Introduction
Mesenchymal stromal cells (MSCs) are defined as
self-renewing progenitor cells with multi-lineage potential that
differentiate into cells of mesodermal origin, including
adipocytes, osteocytes, and chondrocytes, as well as non-mesodermal
cells (1–4). MSCs are easily isolated from bone
marrow (BM), adipose and other tissues, and have immune modulatory
properties, including low antigenicity. Furthermore, MSCs secrete
various chemicals to promote tissue preservation and regeneration,
and to inhibit inflammation and fibrosis (5,6).
Clinical investigations into the therapeutic potential of MSCs in
various diseases are rapidly evolving. Previous studies have
demonstrated that co-administration of MSCs with hematopoietic stem
cells (HSCs) in allogeneic HSC transplantation (HSCT) accelerated
hematopoietic recovery (7,8), ameliorated graft-versus-host disease
(GVHD) (9–11) and promoted tissue regeneration
(12,13).
Allogeneic HSCT is a routine treatment for
intractable hematologic malignancies (14). Previous studies have indicated that
co-transplantation of BM-derived MSCs with donor HSCs promoted
hematopoietic cell engraftment, prevented or treated GVHD, and
accelerated marrow stromal regeneration (11). The use of MSCs in HSCT with HSCs
enhanced long-term engraftment of human cells in animal models
(15,16) and promising results regarding the
enhancement of myelocytic or megakaryocytic engraftment in the
co-transplantation of MSCs with HSCs have also been reported
(17).
MSCs have been previously isolated from human
tonsils and termed tonsil-derived MSCs (T-MSCs) (18). T-MSCs were demonstrated to exhibit
the stem cell characteristics of self-renewal and proliferation,
with the ability to differentiate into adipocytes, osteocytes and
chondrocytes. Furthermore, the cells expressed endodermal markers
and parathyroid cell markers (18), and were differentiated into
hepatocyte-like cells (19). The
authors hypothesized that T-MSCs may be equivalent to BM-derived
MSCs with respect to induction or supplemental effects on BM
reconstitution. T-MSCs were also hypothesized to be beneficial in
hematopoiesis following BM transplantation (BMT). In the present
study, a possible role and beneficial effect for T-MSCs in BM
reconstitution was investigated using an allogeneic BMT mouse
model.
Materials and methods
Animals
Eight-week-old female BALB/c (H-2d) mice and male
C57BL/6 (H-2b) mice were purchased from Orient Bio, Inc. (Seongnam,
South Korea). Mice were housed separately at 21–23°C, 51–54%
humidity with a 12-h light/dark cycle, and had access to food and
water ad libitum. All animal studies were approved by the
Animal Care and Use Committee at Ewha Medical School (Seoul, South
Korea) and conformed to international standards.
Culture of T-MSCs
T-MSC separation was performed as described
previously (18). Surgically
removed palatine tonsils were collected from patients (age, <10
years) undergoing tonsillectomy due to benign hypertrophic tonsils
between September 2011 and December 2012. Informed written consent
was obtained from all patients who participated in the study, and
the study protocol was approved by the Institutional Review Board
of Ewha Womans University Medical Center (Mok-Dong Hospital, Seoul,
South Korea). The tonsil tissues were minced with scissors and
digested in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 210 U/ml collagenase type I
(Invitrogen; Thermo Fisher Scientific, Inc.) and 10 µg/ml
DNase (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37°C.
Mononuclear cells were obtained from the digested tonsil tissue by
Ficoll® Paque density gradient centrifugation (300 × g
for 30 min at room temperature with brake-off; GE Healthcare,
Chalfont, UK). The cells were plated at a density of
1×107 cells/100-mm diameter culture dish in Dulbecco's
modified Eagle's medium containing 10% fetal bovine serum, 100
µg/ml streptomycin and 100 U/ml penicillin all from Welgene,
Inc. (Gyeongsan-si, Korea). After 48 h, non-adherent cells were
removed from the medium and adherent mononuclear cells (the T-MSCs)
were placed in fresh culture medium. T-MSCs were labeled using the
PKH26 MINI kit (Sigma-Aldrich) according to the manufacturer's
protocol. Cells were re-suspended in phosphate-buffered saline
(PBS; Sigma-Aldrich) (5×106 cells/ml) for injection into
mice.
Isolation and preparation of murine
BMCs
Eight-week-old C57BL/6 male mice were sacrificed by
cervical dislocation and the lower limbs (femurs and tibias) were
collected. BMCs were harvested, following dissection and cleaning
of the bones, by removing the ends of each bone and flushing the
medullary cavities with serum-free RPMI-1640 medium (Welgene, Inc.)
using a 25-gauge needle (Korea Vaccine, Ansan-si, Korea). A single
cell suspension was produced by passing the BM suspension through a
sterile 70-µm cell strainer (SPL Life Sciences, Pocheon,
South Korea) and the filtrate was centrifuged at 300 × g for 5 min.
Isolated BMCs were incubated in red blood cell (RBC) lysis solution
(Sigma-Aldrich; 0.15 M NH4Cl, 10 mM NaHCO3
and 10 mM disodium EDTA, all from Sigma-Aldrich) for 2 min followed
by two washes with PBS. Following resuspension with PBS,
2×106 cells/200 µl were intravenously injected
into recipient mice using 1-ml syringes (Sungshim, Bucheon-si,
Korea).
Experimental design for recipient
conditioning and BMT
Eight-week-old female BALB/c recipient mice received
busulfan (Bu; Sigma-Aldrich) and cyclophosphamide (Cy; Baxter, Los
Angeles, CA, USA) combination conditioning therapy. Bu (20 mg/kg)
was injected on days 1 2, 3 and 4, followed by administration of Cy
(100 mg/kg) on days 5 and 6 via intraperitoneal injection.
Following one day of recovery, infusion of T-MSCs or T-MSCs + BMCs
was performed on day 8 according to the following procedure. BMCs
from donor mice were injected via the tail vein into each recipient
mouse. The mice were divided into five groups, as follows: i) The
control, no treatment group; ii) the Bu/Cy chemotherapy group, iii)
the T-MSCs (following Bu/Cy) group; iv) the T-MSCs + BMCs
(following Bu/Cy) group; and the BMCs (following Bu/Cy) group. All
mice were administered with water containing antibiotics for 3
weeks to prevent bacterial infection.
Hematological and histological
analysis
Three weeks after cell transplantation (day 28), the
mice were anesthetized with Zoletil (0.012 ml/20 g body weight;
Virbac, St. Louis, USA) and Rompun (0.008 ml/20 g body weight;
Bayer Korea, Seoul, Korea) and blood (1 ml) was collected by
cardiac puncture into heparin-coated 1-ml syringes (JWphama, Seoul,
Korea). Blood cells were counted using a Neubauer chamber and blood
smear slides were prepared for staining. Peripheral blood smears
were air-dried and dipped in methanol (Duksan, Ansan-si, Korea)
three times for fixation, followed by Diff-Quik staining (Sysmex
Corporation, Kobe, Japan). The femurs of each mouse were isolated
following cervical dislocation, and immediately fixed with 4%
paraformaldehyde (Sigma-Aldrich) and decalcified for embedding in
paraffin (Korea Animal Medical Science Institute, Guri-si, Korea).
BM sections (4 µm) were stained with hematoxylin and eosin
(H&E; Sigma-Aldrich) and images of the tissue sections were
captured using a BX-50 microscope and a DP-71 digital camera and
imaging system (DPController 3.2.276.2) (all from Olympus Corp.,
Tokyo, Japan). Histological analysis was conducted using Image J
software version 1.49 (National Institutes of Health, Bethesda, MD,
USA) by selecting adipocytes in the BM using a color threshold.
Other cells (erythroid and myeloid cells) were selected for BM area
measurement.
Methylcellulose colony-forming assay
To analyze the colony-forming unit derived from
hematopoietic stem cells, 5×105 cells were mixed with 4
ml MethoCult® media (Stemcell Technologies, Inc.,
Vancouver, BC, Canada). The mixture was allowed to stand for 3 min
to remove bubbles and a 1-ml mixture was dispensed by pipette for
each 30-mm cell culture dish (triplicate assay). Cells were
incubated for 3 weeks in a 5% CO2 incubator at 37°C with
humidifying water-containing dishes. After 3 weeks of culture, the
total colony was counted under an optical microscope (Olympus
CKX41; Olympus Corp.) over a grid plate according to the shape and
the size of the colonies.
Statistical analysis
The values are expressed as the mean ± standard
error of the mean. The differences between groups were analyzed via
two-way analysis of variance and Student's t-test using GraphPad
Prism 6.04 software (GraphPad Software Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Co-transplantation of T-MSCs with BMCs
improved survival in the allogeneic mouse of BMT
The degree of BM restoration in the mice was
compared between the groups that received BMCs with or without
T-MSCs following allogeneic BMT. Recipient female BALB/c mice were
injected with Bu/Cy for 6 days (resulting in BM ablation), allowed
to rest for one day, then injected with T-MSCs, with or without
BMCs from C57BL/6 male mice (Fig.
1A). During the 3 weeks following cell transfer, the mice were
followed up and survival was compared between the groups. The mean
survival was 100% in the control group (17/17), 12.5% in the Bu/Cy
ablation group (3/24), 44% in the T-MSCs only group (8/18), 50% in
the T-MSCs + BMCs group (9/18) and 44% in the BMCs group (11/25).
Although the survival rate of the groups injected with T-MSCs +
BMCs or BMC only was improved compared with that in the Bu/Cy only
group, the difference was not statistically significant (Fig. 1B).
Changes in the body weight of the mice were also
monitored from the onset of Bu/Cy treatment, and it was observed
that body weights began decreasing soon after Bu/Cy conditioning
and cell transfer. Although the mice indicated signs of weight
recovery by the end of the first week following cell
transplantation, their body weights subsequently decreased. Upon
completion of the study, the recovered body weights of surviving
mice were comparable with those of the mice in the BMC only groups:
Control group (21.00±0.83 g), the Bu/Cy group (17.75±2.48 g), the
T-MSCs group (17.75±2.19 g), the T-MSCs + BMCs group (17.44±2.63
g), and the BMC group (17.60±2.38 g; Fig. 1C).
T-MSC injection led to recovery of
peripheral mononuclear cells
In order to compare the effect of T-MSCs on the
restoration of peripheral blood cells, blood smear slides were
stained and differential cell counts were analyzed among the
groups. Cell populations from the control group exhibited normal
morphology and the Bu/Cy group demonstrated scant density of RBCs,
leukocytes, and platelets, although the morphology for each of the
cells was near normal (Fig. 2A).
However, RBC counts indicated that the BMCs group
(25.25±3.18×106) almost recovered to the level of the
control (36.50±2.12×106). The Bu/Cy group
(10.00±2.83×106), T-MSCs group
(13.85±6.86×106), and T-MSCs + BMCs group
(20.27±1.41×106) did not fully recover to normal and
were significantly different; Fig.
2B). Leukocyte counts were lowest in the Bu/Cy group
(3.40±0.28×105) and also in the T-MSCs group
(6.00±0.56×105). Notably, in the T-MSCs + BMCs groups,
leukocyte counts (34.13±5.00×105) recovered to above
normal (control group) levels (25.20±5.01×105) while
those of the BMCs group were lower than normal
(8.70±1.27×105; Fig.
2C). Therefore, these results indicate a potential favorable
effect on myelopoiesis resulting from co-transplantation of T-MSCs
with allogeneic BMCs.
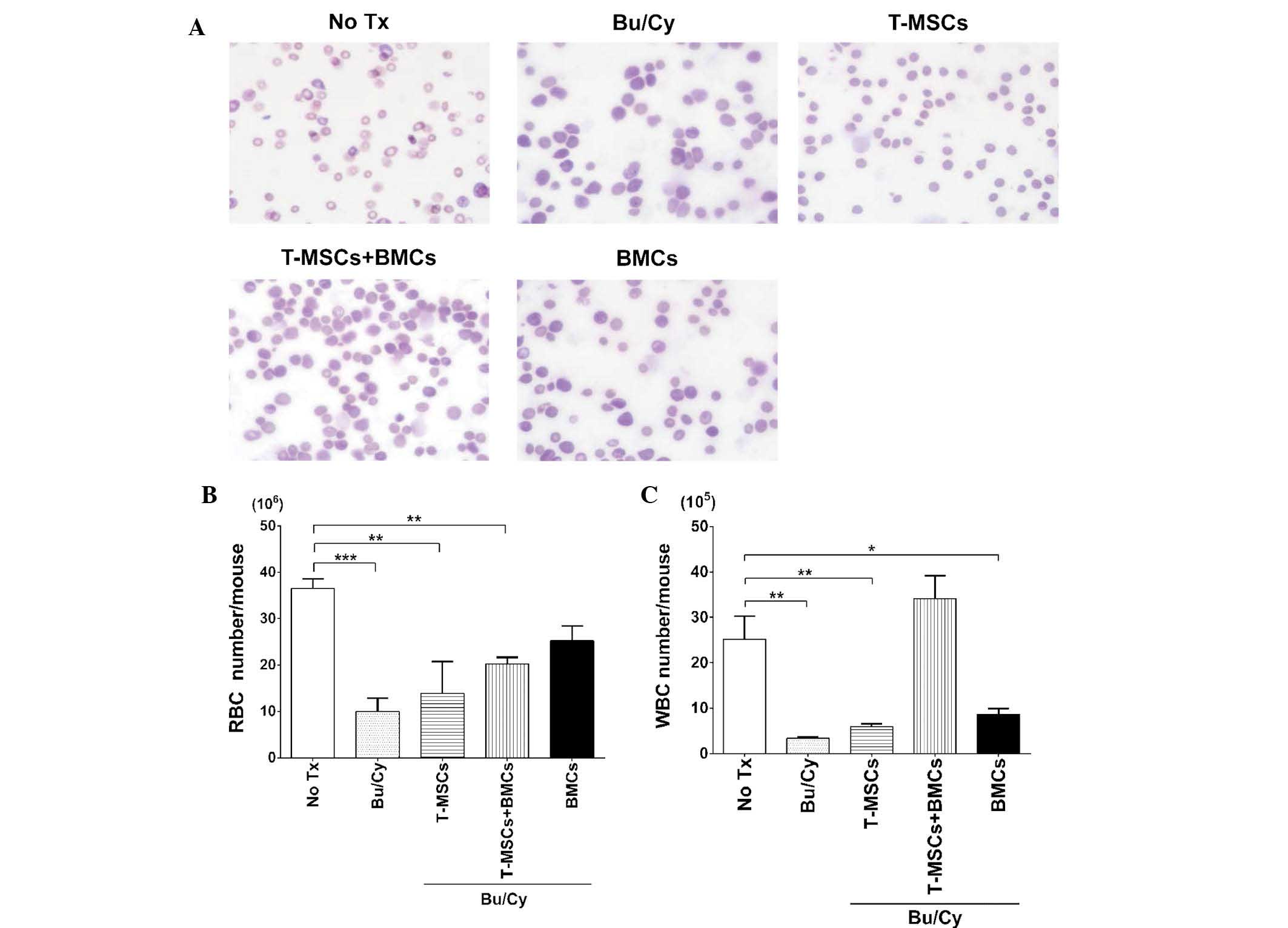 | Figure 2Peripheral blood morphology and
complete blood counts. (A) Peripheral blood smear of control mice
demonstrated normal erythrocytes, WBCs, including polymorphonuclear
leucocytes and mononuclear cells, and platelets. Peripheral blood
smears were stained with Diff-Quik; magnification, ×1,000. (B) RBC
and (C) WBC counts were performed and a t-test was used to compare
each value with the control (*P<0.05,
**P<0.01 and ***P<0.0001). Values are
expressed as the mean ± standard error of the mean. BMT, bone
marrow transplantation; BMC, bone marrow cell; Bu, busulfan; Cy,
cyclophosphamide; T-MSC, tonsil-derived mesenchymal stromal cells;
Tx, treatment; RBC, red blood cell; WBC, white blood cell. |
T-MSCs have a beneficial effect on BM
reconstitution
To evaluate the effect of T-MSCs on BM
reconstitution, the cellular densities of decalcified bone sections
from each group were determined by H&E staining. The BM of mice
from the control group was filled with various erythroid and
myeloid cells, small adipocytes and a small number of
megakaryocytes (Fig. 3A). The
marrow space in the Bu/Cy treatment group was almost completely
replaced by adipocytes and was vacant of cells of hematopoietic
lineage. The T-MSCs group demonstrated increased BMC density
compared with the Bu/Cy group (Fig.
3B). The marrow space of the co-transplantation group, T-MSCs +
BMCs, was also filled with various BMCs, and a number of adipocytes
and megakaryocytes. The BM cellular density was assessed by
calculating the mean ratio of the area of BMCs to adipose cells in
>10 different fields per slide. This quantitative analysis
demonstrated higher BMC density in groups that received T-MSCs +
BMCs compared with any other treatment group (62.81±10.32% vs.
control, 75.71±3.63%) and those of the Bu/Cy group were the lowest
(16.87±5.48%). As indicated in Fig.
3B, the BM cellularity of the T-MSCs + BMCs groups was almost
normal (control; 75.71±3.63%). In the T-MSCs (37.97±8.19%) or BMCs
only groups (33.29±6.98%), cellularity was significantly different
from the normal control.
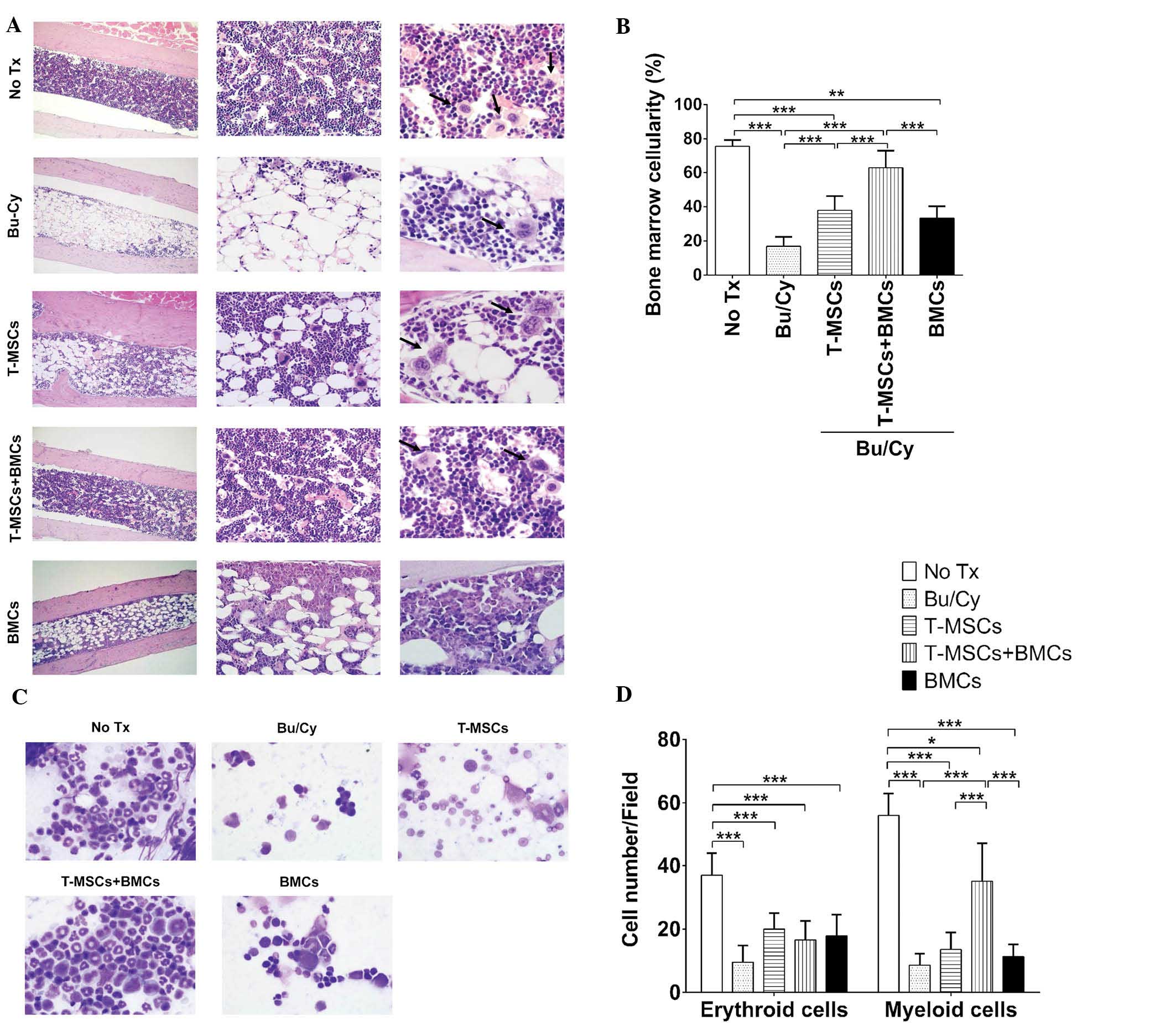 | Figure 3Hematopoiesis in BM. (A)
Representative BM histology and cellularity in the tibias of
experimental mice. Magnification: Left column, ×100; middle column,
×400; right column, ×1,000. Arrows indicate megakaryocytes. (B) BM
cellularity was obtained by calculating the ratio of marrow cells
to adipocytes in 10 different fields from each slide (n=5/group).
BM cellularity was analyzed by Image J software using the ratio of
the area occupied by marrow cells to adipocytes in >10 different
fields on each slide (*P<0.05, **P<0.01
and ***P<0.001). (C) Peripheral blood smears were
stained with Diff-Quik (magnification, ×1,000). (D) BM smear
demonstrated the hematopoietic lineage cells in BM. The data
indicates the cell number for each group obtained from >10
different fields in the samples (*P<0.05 and
***P<0.0001). Values are expressed as the mean ±
standard error of the mean. BM, bone marrow; BMT, bone marrow
transplantation; BMC, bone marrow cell; Bu, busulfan; Cy,
cyclophosphamide; T-MSC, tonsil-derived mesenchymal stromal cells;
Tx, treatment. |
BM smears were stained and analyzed to determine the
composition of cells with erythroid or myeloid lineage in the BM
(Fig. 3C). For erythroid cells,
all Bu/Cy-treated groups demonstrated decreased cell numbers and
insufficient recovery (Fig. 3D).
However, for myeloid cells in the BM smear, the cell numbers
observed in the T-MSCs + BMCs groups were higher than those of
other Bu/Cy-treated groups (the Bu/Cy, T-MSCs and BMCs groups;
Table I).
 | Table ISummary of BMC analysis. |
Table I
Summary of BMC analysis.
| Group | Cell number
|
|---|
| Erythroid | Myeloid |
|---|
| No treatment | 37.00±6.93 | 56.00±6.93 |
| Bu/Cy | 9.50±5.28 | 8.67±3.50 |
| T-MSCs | 20.00±5.03 | 13.57±5.41 |
| T-MSCs + BMCs | 16.57±5.97 | 35.14±11.96 |
| BMCs | 16.33±5.82 | 11.83±3.92 |
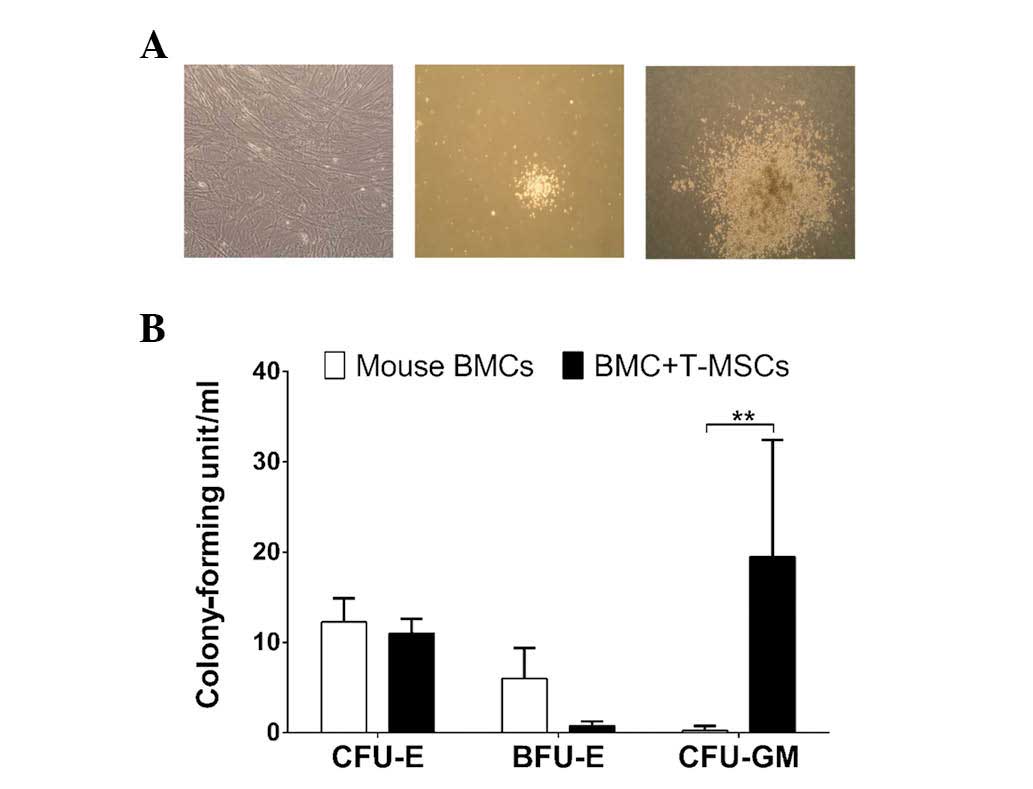
T-MSCs enhanced colony formation in
methylcellulose assay
To determine the effect of T-MSCs on proliferation
and differentiation of BMCs in vitro, a methylcellulose
colony assay (MethoCult®) was performed to evaluate
erythroid and myeloid lineage differentiation from BM cells
(Fig. 4A). Fibroblast-like cells
were grown in MethoCult® dishes with T-MSC-only
conditions and it was observed that the T-MSCs did not
differentiate into hematopoietic lineage cells. For erythroid
lineage cells, there was no significant difference between cells in
the BMCs [colony-forming unit-erythroid (CFU-E), 12.25±2.63 U/ml;
burst-forming unit-erythroid (BFU-E), 6.00±3.37 U/ml] and the
T-MSCs + BMCs groups (CFU-E, 11.00±1.63 U/ml; BFU-E, 0.75±0.50
U/ml). For the myeloid lineage, however, CFU-granulocyte/macrophage
was significantly higher in T-MSCs + BMCs isolated from mice
(19.50±12.92 U/ml) compared with those of BMCs only (0.25±0.50
U/ml; Fig. 4B). Thus, T-MSCs
exerted a greater effect on myeloid differentiation of mice BMCs
than on erythroid lineage cells.
Discussion
In the present study, the effect of human T-MSCs on
BM reconstitution was investigated in a mouse allogeneic BMT model.
Co-transplantation of T-MSCs with BMCs was demonstrated to be
associated with increased myelopoiesis in vivo and in
vitro.
BMT frequently induces GVHD, which results in
engraftment failure; thus, long-term immune suppression treatment
is required to prevent GVHD. Currently, co-transplantation of MSCs
along with HSCs in HSCT or BMT is being actively investigated to
minimize the continuous use of immunosuppressive therapeutic
agents, reduce the incidence of GVHD syndrome and promote allograft
survival (20,21).
To analyze the possible mechanism underlying the
positive effect of T-MSCs on BM reconstruction, PKH26-stained
T-MSCs in the BM were localized following infusion in Bu/Cy-treated
mice. However, PKH-positive cells were not observed in the BM in
these mice (data not shown). Although MSCs have multi-lineage
potential to differentiate into various mature cells in
vitro, they are rarely observed to truly engraft and survive in
damaged host tissue (22). The
present study also aimed to investigate whether T-MSCs
differentiate into hematopoietic cells. The hematopoiesis potential
was analyzed using MethoCult® medium culture, which
indicated that no differentiation of T-MSCs into hematopoietic
lineage cells had taken place, however, myeloid lineage cells (such
as CFU-GM) increased. To evaluate how T-MSCs positively affected BM
reconstruction, the cellular constitution in mouse BM and in
peripheral blood was analyzed. Results of the current study
demonstrated an increase of myeloid lineage cells in the T-MSCs +
BMCs group, which was consistent with the increase of the CFU-GM
colony in the in vitro experiment.
The present study also investigated the quantity of
adipose tissue occupying the medullary space in the BM following
conditioning chemotherapy. Histological findings indicated a
relative increase in adipose tissue in the BM space of mice in the
Bu/Cy chemotherapy group. A recent review indicated that increased
adipocytes in the BM space damage the micro-environmental balance
necessary to maintain hematopoiesis or osteogenesis, and disturb
the interaction between HSCs and other cells (23). The findings of the present study
suggested that increased adipocytes in the BM interrupt BMC
engraftment, which is prevented or attenuated by T-MSCs. Future
studies are required to define a mechanism for the enhancement of
BM reconstitution by T-MSCs and to determine the mechanism by which
the secretory function of T-MSCs is altered in the BM
microenvironment.
Previous studies demonstrated that infused MSCs
exert their effects via secreted trophic signals without
localization within the damaged tissue (24). These regulatory and trophic
factors, which are secreted by MSCs, include cytokines, chemokines
and growth factors that are broadly defined as the MSC secretome
(25). These trophic factors
provide a supportive micro-environment for the regeneration of
injured tissue, and decrease inflammation via modulation of cell
survival, cell renewal, cell differentiation, inflammation and
angiogenesis (2,5,22,26).
The secretome of T-MSCs may be critical in BM reconstitution and
future studies are required to investigate the secretory function
of T-MSCs in vivo.
In conclusion, results from the current study
suggest that the co-transplantation of T-MSCs with HSCs promotes
successful BM engraftment in delayed or defective BM engraftment
patients by enhancing myelopoiesis and possibly
megakaryocytosis.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
which was funded by the Ministry of Education, Science, and
Technology (grant no. 2012M3A9C6049823).
References
|
1
|
Dexter TM, Spooncer E, Schofield R, Lord
BI and Simmons P: Haemopoietic stem cells and the problem of
self-renewal. Blood Cells. 10:315–339. 1984.PubMed/NCBI
|
|
2
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toma C, Pittenger MF, Cahill KS, Byrne BJ
and Kessler PD: Human mesenchymal stem cells differentiate to a
cardiomyocyte phenotype in the adult murine heart. Circulation.
105:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanchez-Ramos J, Song S, Cardozo-Pelaez F,
Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W,
Patel N, et al: Adult bone marrow stromal cells differentiate into
neural cells in vitro. Exp Neurol. 164:247–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim EJ, Kim N and Cho SG: The potential
use of mesenchymal stem cells in hematopoietic stem cell
transplantation. Exp Mol Med. 45:e22013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kern S, Eichler H, Stoeve J, Klüter H and
Bieback K: Comparative analysis of mesenchymal stem cells from bone
marrow, umbilical cord blood, or adipose tissue. Stem Cells.
24:1294–1301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le Blanc K, Samuelsson H, Gustafsson B,
Remberger M, Sundberg B, Arvidson J, Ljungman P, Lönnies H, Nava S
and Ringdén O: Transplantation of mesenchymal stem cells to enhance
engraftment of hematopoietic stem cells. Leukemia. 21:1733–1738.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Macmillan ML, Blazar BR, DeFor TE and
Wagner JE: Transplantation of ex-vivo culture-expanded parental
haploidentical mesenchymal stem cells to promote engraftment in
pediatric recipients of unrelated donor umbilical cord blood:
Results of a phase I–II clinical trial. Bone Marrow Transplant.
43:447–454. 2009. View Article : Google Scholar
|
|
9
|
Joo SY, Cho KA, Jung YJ, Kim HS, Park SY,
Choi YB, Hong KM, Woo SY, Seoh JY, Cho SJ and Ryu KH: Mesenchymal
stromal cells inhibit graft-versus-host disease of mice in a
dose-dependent manner. Cytotherapy. 12:361–370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Le Blanc K, Frassoni F, Ball L, Locatelli
F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger
M, et al: Developmental Committee of the European Group for Blood
and Marrow Transplatation: Mesenchymal stem cells for treatment of
steroid-resistant, severe, acute graft-versus-host disease: A phase
II study. Lancet. 371:1579–1586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Blanc K, Rasmusson I, Sundberg B,
Götherström C, Hassan M, Uzunel M and Ringdén O: Treatment of
severe acute graft-versus-host disease with third party
haploidentical mesenchymal stem cells. Lancet. 363:1439–1441. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dalle Carbonare L, Valenti MT, Zanatta M,
Donatelli L and Lo Cascio V: Circulating mesenchymal stem cells
with abnormal osteogenic differentiation in patients with
osteoporosis. Arthritis Rheum. 60:3356–3365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang GT, Gronthos S and Shi S:
Mesenchymal stem cells derived from dental tissues vs. those from
other sources: Their biology and role in regenerative medicine. J
Dent Res. 88:792–806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blazar BR, Murphy WJ and Abedi M: Advances
in graft-versus-host disease biology and therapy. Nat Rev Immunol.
12:443–458. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Almeida-Porada G, Flake AW, Glimp HA and
Zanjani ED: Cotransplantation of stroma results in enhancement of
engraftment and early expression of donor hematopoietic stem cells
in utero. Exp Hematol. 27:1569–1575. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nolta JA, Hanley MB and Kohn DB: Sustained
human hematopoiesis in immunodeficient mice by cotransplantation of
marrow stroma expressing human interleukin-3: Analysis of gene
transduction of long-lived progenitors. Blood. 83:3041–3051.
1994.PubMed/NCBI
|
|
17
|
Angelopoulou M, Novelli E, Grove JE,
Rinder HM, Civin C, Cheng L and Krause DS: Cotransplantation of
human mesenchymal stem cells enhances human myelopoiesis and
megakaryocytopoiesis in NOD/SCID mice. Exp Hematol. 31:413–420.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryu KH, Cho KA, Park HS, Kim JY, Woo SY,
Jo I, Choi YH, Park YM, Jung SC and Chung SM: Tonsil-derived
mesenchymal stromal cells: Evaluation of biologic, immunologic and
genetic factors for successful banking. Cytotherapy. 14:1193–1202.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SJ, Park MH, Moon HJ, Park JH, Ko Y
and Jeong B: Polypeptide thermogels as a three dimensional culture
scaffold for hepatogenic differentiation of human tonsil-derived
mesenchymal stem cells. ACS Appl Mater Interfaces. 6:17034–17043.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lechler RI, Sykes M, Thomson AW and Turka
LA: Organ transplantation - how much of the promise has been
realized? Nat Med. 11:605–613. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bluestone JA, Thomson AW, Shevach EM and
Weiner HL: What does the future hold for cell-based tolerogenic
therapy? Nat Rev Immunol. 7:650–654. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kupcova Skalnikova H: Proteomic techniques
for characterisation of mesenchymal stem cell secretome. Biochimie.
95:2196–2211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adler BJ, Kaushansky K and Rubin CT:
Obesity-driven disruption of haematopoiesis and the bone marrow
niche. Nat Rev Endocrinol. 10:737–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F,
de Kleijn DP, Choo A and Lim SK: Proteolytic potential of the MSC
exosome proteome: Implications for an exosome-mediated delivery of
therapeutic proteasome. Int J Proteomics. 2012:9719072012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ranganath SH, Levy O, Inamdar MS and Karp
JM: Harnessing the mesenchymal stem cell secretome for the
treatment of cardiovascular disease. Cell Stem Cell. 10:244–258.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Le Blanc K and Pittenger M: Mesenchymal
stem cells: Progress toward promise. Cytotherapy. 7:36–45. 2005.
View Article : Google Scholar : PubMed/NCBI
|