Introduction
Verrucous carcinoma was first reported in 1948 as a
non-metastatic variant of squamous cell carcinoma and its unique
biological behavior has attracted increasing research attention
(1,2). This tumor is frequently prevalent in
the oral cavity; however, it may also occur in the throat,
esophagus, nasal and paranasal sinuses, external genital organs and
on the skin (3,4). Oral verrucous carcinoma (OVC)
proliferates slowly, with a high degree of differentiation and male
smokers >60 years of age are the most commonly affected group
(5). The present treatment options
include surgery, chemotherapy, and radiotherapy or combination
therapy. Although the disease has a good prognosis, the rate of
recurrence is high (6). The
primary etiologic factors for this tumor have been reported to be
tobacco and betel nut chewing, and infection with the human
papilloma virus (7). The genes
encoding vascular endothelial growth factor, E-cadherin and murine
double minute 2 are also important in the development of OVC
(8–11); however, the molecular mechanism
underlying their involvement in its pathogenesis remains to be
elucidated.
A microRNA (miRNA) is an endogenous small non-coding
RNA of ~22 nucleotides in length, which negatively regulates target
genes during translation (12). It
is estimated that one-third of all miRNAs are involved in the
regulation of cellular proliferation, apoptosis, DNA repair and
various other physiological processes (13). Abnormal miRNA expression has been
identified in various types of cancer, including breast, lung,
liver, and head and neck cancer (14). Overexpression of miR-181b, a member
of the miR-181s family, in tumor cells enhanced the growth and
invasive capabilities of the tumor and was able to inhibit the
apoptosis of these tumor cells. Thus, this has led to the
identification of miR-181b as an oncogene in gastric cancer, liver
cancer and oral squamous cell carcinoma (OSCC) (15–17).
In addition, it has been reported that miR-181 is important for the
malignant transformation of oral premalignant lesions (17,18).
It is of note, that there is evidence to the contrary, suggesting
that miR-181 promotes the apoptosis of tumor cells via the
downregulation of Bcl-2 expression levels, which has been has an
anti-apoptotic function in various types of cancer (19,20).
The importance and expression level of miR-181b in OVC requires
further investigation.
B-cell lymphoma 2 (Bcl-2) is an important regulator
of apoptosis by blocking the release of cytochrome c and
inhibiting the caspases (21).
Overexpression of Bcl-2 has been demonstrated in numerous types of
cancer. The leucine-rich repeats and immunoglobulin-like domains 1
(LRIG1) protein may act as a master molecule, that regulates stem
cells in various cancers and is able to inhibit the growth of
different types of cancer. The mechanism underlying its inhibition
of proliferation has been reported to involve the downregulation of
Bcl-2 expression levels (22,23).
LRIG1 is also a target of miR-181b. Previous studies have
determined that miR-181s is important for the apoptotic process of
tumor cells (16). However, this
claim is debatable and the exact molecular mechanism remains to be
elucidated.
The present study investigated the expression of
miR-181b and LRIG1 in OVC, OSCC para-tumor and normal mucosal
tissues. It was determined that decreased expression levels of
LRIG1 and increased expression levels of miR-181b were present in
OVC. In addition, it was observed that expression levels of Bcl-2
were negatively correlated with the expression of LRIG1 in OVC.
Materials and methods
Tissue specimens
The study was approved by the ethics committee of
Central South University (Changsha, China). A total of 30 samples
were obtained from patients between June 2009 to January 2013, all
underwent surgery in the Oral and Maxillofacial Surgery department
at the Xiangya Hospital of Central South University (Changsha,
China). The tumor samples and their corresponding adjacent tissues
were obtained from 6 patients with exogenous OVC (a tumor the
occurs on the surface of the oromaxillo-facial region) and 6
patients with well-differentiated OSCC. Normal tissues were
obtained from 6 patients with maxillofacial trauma. The diagnosis
was conducted by two independent pathologists. The patients did not
receive preoperative medication and all were informed of the
preoperative surgical planning and purpose of the experiment.
Informed consent for the present study was obtained from each
participant. In the OVC group, there were 4 males and 2 females
with a mean age of 51±12.8 years, in the OSCC group, there were 5
males and 1 female with mean age of 48±14.6 years, in the NM group,
there were 4 males and 2 females with mean age of 31±9.4 years. All
tissue samples were immediately frozen in liquid nitrogen and
stored at −80°C until used.
A total of 105 paraffin-embedded samples dating
between 1996 and 2014 were obtained from archival specimens in the
Department of Pathology, Xiangya Hospital, Central South University
for immunohistochemistry (IHC), this included 15 OVC and 30
well-differentiated OSCC samples (including tumor samples and
adjacent para-tumor tissues) and 15 normal oral mucosa samples. In
the OVC group, there were 11 males and 4 females with a mean age of
54±11.3 years, in the OSCC group, there were 26 males and 4 females
with a mean age of 57±9.3 years, in the NM group, there were 6
males and 9 females with a mean age of 35±13.3 years.
Total RNA extraction and reverse
transcription (RT)
Total RNA was extracted from patient and control
tissues using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) following the manufacturer's protocol. The
concentration and quality of the RNA was determined using a
Nanodrop 2000 spectrophotometer. First-strand cDNA was synthesized
using 2 µg of total RNA and M-MLV reverse transcriptase
(Promega Corporation, Madison, WI, USA). A DNase step was performed
using DNase I (Invitrogen; Thermo Fisher Scientific, Inc.) Specific
Bulge-Loop miRNA qPCR primers were obtained from RiboBio Co., Ltd.
(Guangzhou, China).
Quantitative polymerase chain reaction
(qPCR)
The expression levels of miR-181b and the internal
reference gene U6 were determined using SYBR Premix Ex Taq II
(Takara Biotechnology Co., Ltd., Dalian, China) on a TP800
thermocycler. The total reaction volume was 20 µl and was
composed of 10 µl SYBR Master Mixture, 0.5 µl forward
primer, 0.5 µl reverse primer, 1 µl cDNA and 8
µl ddH2O. The conditions for the reaction were:
95°C for 10 min, 95°C for 15 s, 55°C for 30 s, 70°C for 30 sec, 40
cycles from step 2. The fluorescence signal was detected at 70°C.
The relative quantity of gene expression was calculated using the
2‒ΔΔCq formula (24).
The following primers were used for qPCR: miR-181b, forward
5′-CTTGGTACCGAGCTCTCCTAGAGCTCTGTTCGCCT-3′, reverse
5′-TGCTGGATATCTGCACGAACATTCACATGAGGGCG-3′; and U6, forward
5′-CTCGCTTCGGCAGCACA-3′, reverse 5′-AACGCTTCACGAATTTGCGT-3′.
IHC staining
Slices of ~4 µm were cut and dried at 60°C
for 2.5 h, then dewaxed and hydrated in accordance with routine
procedures. The slices were boiled in 0.01 M citric acid buffer
solution (pH 6.0) for 90 sec at high pressure. Droplets of 3%
hydrogen peroxide were placed on the slice and left standing at
37°C temperature for 20 min to remove endogenous peroxidase. The
tissues were then blocked for non-specific binding with 10% normal
goat serum (OriGene Technologies, Inc., Beijing, China) for 1 h at
37°C. Incubation with primary antibodies was performed overnight at
4°C, the antibodies used were as follows: Monoclonal mouse
anti-Bcl-2 (1:200, Cell Signaling Technology, Danvers, MA, USA;
cat. no. 15071S), polyclonal rabbit anti-LRIG1 (1:150; Abcam,
Cambridge, UK; cat. no. ab36707). Subsequently, a secondary
biotinylated IgG antibody solution (cat. no. KIT-9710; Fuzhou
Maixin Biotech Co., Ltd., Fuzhou, China) and an
avidin-biotin-peroxidase reagent (OriGene Technologies, Inc.) were
used to incubate the slices. The color was developed with
3,3′-diaminobenzidine and counterstained with hematoxylin. The
slices were washed with phosphate-buffered saline (PBS). An
incubation with PBS instead of the antibody was used for the
negative control.
Staining evaluation
Each slice was scanned using an Aperio ScanScope CS
scanner (Leica Microsystems, Inc., Buffalo Grove, IL, USA) with
background subtraction. Aperio Quantification software version 9.1
(Leica Microsystems, Inc.) was used to quantify nuclear, membrane,
or total expression levels (25).
Epithelial and cancerous areas were selected for quantification.
The expression scores (3+, 2+ and 1+ indicated strong, medium and
weak positive staining, respectively) of nuclear and membrane
staining were calculated as a percentage of the positive cells
using the following formula: (3+)×3+(2+)×2+(1+)×1. The expression
scores of total quantification was scored as total intensity or
total cell number (26). The
standard controls were provided by Aperio to set the threshold for
scanning of the positive cells (26–28).
Statistical analysis
Data were analyzed using the GraphPad Prism
software, version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
The difference in the immunostaining between groups was determined
using one-way analysis of variance followed by Tukey's or
Bonferroni post-hoc tests for multiple comparisons. The correlation
between the expression levels of LRIG1 and Bcl-2 was determined
using two-tailed Pearson's correlation, subsequent to confirmation
that the data had a Gaussian distribution. All data were expressed
as the mean ± standard error and P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-181b is overexpressed in OVC
tissue
The expression level of miR-181b was evaluated using
qRT-PCR and was significantly greater in tissue samples from
patients with OVC (P<0.05) and OSCC (P<0.01) when compared
with the NM tissues (Fig. 1).
However, expression levels of miR-181b in OVC samples were
significantly reduced when compared with OSCC samples (P<0.05).
No significant difference was identified between the expression
levels of miR-181b in the adjacent para-tumor OVC (para-OVC) and
para-tumor OSCC (para-OSCC) tissue samples (Fig. 1; P>0.05); however, it was lower
in comparison with to the tumor tissue samples. In addition,
miR-181b expression levels were significantly greater in para-OSCC
tissues when compared with NM (P<0.01). These findings indicated
that the aberrant expression of miR-181b may be important for the
initiation of OVC and its malignant progression.
 | Figure 1Expression of miR-181b in human NM,
OVC and OSCC tissues. miR-181b expression in OVC and OSCC tissue
samples was evaluated using reverse transcription-polymerase chain
reaction and normalized using the expression level of U6.
*P<0.05, OVC vs. NM; **P<0.01, OSCC vs.
NM, para-OSCC vs. NM; #P<0.05, OVC vs. OSCC. miR,
microRNA; NM, normal oral mucosa; OVC, oral verrucous carcinoma;
para-OVC, para-tumor tissues of oral verrucous carcinoma; OSCC,
oral squamous cell carcinoma; Para-OSCC, para-tumor tissues of oral
squamous cell carcinoma. |
Bcl-2 is upregulated in OVC tissue
miR-181b has been demonstrated to be important for
the apoptosis of tumor cells and Bcl-2 is an established target of
miR-181b (15). Therefore, Bcl-2
expression was also subsequently examined by IHC staining (Fig. 2) in order to determine whether
miR-181b targeted Bcl-2, which is an important anti-apoptotic
protein (29). IHC analysis
indicated that the expression level of Bcl-2 in NM tissues was
significantly reduced when compared with OVC (P<0.05; Fig. 2D) and OSCC (P<0.01; Fig. 2D) tissues. In addition, Bcl-2
protein levels in OVC and OSCC tissues were significantly higher
when compared with their corresponding adjacent para-tumor tissues
(para-OVC vs. OVC, P<0.05 and para-OSCC vs. OSCC, P<0.05;
Fig 2D). Bcl-2 expression levels
in OVC tissue samples were significantly higher compared with NM
tissues (P<0.05; Fig. 2D).
However, they were significantly lower when OSCC tissue samples
were compared with OVC tissues (P<0.05; Fig. 2D).
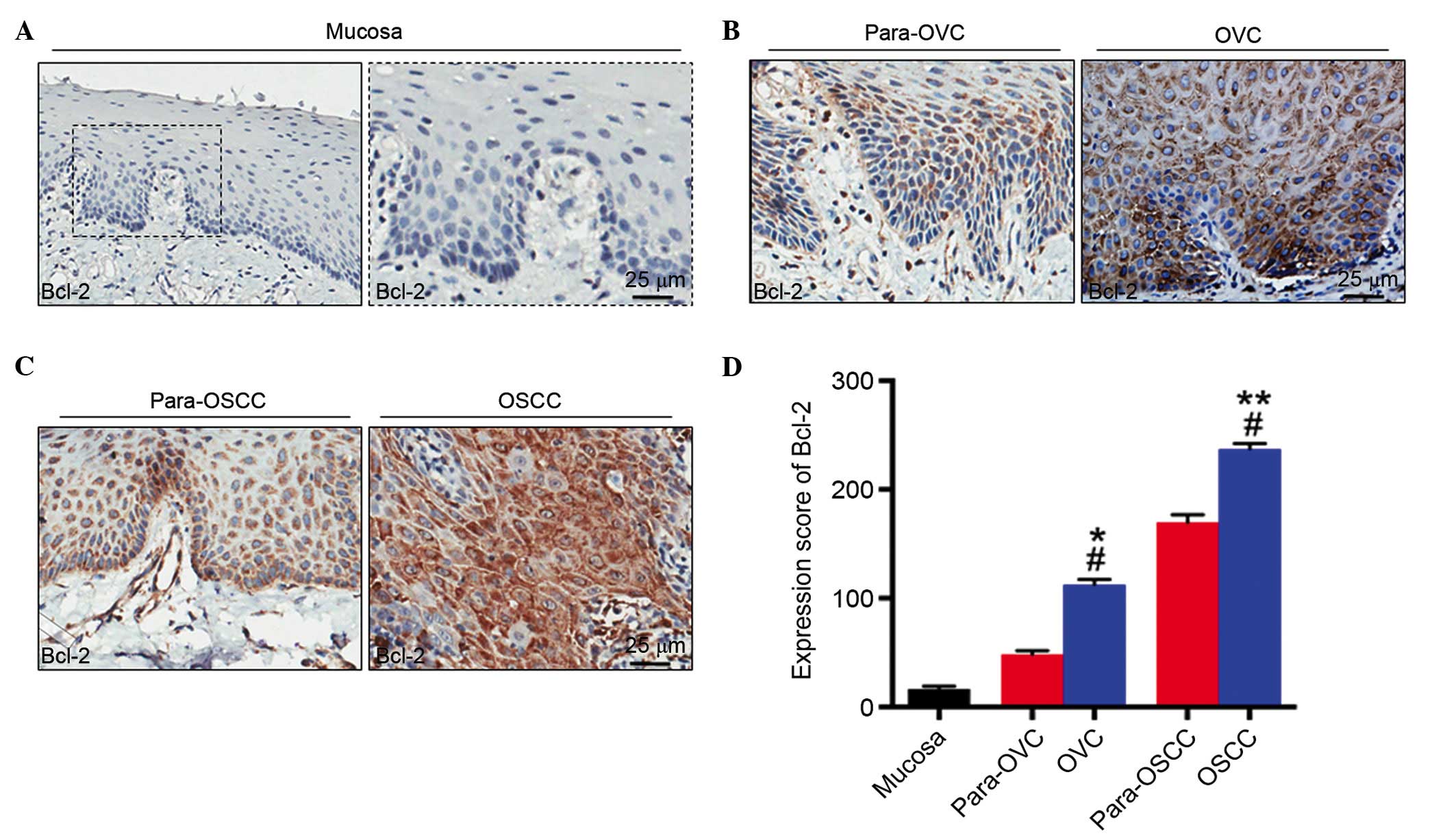 | Figure 2IHC staining of Bcl-2 in human (A)
NM, (B) OVC and (C) OSCC. (D) Semi-quantitative analysis of
histoscore of Bcl-2 expression in human NM, OVC and OSCC tissues.
Bcl-2 expression was absent in NM tissues; however, it was
expressed predominantly in the cytoplasm of OVC and OSCC cells. In
NM, OVC and OSCC, the expression of Bcl-2 intensity increased.
*P<0.05, OVC vs. NM; **P<0.01, OSCC vs.
NM; #P<0.05, para-OVC vs. OVC, para-OSCC vs. OSCC,
OVC vs. OSCC. Magnification, x400. NM, normal oral mucosa; OVC,
oral verrucous carcinoma; para-OVC, para-tumor tissues of oral
verrucous carcinoma; OSCC, oral squamous cell carcinoma; Para-OSCC,
para-tumor tissues of oral squamous cell carcinoma; Bcl-2, B-cell
lymphoma 2; IHC, immunohistochemistry. |
Downregulation of LRIG1 expression in OVC
tissue
The expression levels of LRIG1 in OVC and OSCC were
investigated by staining the mucosa, including OVC and OSCC tissue
samples with an antibody targeting LRIG1 (Fig. 3). The expression was quantified by
scoring the intensity of staining and the results demonstrated that
the expression of LRIG1 in NM tissues was significantly greater
when compared with OVC (P<0.05; Fig. 3D) and OSCC (P<0.01; Fig. 3D) tissue samples. In addition, the
expression levels of LRIG1 in para-OVC and para-OSCC samples were
significantly higher compared with the tumor tissues (para-OVC vs.
OVC, P<0.05 and para-OSCC vs. OSCC, P<0.01; Fig. 3D). These results support the
hypothesis that LRIG1 may act as a potential tumor suppressor gene
in OVC and OSCC tissues.
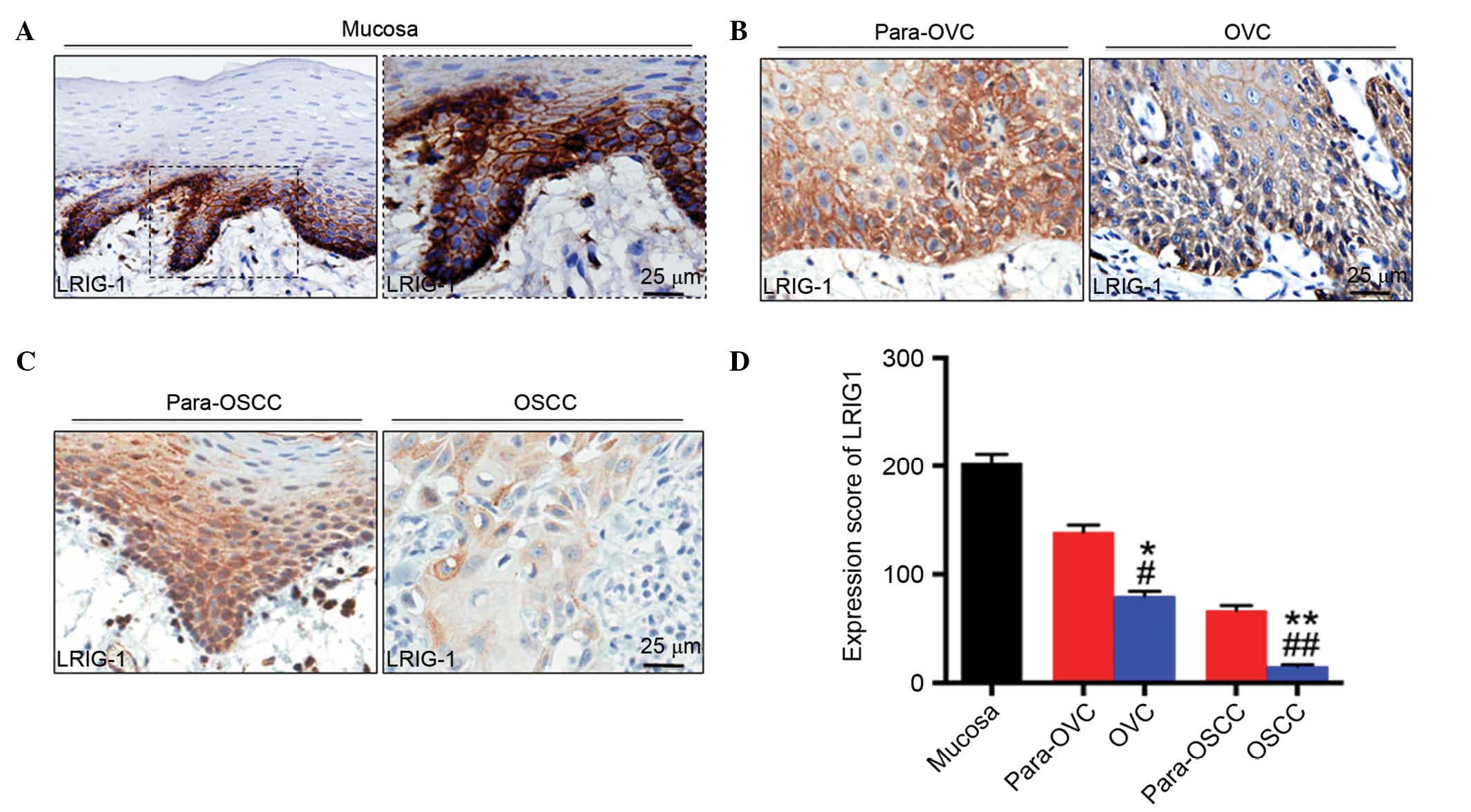 | Figure 3IHC staining of LRIG1 in human (A)
NM, (B) OVC and (C) OSCC tissues. Representative IHC staining
determined that LRIG1 was primarily located in the cell membrane
and the cytoplasm of the tissues. Magnification, ×400. (D)
Semi-quantitative analysis of histoscore of LRIG1 expression in
human NM, OVC and OSCC tissues. In NM, OVC and OSCC, the expression
of LRIG1 intensity was decreased. *P<0.05, OVC vs.
NM; **P<0.01, OSCC vs. NM; #P<0.05,
para-OVC vs. OVC; ##P<0.01, para-OSCC vs. OSCC, OVC
vs. OSCC. NM, normal oral mucosa; OVC, oral verrucous carcinoma;
para-OVC, para-tumor tissues of oral verrucous carcinoma; OSCC,
oral squamous cell carcinoma; para-OSCC, para-tumor tissues of oral
squamous cell carcinoma; LRIG1, leucine rich repeats and
immunoglobulin like domains 1. |
Correlation of LRIG1 and Bcl-2 expression
in NM, OVC and OSCC tissues
The association between Bcl-2 and LRIG1 expression
was determined using a two-tailed Pearson's correlation. The
results indicated that LRIG1 expression was negatively correlated
with Bcl-2 expression in NM, OVC and OSCC tissue samples (Fig. 4; r=−0.8752; P<0.001).
Discussion
The first miRNA was characterized in
Caenorhabditis elegans and reported in 1993, subsequently
thousands of miRNAs have been identified (30). miRNAs regulate the expression of
their target mRNAs via complementarily binding within the
3′-untranslated region of the target mRNA. This binding results in
the degradation of the mRNA under conditions of perfect
complementarity, or the inhibition of translation under conditions
of partial complementarity (31).
Abnormal miRNA expression of has been indicated in numerous types
of cancer, including glioma, gastric, liver, prostate, head and
neck cancer and OSCC. In terms of their function in cancer
pathology miRNAs may be divided into two types, a number act as
tumor promoters and others as tumor suppressors; however, both are
involved in regulation of tumor cell proliferation, invasion and
apoptosis (32). The miR-181s
family members include miR-181a, miR-181b, miR-181c and miR-181d
(33), their abnormal expression
has been reported in various tumors. Upregulation of miR-181b may
promote tumor cell invasion and metastasis in breast cancer
(34). In hepatocellular
carcinoma, the upregulation of miR-181b expression levels has been
indicated to enhance the invasion, metastasis and strengthen the
resistance to antitumor therapeutic agents (15).
The expression of miR-181b in OSCC tissues was
higher and correlated with tumor lymph node metastasis and
invasiveness in a previous study by Yang et al (20), indicating that miR-181b may have
oncogenic potential (20). In the
present study, miR-181b was upregulated in OVC, consistent with the
previous finding in OSCC (20).
However, no significant difference was identified in the expression
level of miR-181b in tumor tissues compared with samples from
adjacent tissues. This may be due to the exposure of the
neighboring cells to same microenvironment and may indicate the
importance of miR-181b in the progression of malignancy. The
expression levels of miR-181b in OVC tissue were significantly
lower compared with OSCC tissue. The expression levels of miR-181b
in tumor-adjacent OSCC tissue samples were significantly different
compared with NM tissue. These results suggest that miR-181b
expression may be associated with the degree of malignancy, which
is in agreement with previous studies in oral leukoplakia and
dysplastic lesions (17,18), as OVC is regarded as a low-grade
malignant tumor, which is less prone to invasion and metastasis and
has a better prognosis, in comparison with OSCC.
Bcl-2 has been identified as a target of miR-181b.
In addition, it has been observed that the downregulation of
miR-181b was inversely correlated with an increase in the protein
levels of BCL2 family apoptosis regulator (MCL1) and Bcl-2, which
are target genes in chronic lymphocytic leukemia (35). In astrocytes, miR-181 affects the
cellular apoptosis and mitochondrial function by targeting multiple
Bcl-2 family members, including BCL2 like 11, MCL1 and Bcl-2
(36,37). It has been reported that miR-181b
acts as a tumor suppressor gene in gliomas and that its
downregulation may lead to tumor growth, inhibit tumor cell
invasion and promote apoptosis (38). However, a previous study
demonstrated that the overexpression of miR-181a resulted in
significant downregulation of the Bcl-2 protein levels in malignant
glioma cells exposed to radiation treatment; thus, miR-181 may be a
potential target for enhancing the effect of radiotherapy by
regulating Bcl-2 expression (19).
In addition, the overexpression of miR-181b in gastric cancer may
enhance migration and invasion of gastric cancer cells, thus
promoting the occurrence and development of gastric cancer
(16). Through employing the
miRanda online database (www.microrna.org/microrna/getMirnaForm.do), LRIG1
was identified as a potential target of miR-181b. A previous study
has determined that LRIG1 is a tumor suppressor gene and its
expression is correlated with the grade of the malignancy (23). The present study highlighted
consistent trends in expression of miR-181b and Bcl-2 in OVC and
OSCC tissues, where their expression levels were significantly
higher compared with NM tissue. This is consistent with previous
studies by Singh et al (39) and Su et al (40) in OSCC tissues. However, this is not
consistent with the results of previous study in chronic lymphoid
leukemia (35). In addition, the
present study determined that LRIG1 expression is important in OVC
and OSCC tissues. Bioinformatics prediction has indicated LRIG1 is
one of the target genes of miR-181b, in addition to Bcl-2.
LRIG1 expression has also been associated with Bcl-2
expression in human ependymomas (41). This may suggest the existence of an
alternative regulation pathway. Therefore, it is possible that
miR-181b may indirectly regulate Bcl-2 expression via a signaling
pathway that involves LRIG1. The current study determined that
there were decreased expression levels of LRIG1 in OVC tissues. In
addition, the expression levels of Bcl-2 were negatively correlated
with LRIG1 expression levels in NM, OVC and OSCC tissues. LRIG1 may
be have anti-apoptotic functions in OVC. Initially, it was
determined that miR-181b had increased expression levels in OVC.
The specific molecular mechanism that is responsible for the
changes in miR-181b and LRIG1 expression levels requires further
experimental verification.
In conclusion, the expression of miR-181b and Bcl-2
in OVC tissues was significantly higher compared with NM tissue;
however lower when OVC was compared with OSCC tissues. The
expression levels of the target of miR-181b, LRIG1, in OVC tissue
were significantly reduced when compared with NM. However, LRIG1
expression levels in were determined to be higher in OVC tissues
compared with OSCC tissues. The expression levels of Bcl-2 were
negatively correlated with the expression of LRIG1 in NM, OVC and
OSCC tissues. Therefore, LRIG1 may have an anti-apoptotic function
in OVC.
Acknowledgments
The present study was supported by the Fundamental
Research Funds for the Central Universities of Central South
University (grant nos. 2012zzts135 and 2016zzts117), The Nature
Sciences Foundation of Qinghai Province (grant no. 2013-z-908) and
The Nature Sciences Foundation of Hunan Province (grant no.
S2013J504B).
References
|
1
|
Ackerman LV: Verrucous carcinoma of the
oral cavity. Surgery. 23:670–678. 1948.PubMed/NCBI
|
|
2
|
Arduino PG, Carrozzo M, Pagano M, Gandolfo
S and Broccoletti R: Verrucous oral carcinoma: Clinical findings
and treatment outcomes in 74 patients in Northwest Italy. Minerva
Stomatol. 57:335–339. 339–341. 2008.PubMed/NCBI
|
|
3
|
Impola U, Uitto VJ, Hietanen J, Hakkinen
L, Zhang L, Larjava H, Isaka K and Saarialho-Kere U: Differential
expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9) and
metalloelastase (MMP-12) in oral verrucous and squamous cell
cancer. J Pathol. 202:14–22. 2004. View Article : Google Scholar
|
|
4
|
Medina JE, Dichtel W and Luna MA:
Verrucous-squamous carcinomas of the oral cavity. A
clinicopathologic study of 104 cases. Arch Otolaryngol.
110:437–440. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walvekar RR, Chaukar DA, Deshpande MS, Pai
PS, Chaturvedi P, Kakade A, Kane SV and D'Cruz AK: Verrucous
carcinoma of the oral cavity: A clinical and pathological study of
101 cases. Oral Oncol. 45:47–51. 2009. View Article : Google Scholar
|
|
6
|
Yeh CJ: Treatment of verrucous hyperplasia
and verrucous carcinoma by shave excision and simple cryosurgery.
Int J Oral Maxillofac Surg. 32:280–283. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel KR, Chernock RD, Zhang TR, Wang X,
El-Mofty SK and Lewis JS Jr: Verrucous carcinomas of the head and
neck, including those with associated squamous cell carcinoma, lack
transcriptionally active high-risk human papillomavirus. Hum
Pathol. 44:2385–2392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quan H, Tang Z, Zhao L, Wang Y, Liu O, Yao
Z and Zuo J: Expression of αB-crystallin and its potential
anti-apoptotic role in oral verrucous carcinoma. Oncol Lett.
3:330–334. 2012.PubMed/NCBI
|
|
9
|
Lin HP, Wang YP and Chiang CP: Expression
of p53, MDM2, p21, heat shock protein 70, and HPV 16/18 E6 proteins
in oral verrucous carcinoma and oral verrucous hyperplasia. Head
Neck. 33:334–340. 2011.
|
|
10
|
Wang YH, Tian X, Liu OS, Fang XD, Quan HZ,
Xie S, Gao S and Tang ZG: Gene profiling analysis for patients with
oral verrucous carcinoma and oral squamous cell carcinoma. Int J
Clin Exp Med. 7:1845–1852. 2014.PubMed/NCBI
|
|
11
|
Liu O, Zhang H, Wang Y, Quan H, Zhang J,
Zhou J, Zuo J, Tang J, Fang X, Wang W, et al: Stereology study of
oral verrucous carcinoma. J Buon. 17:343–349. 2012.PubMed/NCBI
|
|
12
|
Gomes CC and Gomez RS: MicroRNA and oral
cancer: Future perspectives. Oral Oncol. 44:910–914. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allegra A, Alonci A, Campo S, Penna G,
Petrungaro A, Gerace D and Musolino C: Circulating microRNAs: New
biomarkers in diagnosis, prognosis and treatment of cancer
(review). Int J Oncol. 41:1897–1912. 2012.PubMed/NCBI
|
|
14
|
Etheridge A, Lee I, Hood L, Galas D and
Wang K: Extracellular microRNA: A new source of biomarkers. Mutat
Res. 717:85–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang B, Hsu SH, Majumder S, Kutay H, Huang
W, Jacob ST and Ghoshal K: TGFbeta-mediated upregulation of hepatic
miR-181b promotes hepatocarcinogenesis by targeting TIMP3.
Oncogene. 29:1787–1797. 2010. View Article : Google Scholar
|
|
16
|
Guo JX, Tao QS, Lou PR, Chen XC, Chen J
and Yuan GB: miR-181b as a potential molecular target for
anticancer therapy of gastric neoplasms. Asian Pac J Cancer Prev.
13:2263–2267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cervigne NK, Reis PP, Machado J, Sadikovic
B, Bradley G, Galloni NN, Pintilie M, Jurisica I, Perez-Ordonez B,
Gilbert R, et al: Identification of a microRNA signature associated
with progression of leukoplakia to oral carcinoma. Hum Mol Genet.
18:4818–4829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brito JA, Gomes CC, Guimarães AL, Campos K
and Gomez RS: Relationship between microRNA expression levels and
histopathological features of dysplasia in oral leukoplakia. J Oral
Pathol Med. 43:211–216. 2014. View Article : Google Scholar
|
|
19
|
Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P
and Hu W: MicroRNA-181a sensitizes human malignant glioma U87MG
cells to radiation by targeting Bcl-2. Oncol Rep. 23:997–1003.
2010.PubMed/NCBI
|
|
20
|
Yang CC, Hung PS, Wang PW, Liu CJ, Chu TH,
Cheng HW and Lin SC: miR-181 as a putative biomarker for lymph-node
metastasis of oral squamous cell carcinoma. J Oral Pathol Med.
40:397–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barinaga M: Death by dozens of cuts.
Science. 280:32–34. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding J, Liu B, He Y, Yuan X, Tian D, Ji B,
Wang L, Wu L, Dong H, Wang J, et al: LRIG1 improves
chemosensitivity through inhibition of BCL-2 and MnSOD in
glioblastoma. Cell Biochem Biophys. 71:27–33. 2015. View Article : Google Scholar
|
|
23
|
Xu Y, Soo P, Walker F, Zhang HH, Redpath
N, Tan CW, Nicola NA, Adams TE, Garrett TP, Zhang JG and Burgess
AW: LRIG1 extracellular domain: Structure and function analysis. J
Mol Biol. 427:1934–1948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Huang CF, Zhang L, Ma SR, Zhao ZL, Wang
WM, He KF, Zhao YF, Zhang WF, Liu B and Sun ZJ: Clinical
significance of Keap1 and Nrf2 in oral squamous cell carcinoma.
PLoS One. 8:e834792013. View Article : Google Scholar
|
|
26
|
Sun ZJ, Zhang L, Hall B, Bian Y, Gutkind
JS and Kulkarni AB: Chemopreventive and chemotherapeutic actions of
mTOR inhibitor in genetically defined head and neck squamous cell
carcinoma mouse model. Clin Cancer Res. 18:5304–5313. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bian Y, Hall B, Sun ZJ, Molinolo A, Chen
W, Gutkind JS, Waes CV and Kulkarni AB: Loss of TGF-β signaling and
PTEN promotes head and neck squamous cell carcinoma through
cellular senescence evasion and cancer-related inflammation.
Oncogene. 31:3322–3332. 2012. View Article : Google Scholar
|
|
28
|
Ma SR, Wang WM, Huang CF, Zhang WF and Sun
ZJ: Anterior gradient protein 2 expression in high grade head and
neck squamous cell carcinoma correlated with cancer stem cell and
epithelial mesenchymal transition. Oncotarget. 6:8807–8821. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu W, Shan X, Wang T, Shu Y and Liu P:
miR-181b modulates multidrug resistance by targeting BCL2 in human
cancer cell lines. Int J Cancer. 127:2520–2529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu BH, Xiong XP, Jia J and Zhang WF:
MicroRNAs: New actors in the oral cancer scene. Oral Oncol.
47:314–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lynam-Lennon N, Maher SG and Reynolds JV:
The roles of microRNA in cancer and apoptosis. Biol Rev Camb Philos
Soc. 84:55–71. 2009. View Article : Google Scholar
|
|
33
|
Henao-Mejia J, Williams A, Goff LA, Staron
M, Licona-Limón P, Kaech SM, Nakayama M, Rinn JL and Flavell RA:
The microRNA miR-181 is a critical cellular metabolic rheostat
essential for NKT cell ontogenesis and lymphocyte development and
homeostasis. Immunity. 38:984–997. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Neel JC and Lebrun JJ: Activin and TGFβ
regulate expression of the microRNA-181 family to promote cell
migration and invasion in breast cancer cells. Cell Signal.
25:1556–1566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Visone R, Veronese A, Rassenti LZ, Balatti
V, Pearl DK, Acunzo M, Volinia S, Taccioli C, Kipps TJ and Croce
CM: miR-181b is a biomarker of disease progression in chronic
lymphocytic leukemia. Blood. 118:3072–3079. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ouyang YB, Lu Y, Yue S and Giffard RG:
miR-181 targets multiple Bcl-2 family members and influences
apoptosis and mitochondrial function in astrocytes. Mitochondrion.
12:213–219. 2012. View Article : Google Scholar :
|
|
37
|
Lu F, Zhang J, Ji M, Li P, Du Y, Wang H,
Zang S, Ma D, Sun X and Ji C: miR-181b increases drug sensitivity
in acute myeloid leukemia via targeting HMGB1 and Mcl-1. Int J
Oncol. 45:383–392. 2014.PubMed/NCBI
|
|
38
|
Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu
Z and You Y: hsa-mir-181a and hsa-mir-181b function as tumor
suppressors in human glioma cells. Brain Res. 1236:185–193. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singh BB, Chandler FW Jr, Whitaker SB and
Forbes-Nelson AE: Immunohistochemical evaluation of bcl-2
oncoprotein in oral dysplasia and carcinoma. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 85:692–698. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Su L, Wang Y, Xiao M, Lin Y and Yu L:
Up-regulation of survivin in oral squamous cell carcinoma
correlates with poor prognosis and chemoresistance. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 110:484–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yi W, Haapasalo H, Holmlund C, Järvelä S,
Raheem O, Bergenheim AT, Hedman H and Henriksson R: Expression of
leucine-rich repeats and immunoglobulin-like domains (LRIG)
proteins in human ependymoma relates to tumor location, WHO grade
and patient age. Clin Neuropathol. 28:21–27. 2009. View Article : Google Scholar : PubMed/NCBI
|


















