Introduction
Atherosclerosis (AS) is a multifactorial
pathological process of arterial vasculature undergoing gradual
intima thickening, causing decreased elasticity and narrowing. The
causal association between AS and cholesterol metabolism is well
established. The appearance of lipid accumulation and foam cells is
a characteristic histological finding in early AS lesions (1,2).
Oxidized low-density lipoprotein (oxLDL) is highly
proinflammatory and has a key role in the generation and
progression of AS. OxLDL binds to β2-glycoprotein I (β2GPI) and
C-reactive protein (CRP), resulting in the formation of covalently
stable complexes, such as CRP/oxLDL, oxLDL/β2GPI and
CRP/oxLDL/β2GPI. β2GPI is a highly glycosylated plasma protein,
which serves as the main specific antigen for anti-phospholipid
antibodies associated with atherothrombotic complications
frequently observed in patients with systemic autoimmune diseases
(3). OxLDL interacts with β2GPI
via 7-ketocholesterol, which bears a w-carboxyl acyl chain, to form
a covalent oxLDL/β2GPI complex (4,5). CRP
is an acute-phase reactant that has a major role in innate immunity
and inflammation. Multiple clinical studies have demonstrated the
predictive value of increased CRP levels for atherothrombotic
events, suggesting that CRP is a serological marker for AS
(6). CRP is able to bind to oxLDL
but not to native LDL through the recognition of a
phosphorylcholine moiety in oxLDL (7).
Accumulating evidence suggested the participation of
these complexes in AS-associated processes (8,9). In
auto-immune diseases, such as anti-phospholipid syndrome (APS),
oxLDL/β2GPI and its antibody were found in the intima of AS lesions
and the specific immune complexes were taken up avidly by
macrophages via anti-β2GPI auto-antibody-mediated phagocytosis
(8). However, in the serum of
diabetics, anti-β2GPI auto-antibody levels were found to be low
(10), while oxLDL/β2GPI,
CRP/oxLDL and CRP/oxLDL/β2GPI complexes were present at high
concentrations and were all positively correlated with the
thickness of the intima media, a sensitive index for cardiovascular
disease (11). Direct evidence for
the involvement of CRP and β2GPI in AS was provided by
immunohistochemical staining, which demonstrated the presence of
CRP as well as β2GPI co-localized with oxLDL in human AS lesions
(12). A previous study by our
group reported that the CRP/oxLDL/β2GPI complex aggravated AS
progression in diabetic BALB/c mice, which may be mediated through
the p38/mitogen-activated protein kinase (MAPK) pathway (9). However, in vitro, the roles of
these oxLDL complexes in the onset and development of AS in
diabetes have not been elucidated. The present study aimed to
investigate the impact of various oxLDL complexes on lipid
accumulation and inflammatory reactions in RAW264.7 macrophages
under hyperglycemic conditions.
Materials and methods
Materials and reagents
The RAW264.7 mouse macrophage cell line was
purchased from the American Type Culture Collection (ATCC no
TIB-71; Manassas, VA, USA). Dulbecco's modified Eagle's medium
(DMEM) and fetal bovine serum (FBS) were purchased from Beijing
Solarbio Science & Technology Co., Ltd. (Beijing, China). oxLDL
was purchased from XINYUANJIAHE Biotechnology Co., Ltd. (Beijing,
China). CRP was purchased from ProSpec (cat no. pro-557; Rehovot,
Israel). Oil Red O and hematoxylin were purchased from
Sigma-Aldrich (St. Louis, MO, USA). An intracellular total
cholesterol assay kit was purchased from Applygen Techonology Co.,
Ltd (Beijing, China). Interleukin (IL)-1β, IL-6 and tumor necrosis
factor (TNF)-α ELISA kits were purchased from Uscn Life Science
Inc. (Hubei, China). The TRIzol reagent, RevertAid™ First Strand
cDNA Synthesis kit and Micro Bicinchoninc Acid (BCA) Protein Assay
kit were purchased from Invitrogen Life Technologies, Inc.
(Carlsbad, CA, USA). SYBR® Premix Ex TaqTM DNA
polymerase were purchased from Takara Bio. Inc. (Otsu, Japan).
Rabbit monoclonal antibodies to p38/MAPK, phosphorylated
(p)-p38MAPK (Thr180/Tyr182), nuclear factor (NF)-κB (p65) and
p-NF-κB (Ser 276) were purchased from Cell Signaling Technology,
Inc. (Beverly, MA, USA). Mouse monoclonal antibody to β-actin and
horseradish peroxidase (HRP)-labeled goat anti-rabbit and
anti-mouse immunoglobulin (Ig)G antibody were purchased from
Tianjin Sungene Biotec Co., Ltd (Tianjin, China). High-performance
chemiluminescence kit was purchased from Beijing ComWin Biotech
Co., Ltd. (Beijing, China).
Purification of β2GPI
β2GPI was purified from normal human plasma as
described previously (9). The
blood was donated by a healthy voluntary blood donor at the
Metabolic Diseases Hospital of Tianjin Medical University (Tianjin,
China) in April 2014. The present study was approved by the ethics
committee of Metabolic Diseases Hospital fo Tianjin Medical
University. Written informed consent was obtained form the healthy
blood donor. Plasma β2GPI was precipitated using 3% (v/v)
perchloric acid (Sigma-Aldrich) and isolated by Heparin-Sepharose
affinity chromatography (HiTrap Heparin; GE Healthcare, Little
Chalfont, UK). Liquid Chromatography-Mass Spectrometric analysis
(1100LC/MSD; Agilent Technologies, Santa Clara, CA, USA) was used
to confirm this protein. The purity of β2GPI was confirmed by
SDS-PAGE using a 10% Tris-glycine gel. The SDS-PAGE analysis of the
protein sample showed an identical band to that of the standard
β2GPI sample (Crystal Chem, Inc., Downers Grove, IL, USA). The
purified β2GPI was tested to exclude the possibility of
lipopolysaccharide contamination using the Limulus ES-II Test
(13,14). Briefly, 250 µg human β2GPI
was incubated with an extraction solution of chloroform/methanol
(2:1) for 2 h at 25°C with constant mixing on a rotator. Following
centrifugation, the aqueous supernatant containing lipid-free β2GPI
was retained. All treated preparations were sterilized using a 0.2
µm filter and tested for endotoxin activity using a
commercial Limulus amebocyte lysate assay (Associates of Cape Cod,
Inc., East Falmouth, MA, USA), according to the manufacturer's
protocol. The lower limit of endotoxin detection of the LAL assay
was 0.005 endotoxin units/ml. The BCA method was used to determine
the concentration of β2GPI.
Complex preparation and
identification
The preparation of CRP/oxLDL, oxLDL/β2GPI and
CRP/oxLDL/β2GPI complexes was performed as described previously
(9,11,15).
Briefly, CRP/oxLDL and oxLDL/β2GPI complexes were pre-formed by
incubating oxLDL [1 mg apolipoprotein B (apoB) equivalent/ml] and
β2GPI (1 mg/ml) in the absence of CaCl2, as well as
oxLDL (1 mg apoB equivalent/ml) and CRP (1 mg/ml) in the presence
of 2 mM CaCl2, at 37°C for 16 h. Subsequently, the
purified oxLDL/β2GPI (1 mg/ml of apoB equivalent) was further
incubated with CRP (0.1 mg/ml) in the presence of 2 mM
CaCl2 at 37°C for another 16 h to form the covalent
CRP/oxLDL/β2GPI complex. The identity of the oxLDL/β2GPI, CRP/oxLDL
and CRP/oxLDL/β2GPI complexes was confirmed by ELISAs as described
previously (9,11).
Cell culture
RAW264.7 macrophages were cultured in DMEM with 25
mmol/l glucose (Beijing Solarbio Science & Technology Co.,
Ltd.), which was supplemented with 10% (v/v) FBS, 100 U/ml
penicillin and 100 g/ml streptomycin at 37°C in a humidified
incubator with 5% (v/v) CO2. Cells were seeded onto
six-well or 24-well cell culture plates at a density of
1×105/ml cultured for 12 h and then serum-starved for
another 12 h. The cells were then incubated for another 24 h in
FBS-free hyperglycemic DMEM with the following intervention
factors: phosphate-buffered saline (PBS; control), 80 µg/ml
CRP, 80 µg/ml β2GPI, 80 µg/ml oxLDL, 160 µg/ml
CRP/oxLDL, 160 µg/ml oxLDL/β2GPI or 176 µg/ml
CRP/oxLDL/β2GPI, which all contained the same amount of oxLDL. The
concentrations and incubation time with the above reagents are
based on those used in previous studies by our group (9,16).
Following incubation, the culture medium was collected for ELISAs
and the cells were washed twice with PBS prior to subsequent
analysis.
Oil Red O staining
Oil Red O staining of foam cells was performed as
described in a previous study by our group (16). Briefly, the macrophages were fixed
in 10% (v/v) formaldehyde solution for 30 min. Fixed cells were
washed with PBS and then with 60% (v/v) isopropanol solution twice
for 5 min each. Next, the cells were stained with freshly prepared
Oil Red O working solution for 30 min at 60°C. The nuclei were
lightly stained with hematoxylin for 5 min. Stained cells were
washed with distilled water, mounted in neutral balsam (Beijing
Solarbio Science & Technology Co., Ltd.) and then observed
using an inverted microscope (DM4000 B; Leica Microsystems,
Wetzlar, Germany).
Intracellular total cholesterol content
assay
After treatment with the complexes, the culture
medium was removed and cells were washed twice with ice-cold PBS.
The cells were collected and sonicated at 4°C in lysis buffer (1X
Tris-buffered saline, pH 7.5, 10 mM EDTA, 1% Triton X-100, 10 mM
NaF, 1 mM phenylmethanesulfonylfluoride, all Beijing Solarbio
Science & Technology Co., Ltd.; and 1 mM sodium orthovanadate,
Sigma-Aldrich). After homogenization, the supernatant was obtained
by centrifugation (10,000 ×g for 10 min at 4°C). Protein
concentrations were determined according to BCA assay. Total
cholesterol levels were measured using a commercially available
intracellular total cholesterol assay kit according to the
manufacturer's instructions. Cholesterol levels were expressed as
µmol per mg protein.
Total RNA isolation and
reverse-transcription quantitative polymerase chain reaction
(PCR)
Primers for scavenger receptor B (CD36), scavenger
receptor B1 (SRB1), adenosine triphosphate (ATP) binding cassette
receptor A1 (ABCA1), ATP binding cassette receptor G1 (ABCG1) and
β-actin mRNA were designed according to the GenBank database
(http://www.ncbi.nlm.nih.gov/genbank/)
using Primer 5.0 (Premier Biosoft, Palo Alto, CA, USA) and Oligo
6.0 (Molecular Biology Insights, Inc., Colorado Springs, CA, USA)
software. The primers were purchased from Sangon Biotech Co., Ltd.
(Shanghai, China). The primer sequences were as follows: CD36
forward, 5′-GAACCACTGCTTTCAAAAACTGG-3′ and reverse,
5′-TGCTGTTCTTTGCCACGTCA-3′; SRB1 forward,
5′-TTTGGAGTGGTAGTAAAAAGGGC-3′ and reverse,
5′-TGACATCAGGGACTCAGAGTAG-3′; ABCA1 forward,
5′-AGTGATAATCAAAGTCAAAGGCACAC-3′ and reverse,
5′-AGCAACTTGGCACTAGTAACTCTG-3′; ABCG1 forward,
5′-TTCATCGTCCTGGGCATCTT-3′ and reverse,
5′-CGGATTTTGTATCTGAGGACGAA-3′; β-actin forward,
5′-TGGAGAAGAGCTATGAGCTGCCTG-3′ and reverse,
5′-GTGCCACCAGACAGCACTGTGTTG-3′.
Total RNA was extracted from cells using TRIzol
reagent according to the manufacturer's instructions. RNA (2
µg) was reverse transcribed into cDNA using RevertAid™ First
Strand cDNA Synthesis kit, as previously described (16). The PCR was performed using an iQ™
Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). For qPCR, each reaction comprised 5 µl
SYBR® Green II (Takara Bio. Inc.), 0.4 µl
downstream/upstream primers, 1 µl cDNA, and 3.2 µl
diethylpyrocarbonate-treated water. PCR cycling conditions were as
follows: 50°C for 2 min, 94°C for 3 min, followed by 40 cycles at
94°C for 30 sec, 60°C for 30 sec and 72°C for 20 sec. The relative
quantification was based on β-actin to determine fold-differences
in the expression of the target gene. The ΔΔCt-method was used for
the normalization procedure. The final results are expressed as the
2−ΔΔCt value between the experimental and the control
group.
ELISA
RAW264.7 cells were seeded at 1×105/well
in a 24-well plate. After serum-starvation for 12 h, cells were
treated with various reagents as described above. IL-1β, IL-6 and
TNF-α were determined in the supernatants of the cells using
commercially available ELISA kits according to the manufacturer's
instructions. Standard samples provided in the kits were used to
calculate absolute index levels. The protein levels of IL-1β, IL-6
and TNF-α in the cell culture media were expressed in pg/ml.
Western blot analysis
After treatment with the complexes as described
above, the culture medium was removed and the reaction was
terminated by adding 1 ml cold PBS containing 100 µM sodium
vanadate (Sigma-Aldrich). The samples were then placed on ice,
washed with ice-cold PBS and lysed in radioimmunoprecipitation
(RIPA) lysis buffer for 30 min. Lysates were clarified by
centrifugation at 12,000 × g for 15 min at 4°C, and the protein
content in the supernatant was measured using the BCA method. 10
µg protein was subjected to 12% SDS-PAGE and transferred
onto a nitrocellulose membrane. The membrane was blocked in freshly
prepared 5% skimmed milk in Tris-buffered saline/0.05% Tween 20
(TBST) for 2 h at room temperature (RT), subsequently incubated
with primary antibodies against p38/MAPK (cat. no. 14451),
p-p38/MAPK (Thr180/Tyr182; cat. no. 4511), NF-κB (p65) (cat. no.
8242), p-NF-κB (Ser 276) (cat. no. 3037) (all 1:1,000) or β-actin
(cat. no. DKM9001; 1:5,000) diluted in 5% skimmed milk - TBST,
overnight at 4°C. Following three washes for 10 min each with TBST,
the membranes were incubated with HRP-conjugated goat anti-rabbit
(cat. no. LK2001) or anti-mouse (cat. no. LK2003) IgG in 5% skimmed
milk - TBST (1:3,000) for 1 h at RT. The membrane was then
extensively washed with TBST. The immunoreactive bands were
detected using enhanced chemiluminescence reagents, blots were
imaged using radiography with an ultraviolet transmission analyzer
(GE Healthcare, Piscataway, NJ, USA) and images were captured onto
film (Kodak, Inc., Rochester, NY, USA), and analyzed using BandScan
software (Bio-Rad Gel Doc 2000; Bio-Rad Laboratories, Inc.).
β-actin was used as the internal standard protein.
Statistical analysis
All experiments were performed at least three times
in duplicate. SPSS 20.0 software (International Business Machines,
Armonk, NY, USA) was used for statistical analysis. Values are
expressed as the mean ± standard deviation. Comparisons among
multi-groups were accomplished by one-way analysis of variance and
differences between two groups were analyzed using the
Student-Newman-Keuls Test. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
OxLDL and its complexes induce lipid
accumulation in macrophages
Incubation of RAW264.7 cells with oxLDL produced
lipid-rich foam cells characterized by an intense red color
accompanied with an increase in cell size and a decrease in cell
count. Incubation of macrophages with CRP or β2GPI alone did not
produce any observable effects; however, in the CRP/oxLDL,
oxLDL/β2GPI and CRP/oxLDL/β2GPI groups, a considerably larger
amount of red lipid droplets was observed in the cytoplasm
(Fig. 1).
OxLDL and its complexes increase the
intracellular total cholesterol content in RAW264.7
macrophages
Among all of the groups, the total cholesterol (TC)
content in cells treated with oxLDL was highest, which was 6.7-fold
that of the control group (P<0.05). Treatment with CRP or β2GPI
alone produced no obvious change in the cholesterol content
compared with that in the control group. The cholesterol
accumulation induced by the three complexes significantly decreased
in comparison to that in the oxLDL group (P<0.05). TC in the
CRP/oxLDL group was 5.5-fold of that in the control group, which
was higher than that in the oxLDL/β2GPI and CRP/oxLDL/β2GPI groups
(P<0.05) and 3.1- and 4.0-fold of that in the control group,
respectively. TC in the oxLDL/β2GPI group was lower than that in
the CRP/oxLDL and CRP/oxLDL/β2GPI groups (P<0.05) (Table I).
 | Table IEffects of CRP/oxLDL/β2GPI complexes
on intracellular TC content in RAW264.7 macrophages. |
Table I
Effects of CRP/oxLDL/β2GPI complexes
on intracellular TC content in RAW264.7 macrophages.
| Group | TC
concentration
(µmol/mg protein) |
|---|
| Control | 22.6±3.1 |
| CRP | 26.0±4.7b |
| β2GPI | 20.3±2.5b |
| oxLDL | 151.4±6.2a |
| CRP/oxLDL | 124.3±4.0a,b |
| oxLDL/β2GPI | 70.4±3.9a,b |
|
CRP/oxLDL/β2GPI | 91.2±5.8a,b |
oxLDL and its complexes increase CD36,
SRB1, ABCA1 and ABCG1 mRNA expression in macrophages
Compared to that in the control group, the mRNA
expression of SRB1, ABCG1, CD36 and ABCA1 in the oxLDL group was
significantly increased (P<0.05), which was consistent with the
findings of a previous study by our group (16). When compared to oxLDL, all of the
three complexes inhibited the expression of CD36, but had no
significant effect on SRB1 and ABCA1 expression (P<0.05). Only
oxLDL/β2GPI and CRP/oxLDL/β2GPI significantly increased the
expression of ABCG1 compared to that in the oxLDL group
(P<0.05). However, there was no significant difference in the
expression levels of CD36, SRB1, ABCA1 and ABCG1 mRNA among the
three complexes (Fig. 2).
OxLDL and its complexes increase the
secretion of inflammatory factors IL-1β, IL-6 and TNF-α by
macrophages
As oxLDL is highly pro-inflammatory, the present
study assessed its effect on the secretion of inflammatory factors
IL-1β, IL-6 and TNF-α by RAW264.7 macrophages. Compared with the
expression of IL-1β, IL-6 and TNF-α following treatment with CRP or
β2GPI alone, it was significantly increased by their complexes with
oxLDL. OxLDL/β2GPI inhibited the secretion of IL-1β and IL-6
induced by oxLDL (P<0.05). There were no differences in the
expression levels of IL-1β and IL-6 among the oxLDL, CRP/oxLDL and
CRP/oxLDL/β2GPI groups (P>0.05). Furthermore, there were no
significant differences among the levels of TNF-α following
treatment with oxLDL and those following treatment with any of its
complexes (P>0.05) (Fig.
3).
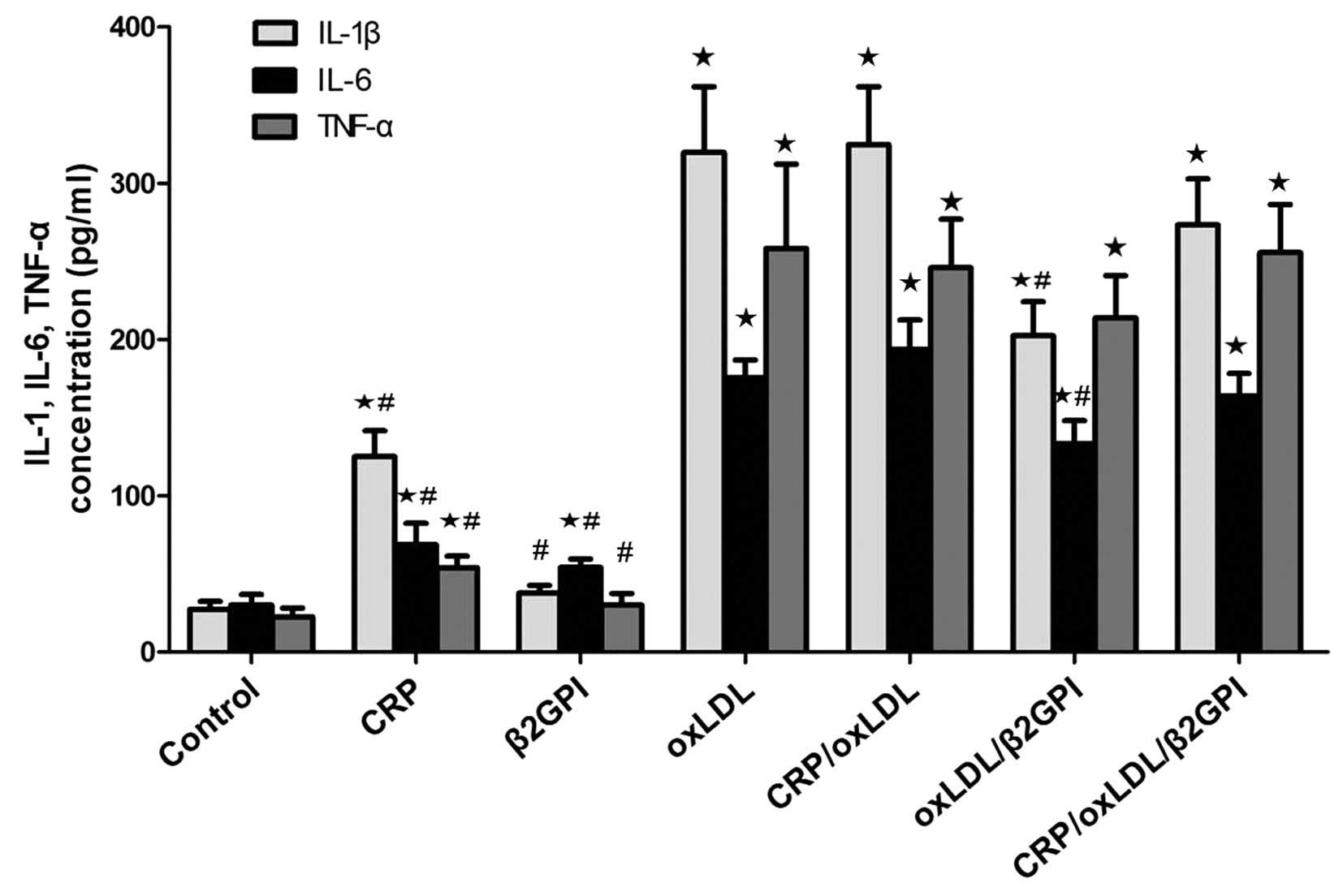 | Figure 3Effects of CRP/oxLDL/β2GPI complexes
on the expression of inflammatory factors IL-1β, IL-6 and TNF-α.
After treatment with CPR, β2GPI, oxLDL, CRP/oxLDL, oxLDL/β2GPI or
CRP/oxLDL/β2GPI for 24 h, culture media of RAW264.7 macrophages
were collected. The levels of IL-1β, IL-6 and TNF-α were measured
using commercial ELISA kits. Values are expressed as the mean ±
standard deviation of experiments performed three times in
duplicate. ⋆P<0.05, compared with control group;
#P<0.05, compared with oxLDL group. oxLDL, oxidized
lipopolysaccharide; CRP, C-reactive protein; β2GPI, β2-glycoprotein
I. TNF, tumor necrosis factor; IL, interleukin. |
P38/MAPK and NF-κB phosphorylation in
RAW264.7 macrophages
OxLDL and its complexes significantly triggered the
phosphorylation of p38/MAPK and NF-κB compared with that in the
control group (P<0.05) (Fig.
4). The increase of p-p38/MAPK and p-NF-κB induced by
oxLDL/β2GPI treatment was lower than that caused by oxLDL
(P<0.05). The effect of CRP/oxLDL/β2GPI on activating the
phosphorylation of p38/MAPK and NF-κB was identical to that of
oxLDL (P>0.05). In the CRP/oxLDL group, phosphorylation of
p38/MAPK and p-NF-κB was decreased compared with that in the oxLDL
group, while only the difference in NF-κB phosphorylation was
significant (P<0.05 vs. oxLDL).
Discussion
In a previous study by our group, animal experiments
using BALB/c mice showed that exogenous CRP/oxLDL/β2GPI complexes
aggravated AS in diabetic BALB/c mice by increasing lipid uptake,
which was indicated to be mediated via the p38/MAPK signaling
pathway (9), while the
contribution of formed auto-immune antibodies to the process could
not be excluded. Given that no in vitro study has been
performed to detect the different roles of oxLDL by-product
complexes on foam-cell formation and inflammatory activation
without the interference of antibodies, the present study aimed to
investigate the effects and the potential mechanisms of CRP, oxLDL
and β2GPI as well as their complexes CRP/oxLDL, oxLDL/β2GPI and
CRP/oxLDL/β2GPI on lipid accumulation and inflammatory cytokine
release in RAW264.7 macrophages.
A previous study by our group has established the
foam cell model in vitro by stimulating murine RAW264.7
macrophages with oxLDL (16). In
the present study, CRP/oxLDL, oxLDL/β2GPI and CRP/oxLDL/β2GPI were
able to induce macrophages to accumulate lipid and develop into
foam cells; however, this effect was less marked than that of oxLDL
alone, as confirmed by assessment of the cells' cholesterol
content. The covalent binding of β2GPI to oxLDL reduced lipid
accumulation to a significant degree, suggesting that β2GPI acts as
an anti-AS agent in the process of foam cell formation and AS. With
regard to the role of CRP/oxLDL, the present study showed that the
levels of cholesterol in the CRP/oxLDL group were lower than those
in the oxLDL group, which was consistent with the findings of other
studies, which reported that macrophage binding/uptake of oxLDL was
decreased when complexed with CRP (17,18).
The effect of CRP/oxLDL/β2GPI on lipid accumulation in macrophages
was lower than that of CRP/oxLDL, while being higher than that of
oxLDL/β2GPI; however these differences were not significant. Thus,
the binding of CRP or β2GPI to oxLDL was able to induce foam-cell
formation, which was less marked than the effect of oxLDL on
macrophage lipid uptake.
The present study further investigated the possible
mechanism of lipid accumulation in macrophages. The intracellular
load of oxLDL is mainly mediated by scavenger receptors, including
SRBl, CD36 and lectin-like oxidized LDL receptor 1, which are
expressed on the surface of macrophages, resulting in the formation
of foam cells (19). ABCA1 and
ABCG1 are key participants in reverse cholesterol transport,
mediating cholesterol efflux directly to high-density lipoprotein
particles (20). The results of
the present study showed that oxLDL promoted the accumulation of
lipids within the cells by upregulating the expression of CD36,
SRB1, ABCA1 and ABCG1 mRNA. All of the three complexes promoted
CD36 mRNA expression, while their effects were less marked than
that of oxLDL, which is thought to have a vital role in promoting
foam-cell formation. β2GPI was indicated to have marked anti-AS
properties, as expression of CD36 mRNA in the β2GPI group was as
low as that in the control group, and in the oxLDL/β2GPI group,
CD36 expression was significantly reduced compared with that in the
oxLDL group.
Given that CRP and oxLDL are potent pro-inflammatory
substances, the present study analyzed the secretion levels of
IL-1β, IL-6 and TNF-α by macrophage cells in order to assess their
inflammatory response induced by the complexes. IL-1β is a potent
pro-inflammatory and atherogenic cytokine (21,22);
in addition, macrophages are the primary IL-6 and TNF-α-producing
cells. In the present study, the secretion of IL-1β and IL-6 in the
oxLDL/β2GPI group was reduced compared with that in the oxLDL
group. The decreased inflammatory effect of oxLDL/β2GPI implied
that β2GPI binds to oxLDL, mitigating its harmful inflammatory
impact and possibly exerting a protective effect in the process of
foam-cell formation and AS. However, a previous in vivo
study by our group showed that oxLDL/β2GPI increased
pro-inflammatory cytokine expression under diabetic conditions when
compared to oxLDL (9), which
appears to contradict the results of the present in vitro
study. The discrepancy between the in vitro and in
vivo findings may be due to exogenous oxLDL/β2GPI acting as an
antigen, which induced an auto-immune response and promoted the
secretion of inflammatory molecules; however, this notion is
required to be verified by further studies. The secretion of IL-1β,
IL-6 and TNF-α stimulated by CRP/oxLDL and CRP/oxLDL/β2GPI was
almost the same as that following stimulation with oxLDL;
therefore, the two complexes can be regarded as pro-inflammatory
agents.
Intracellular MAPK signaling cascades are important
in the pathogenesis of cadiovascular diseases (23). Three MAPK families (extracellular
signal-regulated kinase 1/2, p38/MAPK and c-Jun N-terminal kinase)
are signaling molecules that react to extracellular stimuli and
regulate immune responses (24).
NF-κB activation has been linked with multiple aspects of
biological functions, including inflammation, stress, cell
proliferation, migration and tumour angiogenesis. A previous study
by our group demonstrated that CRP/oxLDL/β2GPI increased the
development and formation of AS lesions through the p38/MAPK
signaling pathway in BALB/c mice (9). Furthermore, andrographolide was
indicated to reduce foam cell formation by blocking the p38/MAPK
and NF-κB pathways in mouse peritoneal macrophages (25). The present study explored the
contribution of CRP/oxLDL/β2GPI complexes to the activation of
p38/MAPK and NF-κB. The results revealed that p38/MAPK and NF-κB
were involved in foam cell formation and inflammatory factor
production induced by oxLDL and its complexes in RAW264.7 cells.
The CRP/oxLDL complex mainly promoted the phosphorylation of
p38MAPK, while CRP/oxLDL/β2GPI increased the phosphorylation of
p38/MAPK as well as NF-κB in vitro. Hence, the induction of
AS by CRP/oxLDL and CRP/oxLDL/β2GPI may be mediated via the
activation of the p38MAPK and NF-κB pathways.
AS, which is a major health concern of worldwide
importance, is a multifactorial process with not only lipoprotein
metabolism but also inflammatory and immunological mechanisms
linked to its initiation and progression (26). oxLDL is an accepted key factor in
macrophage lipid accumulation and foam cell establishment, leading
to the formation of atherogenic lesions and plaques (27). Circulating oxLDL has been reported
to be a predictor of coronary disease in healthy middle-aged
people, as well as in people with cardiovascular diseases and
diabetes (28,29). While oxLDL alone is unstable in the
blood circulation and is cleared rapidly, it is able to bind to
β2GPI and/or CRP to form covalently stable complexes, which in turn
trigger further pro-inflammatory and pro-atherogenic
mechanisms.
Cu2+-oxLDL-derived ligands, such as
7-ketocholesteryl-9- carboxynonanoate, mediate the interaction with
β2GPI through its positively charged V domain in a time- and
temperature-dependent manner (4).
It has been confirmed that oxLDL/β2GPI is present in the blood
circulation of patients with diseases, including systemic lupus
erythematosus (SLE), APS, systemic sclerosis (30), diabetes mellitus (DM) (10) and chronic renal diseases (31), as well as acute coronary syndromes
(32). OxLDL/β2GPI is regarded to
be a pro-atherogenic auto-antigen accounting for pre-mature or
accelerated arteriosclerotic cardiovascular disease in systemic
auto-immune disease (33).
Anti-β2GPI antibodies accelerate macrophage uptake and accumulation
of oxLDL in the presence of β2GPI in vitro (34). OxLDL/β2GPI/anti-β2GPI was
internalized by macrophages via anti-β2GPI antibody-mediated
phagocytosis through Fc-γ- and Toll-like receptors, suggesting that
the uptake of oxLDL/β2GPI. CRP has a crucial role in the
progression and vulnerability of AS lesions (35) by inducing the production of
cytokines and inflammatory mediators, including IL-1, IL-6, IL-8,
TNF-α, intercellular adhesion molecule 1 and vascular cell adhesion
molecule 1, which are involved in the development of AS depending
on the activation of p38/MAPK, Akt and NF-κB (36,37).
CRP binds to oxLDL by recognition of a phosphorylcholine moiety of
oxLDL in a Ca2+- and pH-dependent manner, which is a
regulated mechanism of macrophages to clear CRP (7).
Unlike SLE and APS patients, in which oxLDL
complexes and associated auto-antibodies are present, IgG
auto-antibodies against oxLDL/β2GPI were merely observed in
patients with non-autoimmune diseases, such as type 2 DM (10). CRP/oxLDL and CRP/oxLDL/β2GPI
complexes have been reported to be markedly elevated in patients
with DM, rheumatoid arthritis and pyrogenic diseases, as compared
with in healthy control subjects. Furthermore, the elevated
complexes were reported to be associated with arterial inflammation
and intima media thickness, suggesting that they may represent
predictive markers with enhanced specificity for evaluating the
severity of diabetic AS (11). A
recent study confirmed that patients with type 2 DM exhibited
significantly higher serum levels of oxLDL/β2GPI complexes than
age- and gender-matched healthy control subjects. Furthermore,
serum oxLDL/β2GPI levels were correlated with the severity of
coronary heart disease (32).
The present study showed that oxLDL/β2GPI was able
to repress macrophage phagocytosis of lipid and decrease the
expression of inflammatory factors induced by oxLDL, which is
consistent with a previous study by our group, which reported that
β2GPI protects macrophages from oxLDL-induced foam-cell formation
and apopotosis (16). CRP/oxLDL
and CRP/oxLDL/β2GPI exhibited a significant ability to induce
foam-cell formation and activate inflammatory responses via p38MAPK
and NF-κB phosphorylation, which was, however, decreased compared
with that of oxLDL. CRP/oxLDL, oxLDL/β2GPI and CRP/oxLDL/β2GPI
complexes can be distinguished from pyrogenic non-complexed CRP
isoforms or unstable oxLDL and may therefore represent more
specific and reliable predictive markers for AS. Thus, strategies
aimed at detecting the complexes may prove to be beneficial in
detecting and preventing atherothrombotic events in early stages.
Among CRP/oxLDL, oxLDL/β2GPI and CRP/oxLDL/β2GPI, the complex which
is most suitable as a predictive marker remains to be
identified.
In conclusion, the present study indicated that
CRP/oxLDL, oxLDL/β2GPI and CRP/oxLDL/β2GPI complexes induced the
transformation of macrophages into foam cells by activating
inflammatory responses via p38/MAPK and NF-κB signaling pathways.
oxLDL/β2GPI had an anti-AS effect, but to a lesser extent than
oxLDL alone. It may be possible that β2GPI has an anti-AS effect by
forming a complex with oxLDL; however, this was not demonstrated in
the present study. Due to their enhanced stability in the blood
compared with oxLDL, complexes of oxLDL may be suitable as
predictive biomarkers of AS.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81070645), The Key
Projects in the Tianjin Science & Technology Pillar Program
(no. 13ZCZDSY01300), The Tianjin Natural Science Foundation (no.
10JCYBJC12000) and the Science and Technology Foundation of Tianjin
Health Bureau (no. 2012KG135). The authors greatly appreciate the
comprehensive and in-depth professional English language editing
performed by Professor Gareth Denyer (School of Molecular
Biosciences, Sydney University, Sydney, Australia).
References
|
1
|
Galkina E and Ley K: Immune and
inflammatory mechanisms of atherosclerosis (*). Ann Rev
Immunol. 27:165–197. 2009. View Article : Google Scholar
|
|
2
|
Heinecke JW: Mechanisms of oxidative
damage of low density lipoprotein in human atherosclerosis. Curr
Opin Lipidol. 8:268–274. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miyakis S, Giannakopoulos B and Krilis SA:
Beta 2 glycoprotein I - function in health and disease. Thromb Res.
114:335–346. 2004. View Article : Google Scholar
|
|
4
|
Kobayashi K, Matsuura E, Liu Q, Furukawa
J, Kaihara K, Inagaki J, Atsumi T, Sakairi N, Yasuda T, Voelker DR
and Koike T: A specific ligand for beta (2)-glycoprotein I mediates
autoantibody-dependent uptake of oxidized low density lipoprotein
by macrophages. J Lipid Res. 42:697–709. 2001.PubMed/NCBI
|
|
5
|
Kobayashi K, Kishi M, Atsumi T,
Bertolaccini ML, Makino H, Sakairi N, Yamamoto I, Yasuda T,
Khamashta MA, Hughes GR, et al: Circulating oxidized ldl forms
complexes with beta2-glyco-protein I: Implication as an atherogenic
autoantigen. J Lipid Res. 44:716–726. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Libby P and Ridker PM: Inflammation and
atherosclerosis: Role of c-reactive protein in risk assessment. Am
J Med. 116(Suppl 6A): 9S–16S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang MK, Binder CJ, Torzewski M and
Witztum JL: C-reactive protein binds to both oxidized LDL and
apoptotic cells through recognition of a common ligand:
Phosphorylcholine of oxidized phospholipids. Proc Natl Acad Sci
USA. 99:13043–13048. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuura E, Kobayashia K, Koikeb T,
Shoenfeld Y, Khamashta MA and Hughes GR: Atherogenic autoantigen:
Oxidized LDL complexes with beta2-glycoprotein I. Immunobiology.
207:17–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang R, Zhou SJ, Li CJ, Wang XN, Tang YZ,
Chen R, Lv L, Zhao Q, Xing QL, Yu DM and Yu P: C-reactive
protein/oxidised low-density lipoprotein/β2-glycoprotein I complex
promotes atherosclerosis in diabetic BALB/c mice via
p38mitogen-activated protein kinase signal pathway. Lipids Health
Dis. 12:422013. View Article : Google Scholar
|
|
10
|
Lopez LR, Hurley BL, Simpson DF and
Matsuura E: Oxidized low-density lipoprotein/beta2-glycoprotein I
complexes and autoantibodies in patients with type 2 diabetes
mellitus. Ann NY Acad Sci. 1051:97–103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tabuchi M, Inoue K, Usui-Kataoka H,
Kobayashi K, Teramoto M, Takasugi K, Shikata K, Yamamura M, Ando K,
Nishida K, et al: The association of c-reactive protein with an
oxidative metabolite of LDL and its implication in atherosclerosis.
J Lipid Res. 48:768–781. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meuwissen M, van der Wal AC, Niessen HW,
Koch KT, de Winter RJ, van der Loos CM, Rittersma SZ, Chamuleau SA,
Tijssen JG, Becker AE and Piek JJ: Colocalisation of intraplaque C
reactive protein, complement, oxidised low density lipoprotein, and
macrophages in stable and unstable angina and acute myocardial
infarction. J Clin Pathol. 59:196–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldstein JL, Basu SK and Brown MS:
Receptor-mediated endocytosis of low-density lipoprotein in
cultured cells. Methods Enzymol. 98:241–260. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laplante P, Amireault P, Subang R, Dieudé
M, Levine JS and Rauch J: Interaction of β2-glycoprotein I with
lipopolysaccharide leads to Toll-like receptor 4 (TLR4)-dependent
activation of macrophages. J Biol Chem. 286:42494–42503. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Q, Kobayashi K, Furukawa J, Inagaki J,
Sakairi N, Iwado A, Yasuda T, Koike T, Voelker DR and Matsuura E:
Omega-carboxyl variants of 7-ketocholesteryl esters are ligands for
beta (2)-glycoprotein I and mediate antibody-dependent uptake of
oxidized ldl by macrophages. J Lipid Res. 43:1486–1495. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang WL, Meng ZX, Zhou SJ, Li CJ, Chen R,
Lv L, Ma ZJ, Yu DM and Yu P: Reduced beta2-glycoprotein I protects
macrophages from ox-LDL-induced foam cell formation and cell
apoptosis. Lipids Health Dis. 12:1742013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji SR, Wu Y, Potempa LA, Qiu Q and Zhao J:
Interactions of C-reactive protein with low-density lipoproteins:
Implications for an active role of modified C-reactive protein in
atherosclerosis. Int J Biochem Cell Biol. 38:648–661. 2006.
View Article : Google Scholar
|
|
18
|
Chang MK, Hartvigsen K, Ryu J, Kim Y and
Han KH: The pro-atherogenic effects of macrophages are reduced upon
formation of a complex between C-reactive protein and
lysophosphatidylcholine. J Inflamm (Lond). 9:422012. View Article : Google Scholar
|
|
19
|
Moore KJ and Freeman MW: Scavenger
receptors in atherosclerosis: Beyond lipid uptake. Arterioscler
Thromb Vasc Biol. 26:1702–1711. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Collins HL, Ranalletta M, Fuki IV,
Billheimer JT, Rothblat GH, Tall AR and Rader DJ: Macrophage ABCA1
and ABCG1, but not SR-BI, promote macrophage reverse cholesterol
transport in vivo. J Clin Invest. 117:2216–2224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsuura E, Atzeni F, Sarzi-Puttini P,
Turiel M, Lopez LR and Nurmohamed MT: Is atherosclerosis an
autoimmune disease? BMC Med. 12:472014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dinarello CA: Interleukin-1 in the
pathogenesis and treatment of inflammatory diseases. Blood.
117:3720–3732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muslin AJ: MAPK signalling in
cardiovascular health and disease: Molecular mechanisms and
therapeutic targets. Clin Sci (Lond). 115:203–218. 2008. View Article : Google Scholar
|
|
24
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
25
|
Li FX and Li SS: Effects of
andrographolide on the activation of mitogen activated protein
kinases and nuclear factor-κB in mouse peritoneal
macrophage-derived foam cells. Chin J Integr Med. 18:391–394. 2012.
View Article : Google Scholar
|
|
26
|
Weber C and Noels H: Atherosclerosis:
Current pathogenesis and therapeutic options. Nat Med.
17:1410–1422. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobayashi K, Lopez LR, Shoenfeld Y and
Matsuura E: The role of innate and adaptive immunity to oxidized
low-density lipoprotein in the development of atherosclerosis. Ann
NY Acad Sci. 1051:442–454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meisinger C, Baumert J, Khuseyinova N,
Loewel H and Koenig W: Plasma oxidized low-density lipoprotein, a
strong predictor for acute coronary heart disease events in
apparently healthy, middle-aged men from the general population.
Circulation. 112:651–657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsuzura S, Ikeda Y, Suehiro T, Ota K,
Osaki F, Arii K, Kumon Y and Hashimoto K: Correlation of plasma
oxidized low-density lipoprotein levels to vascular complications
and human serum paraoxonase in patients with type 2 diabetes.
Metabolism. 53:297–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lopez LR, Simpson DF, Hurley BL and
Matsuura E: OxLDL/beta2GPI complexes and autoantibodies in patients
with systemic lupus erythematosus, systemic sclerosis and
antiphospholipid syndrome: Pathogenic implications for vascular
involvement. Ann NY Acad Sci. 1051:313–322. 2005. View Article : Google Scholar
|
|
31
|
Kasahara J, Kobayashi K, Maeshima Y,
Yamasaki Y, Yasuda T, Matsuura E and Makino H: Clinical
significance of serum oxidized low-density
lipoprotein/beta2-glycoprotein I complexes in patients with chronic
renal diseases. Nephron Clin Pract. 98:c15–c24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Greco TP, Conti-Kelly AM, Anthony JR,
Greco TJ, Doyle R, Boisen M, Kojima K, Matsuura E and Lopez LR:
Oxidized-LDL/beta (2)-glycoprotein I complexes are associated with
disease severity and increased risk for adverse outcomes in
patients with acute coronary syndromes. Am J Clin Pathol.
133:737–743. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sherer Y and Shoenfeld Y: Mechanisms of
disease: Atherosclerosis in autoimmune diseases. Nat Clin Pract
Rheumatol. 2:99–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu Y, Kong X, Zhou H, Zhang X, Liu J, Yan
J, Xie H and Xie Y: OxLDL/β2GPI/anti-β2GPI complex induced
macrophage differentiation to foam cell involving TLR4/NF-KappaB
signal transduction pathway. Thromb Res. 134:384–392. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han KH, Hong KH, Park JH, Ko J, Kang DH,
Choi KJ, Hong MK, Park SW and Park SJ: C-reactive protein promotes
monocyte chemoattractant protein-1-mediated chemotaxis through
upregulating CC chemokine receptor 2 expression in human monocytes.
Circulation. 109:2566–2571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Devaraj S, Kumaresan PR and Jialal I:
Effect of C-reactive protein on chemokine expression in human
aortic endothelial cells. J Mol Cell Cardiol. 36:405–410. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galve-de Rochemonteix B, Wiktorowicz K,
Kushner I and Dayer JM: C-reactive protein increases production of
IL-1 alpha, IL-1 beta and TNF-alpha, and expression of mRNA by
human alveolar macrophages. J Leukoc Biol. 53:439–445.
1993.PubMed/NCBI
|


















