release of cytochrome c and activation of caspase-9,
and subsequently leads to the apoptosis of cells (14). Mitochondria are also the energy
powerhouses of cells and efficiently utilize the cell's ATP. Tumor
cells consume a substantial quantities of energy supplied as ATP,
therefore, any disruption in this supply is likely to cause cell
death. As expected, OC reduced the MMP in a dose-dependent manner,
as indicated by the reduction of JC-1 fluorescence at 590/520 nm
(Fig. 3A). OC also reduced the
production of ATP (Fig. 3B).
Pharmacological regulation of autophagy
affects OC-induced apoptosis and MtD in QBC939 cells
Using pharmacological drugs to demonstrate the
involvement of autophagy in the regulation of cell apoptosis has
been widely acknowledged. In the present study, 3-MA, which
inhibits autophagosomal cargo sequestration, and RP, an autophagy
enhancer, were used to evaluate the effect of autophagy on
OC-induced apoptosis and MtD in QBC939 cells. As the lowest level
of induction was found in the 10 μM OC treatment group, 10
μM OC was used as a stimulation factor in the experiments.
As expected, 3-MA inhibited autophagy, as demonstrated by the
further accumulation of p62 and decrease in LC3II/LC3I, compared
with the cells treated with OC alone (Fig. 4A and B). However, RP had the
opposite effect (Fig. 4A and B).
As shown in Fig. 4C–E, 3-MA
treatment increased the OC-induced apoptosis and MtD of the QBC939
cells, whereas RP significantly improved the OC-induced
changes.
Discussion
Cholangiocarcinoma remains one of the most difficult
types of tumor to treat in clinical practice and novel therapeutic
modalities are required, with the induction of cholangiocarcinoma
apoptosis considered to be one promising therapeutic strategy.
Several toxic compounds from traditional Chinese
medicines (15–17) exhibit antitumor effects and have
been used for cancer therapy. OC has been acknowledged to activate
a mitochondrial apoptotic pathway in human cervical cancer cells
(11). However the effect of OC on
cholangiocarcinoma remains to be elucidated. In the present study,
it was shown that OC significantly reduced the viability and
induced the apoptosis of the QBC939 cells (Fig. 1), which suggested that OC may be
examined as an effective chemotherapeutic agent against
cholangiocarcinoma.
It has been recognized that controlling the
progression of autophagy in cancer cells is an effective strategy
to inhibit tumor growth (18,19),
and molecular analyses of human cancer have revealed that autophagy
regulators are frequently deregulated in the majority of common
malignancies (20,21). For this reason, the inhibition of
autophagy has been regarded as a promising anticancer strategy to
inhibit multiple cellular processes. The in vitro data
obtained in the present study demonstrated that the treatment of
QBC939 cells with OC, a novel autophagic flux inhibitor, inhibited
the progression of autophagy (Fig.
2). Additionally, the pharmacological inhibition of autophagy
increased OC-induced apoptosis, wheareas the promotion of autophagy
attenuated OC-induced apoptosis, indicating that the inhibition of
autophagy was one of the mechanisms by which OC induced the
apoptosis of QBC939 cells (Fig.
4).
MtD represents a malfunction in biochemical
processes, characterized by MMP collapse, which has been shown to
be essential in the mediation of apoptosis (14). Furthermore, mitochondria are the
energy powerhouses of cells and efficiently utilize the cell's ATP.
Cancer cells consume a substantial quantity of energy supplied as
ATP, therefore, any disruption in this supply is likely to cause
the apoptosis of cells. An increasing number of studies have shown
that the inhibition of autophagy often leads to MtD, which is
usually associated with cell death (22,23).
In the present study, it was found that OC inhibited autophagy,
accompanied by MtD (Fig. 2), and
that inhibiting autophagy increased OC-induced MtD, whereas
treatment with RP attenuated this process (Fig. 4). These results indicated that MtD
resulted from the inhibition of autophagy and may have contributed
to the OC-induced apoptosis of QBC939 cells.
In conclusion, the present study provided the first
evidence, to the best of our knowledge, that OC-induced apoptosis
resulted from inhibition of autophagy and MtD in human
cholangiocarcinoma QBC939 cells, and OC may offer potential as an
effective agent for the prevention of cholangiocarcinoma.
Acknowledgments
This study was financially supported by the
Scientific Research Program from Nanjing Medical University (grant
nos. 2011NJMU251 and 2014NJMUZD068).
References
|
1
|
Shaib YH, Davila JA, McGlynn K and
El-Serag HB: Rising incidence of intrahepatic cholangiocarcinoma in
the United States: A true increase? J Hepatol. 40:472–477. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Landis SH, Murray T, Bolden S and Wingo
PA: Cancer statistics, 1998. CA Cancer J Clin. 48:6–29. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mittal B, Deutsch M and Iwatsuki S:
Primary cancers of extrahepatic biliary passages. Int J Radiat
Oncol Biol Phys. 11:849–854. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pitt HA, Nakeeb A, Abrams RA, Coleman J,
Piantadosi S, Yeo CJ, Lillemore KD and Cameron JL: Perihilar
cholangiocarcinoma. Postoperative radiotherapy does not improve
survival. Ann Surg. 221:788–797; discussion 797–798. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boerma EJ: Research into the results of
resection of hilar bile duct cancer. Surgery. 108:572–580.
1990.PubMed/NCBI
|
|
6
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim MJ, Choi OK, Chae KS, Kim MK, Kim JH,
Komatsu M, Tanaka K, Lee H, Chung SS, Kwak SH, et al: Mitochondrial
complexes I and II are more susceptible to autophagy deficiency in
mouse β-cells. Endocrinol Metab (Seoul). 30:65–70. 2015. View Article : Google Scholar
|
|
8
|
Dhingra R and Kirshenbaum LA: Regulation
of mitochondrial dynamics and cell fate. Circ J. 78:803–810. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang YJ, Park KK, Chung WY, Hwang JK and
Lee SK: Xanthorrhizol, a natural sesquiterpenoid, induces apoptosis
and growth arrest in HCT116 human colon cancer cells. J Pharmacol
Sci. 111:276–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang JS, Lee SW, Kim MS, Yun BR, Park MH,
Lee SG, Park SJ, Lee WS and Rho MC: Manassantin A and B from
Saururus chinensis inhibit interleukin-6-induced signal transducer
and activator of transcription 3 activation in Hep3B cells. J
Pharmacol Sci. 115:84–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng C, Zhou LY, Yu T, Xu G, Tian HL, Xu
JJ, Xu HX and Luo KQ: A new anticancer compound, oblongifolin C,
inhibits tumor growth and promotes apoptosis in HeLa cells through
Bax activation. Int J Cancer. 131:1445–1454. 2012. View Article : Google Scholar
|
|
12
|
Lao Y, Wan G, Liu Z, Wang X, Ruan P, Xu W,
Xu D, Xie W, Zhang Y, Xu H and Xu N: The natural compound
oblongifolin C inhibits autophagic flux and enhances antitumor
efficacy of nutrient deprivation. Autophagy. 10:736–749. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Acton BM, Jurisicova A, Jurisica I and
Casper RF: Alterations in mitochondrial membrane potential during
preimplantation stages of mouse and human embryo development. Mol
Hum Reprod. 10:23–32. 2004. View Article : Google Scholar
|
|
14
|
Mao WP, Ye JL, Guan ZB, Zhao JM, Zhang C,
Zhang NN, Jiang P and Tian T: Cadmium induces apoptosis in human
embryonic kidney (HEK) 293 cells by caspase-dependent and
-independent pathways acting on mitochondria. Toxicol In Vitro.
21:343–354. 2007. View Article : Google Scholar
|
|
15
|
He W, Wang B, Zhuang Y, Shao D, Sun K and
Chen J: Berberine inhibits growth and induces G1 arrest and
apoptosis in human cholangiocarcinoma QBC939 cells. J Pharmacol
Sci. 119:341–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukuda T, Oda K, Wada-Hiraike O, Sone K,
Inaba K, Ikeda Y, Miyasaka A, Kashiyama T, Tanikawa M, Arimoto T,
et al: The anti-malarial chloroquine suppresses proliferation and
overcomes cisplatin resistance of endometrial cancer cells via
autophagy inhibition. Gynecol Oncol. 137:538–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang L, Zhao MN, Liu TY, Wu XS, Weng H,
Ding Q, Shu YJ, Bao RF, Li ML, Mu JS, et al: Bufalin induces cell
cycle arrest and apoptosis in gallbladder carcinoma cells. Tumour
Biol. 35:10931–10941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma N, Thomas S, Golden EB, Hofman FM,
Chen TC, Petasis NA, Schönthal AH and Louie SG: Inhibition of
autophagy and induction of breast cancer cell death by mefloquine,
an antimalarial agent. Cancer Lett. 326:143–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Egger ME, Huang JS, Yin W, McMasters KM
and McNally LR: Inhibition of autophagy with chloroquine is
effective in melanoma. J Surg Res. 184:274–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schonewolf CA, Mehta M, Schiff D, Wu H,
Haffty BG, Karantza V and Jabbour SK: Autophagy inhibition by
chloroquine sensitizes HT-29 colorectal cancer cells to concurrent
chemoradiation. World J Gastrointest Oncol. 6:74–82.
2014.PubMed/NCBI
|
|
21
|
Liu F, Shang Y and Chen SZ: Chloroquine
potentiates the anti-cancer effect of lidamycin on non-small cell
lung cancer cells in vitro. Acta Pharmacol Sin. 35:645–652. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hall AM and Unwin RJ: The not so 'mighty
chondrion': Emergence of renal diseases due to mitochondrial
dysfunction. Nephron Physiol. 105:p1–p10. 2007. View Article : Google Scholar
|
|
23
|
Hagiwara M, Yamagata K, Capaldi RA and
Koyama A: Mitochondrial dysfunction in focal segmental
glomerulosclerosis of puromycin aminonucleoside nephrosis. Kidney
Int. 69:1146–1152. 2006. View Article : Google Scholar : PubMed/NCBI
|