Introduction
Impairment of articular hyaline cartilage, can lead
to different grades of disability depending on the functionality of
the joint, and the grade of cartilage degeneration and destruction
(arthritis). Acute trauma of a joint due to a sporting injury or
accident, or chronic degeneration of the cartilage due to age, are
the main and most frequent causes of severe impairment of articular
cartilage that can lead to final stages of osteoarthritis and
serious disability (1–3). The most common therapies [abrasion
arthroplasty, autologous chondrocyte implantation (ACI),
microfractures and arthroplasties] may not lead to complete
rehabilitation and there is always the risk of side effects, such
as infection of total arthroplasty and dislocation of the joint due
to stem misorientation or loosening (1–5).
On these grounds, to avoid severe osteoarthritis and
the requirement for arthroplasty surgery, research is being
conducted in the field of neochondrogenesis (2,5,6).
Neochondrogenesis is a procedure during which the body is
stimulated to form newly synthesized cartilage from mesenchymal
stem cells (MSCs), at a specific location (2,4).
Several lines of evidence suggest that neochondrogenesis may become
a promising alternative to prevent osteoarthritis. Studies on
skeleton metabolism have led to the identification of a number of
factors involved in cartilage formation by causing the
differentiation of MSCs into chondrocytes (7–10).
There are several approaches for administering these factors into
the point of interest in laboratory animals and humans. It has been
suggested that these factors can be administered directly into the
point of interest (damaged cartilage) with rather encouraging
results or in the form of a factor saturated scaffold (11,12).
In addition, in certain cases, apart from being saturated with
growth factors, scaffolds contain premature chondrocytes obtained
after the in vitro differentiation of autologous MSCs
isolated from the patient (13–17).
One of the most potent factors that has been
investigated for its ability to stimulate chondrogenesis in human
MSCs (hMSCs) is transforming growth factor β1 (TGF-β1) (18). MSC transformation into chondrocytes
in this case is stimulated though activation (phosphorylation) of
extracellular signal-regulated kinase 1/2 (ERK 1/2) (19,20).
Insulin-like growth factor 1 (IGF-1) is a potent
growth and survival factor in human cancer, and the gene gives rise
to multiple heterogeneous transcripts, IGF-1Ec among them, all
resulting in the mature form of IGF-1. While the physiological role
of IGF-1Ec is under debate, it has been known to participate in
skeletal muscle repair, as well as in the cardiac remodeling/repair
process (21,22).
Recent evidence suggests that the Ec peptide (PEc),
resulting from the proteolytic cleavage of the COO−
terminal of the IGF-1Ec isoform, is associated with ERK 1/2
activation in various cell lines with adverse effects, including
cellular proliferation, differentiation and satellite stem cell
mobilization prior to repair (23–27).
Also, there are indications that PEc participates in the healing
process by affecting the expression pattern of osteogenic and
adipogenic genes in MSCs, thus affecting differentiation during
wound healing, while the mechanism by which it promotes
proliferation and survival in cancer cells may in fact be mediated
by a unique receptor (28,29). Additionally, it has also been
suggested that IGF-1Ec expression is stimulated by tissue damage
leading to MSC attraction through PEc prior to repair, as was
determined by in vitro assays (30).
The aim of the present study was to examine the
effects of PEc on hMSCs and the possibility of increasing hyaline
cartilage formation, using PEc in combination with TGF-β1 to
stimulate MSC migration (PEc) and differentiation (TGF-β1)
simultaneously.
Materials and methods
hMSC isolation
Human bone marrow was collected from the scrape
material of three healthy donors aged 32, 37 and 29 years old, with
open femur fractures. The material was used after all the patients
filled in a written informed consent form. This study was approved
by the institutional ethics committee and all experimental
procedures conformed to the Declaration of Helsinki and was given
and explained to the patients after the surgery and during their
rehabilitation. The acquired marrow was briefly filtered through a
70-mm mesh and the resulting cells were cultured at a density of
25×106 per 75-cm2 flask in Dulbecco's
modified Eagle's medium (DMEM) and 20% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells
were incubated in a standard issue tissue culture incubator (37°C
and 5% CO2). The media was changed 3 h after initial
culture and then every 8 h for the remaining 72 h. The adherent
cells were then trypsinized for 2 min at 25°C and examined for
expression of the epithelial marker E-cadherin and the mesenchymal
marker vimentin (Fig. 1A)
(31).
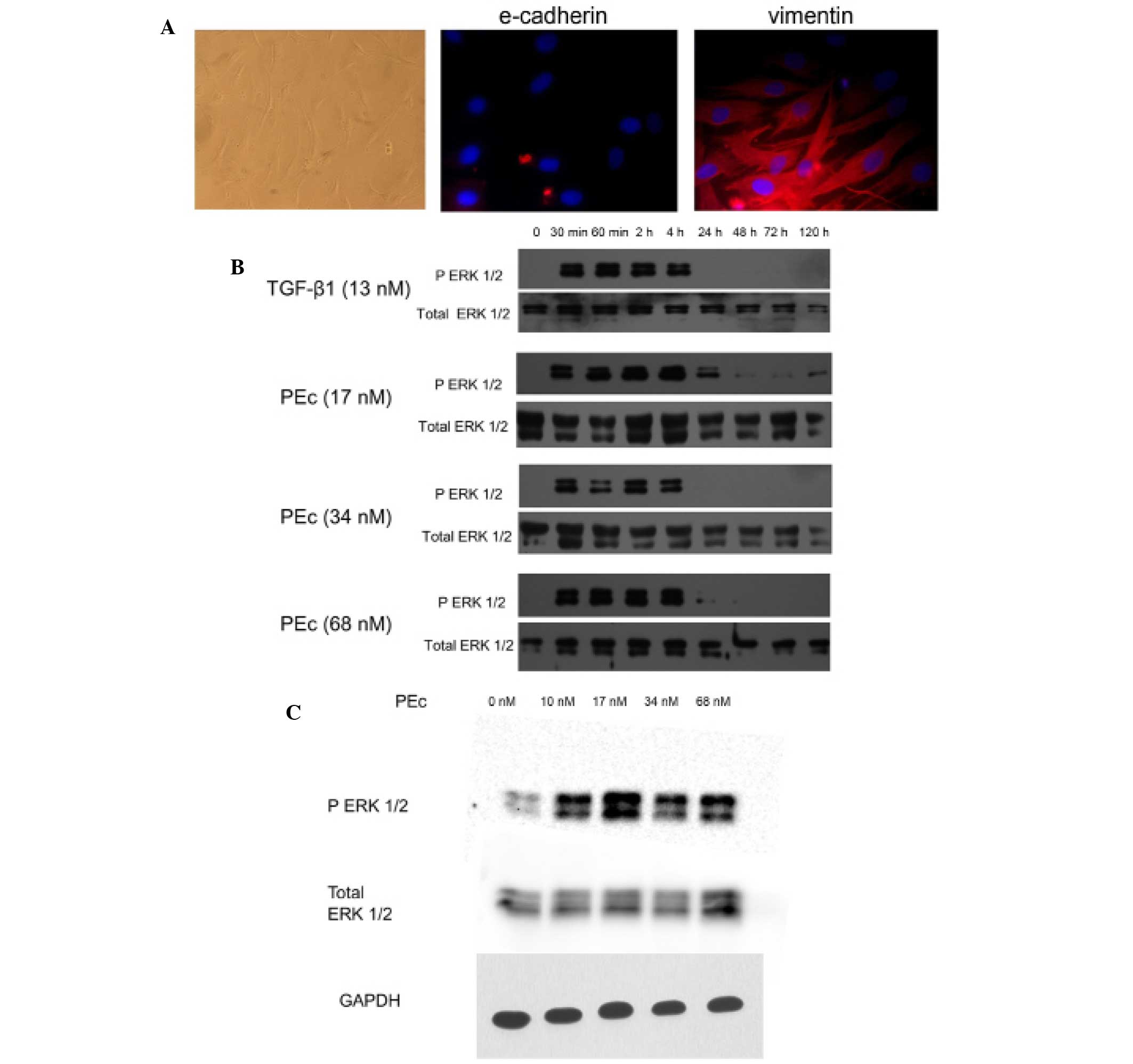 | Figure 1Effects of PEc on hMSC activation. (A)
Characterization of the freshly isolated hMSCs by
immunofluorescence (magnification, ×200). The cells obtained from
human bone marrow were examined for E-cadherin and Vimentin
expression. It was determined that these cells were not expressing
E-cadherin but were expressing vimentin at the protein level. In
addition, the cellular morphology as was determined by light
microscopy, was confirmatory to the mesenchymal nature of these
cells. (B) The effect of different concentrations of PEc on hMSCs
was examined by western blot analysis of ERK 1/2 phosphorylation,
at different time intervals. It was determined that PEc activates
ERK 1/2 whereas its optimum concentration (17 nM) was able to
maintain the ERK 1/2 phosphorylation for 24 h, longer than that
obtained with TGF-β1 treatment (4 h). (C) The optimal PEc
concentration (17 nM) was determined by western blot analysis of
ERK 1/2 phosphorylation at 30 min. hMSCs, human mesenchymal stem
cells; PEc, Ec peptide; ERK, extracellular signal-regulated kinase;
TGF-β1, transforming growth factor-β; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
Immunofluorescent staining
Cells cultured on culture slides (8-well, BD
Biosciences, Franklin Lakes, NJ, USA) were stained by an indirect
immunofluorescence method. Briefly, cells were rinsed in 1X
phosphate-buffered saline (PBS) and fixed with ice-cold 80%
methanol for 10 min at room temperature. They were permeabilized
with 1X PBS plus 0.5% Triton X-100 (Sigma-Aldrich, St. Louis, MO,
USA) for 10 min. They were then incubated with primary antibodies
overnight at 4°C: Polyclonal rabbit anti-E-cadherin (1:100;
ab15148; Abcam, Cambridge, UK) or monoclonal mouse anti-vimentin
(1:100; ac8978330; Abcam, Cambridge, UK) in 1X PBS. After 3 washes
with 1X PBS, each lasting 5 min at room temperature, cells were
incubated for 30 min with goat anti-rabbit IgG conjugated to the
fluorescent Alexa Fluor 488 dye (1:2,000, ab150077, Abcam,
Cambridge, UK) or with goat anti-mouse IgG conjugated to the
fluorescent Alexa 555 dye (1:2,000, A-21422, Thermo Fisher
Scientific, Inc.) in 1X PBS. After 3 washes with 1X PBS, each
lasting 5 min at room temperature, samples were stained with DAPI
(1 μg/ml; Leica Microsystems, Inc., Buffalo Grove, IL, USA)
for viewing with an Olympus BX40 microscope (Olympus, Tokyo,
Japan).
Cell proliferation
For the Trypan Blue Exclusion assays, hMSCs were
plated at a cell density of 3.5×104 cells/well in 6-well
plates and cultured in DMEM and 10% FBS. After 24 and 48 h of
culture, the cell number was counted using a hemocytometer
(Celeromics Technologies, Cambridge, UK). A 0.4% solution of trypan
blue in PBS was prepared and 0.1 ml trypan blue solution was added
to 1 ml of cells. Immediately after, a hemocytometer was used to
count the number of blue stained cells and the number of total
cells. Blue staining cells are considered to be non-viable. Trypan
blue exclusion assays determine the actual number of living
cells.
Western blot analysis
Investigation of phosphorylated (p)-ERK 1/2 and
total ERK 1/2 expression in the isolated hMSCs. TGF-β1 (13 nM;
Biognos, Waterven, Belgium) and PEc (17, 34 or 68 nM; Eastern
Quebec Proteomic Core Facility, Sainte-Foy, Canada) were added in
tissue culture wells with hMSCs at intervals of 0, 0.5, 1, 2, 4,
24, 48, 72 and 120 h. Proteins were then isolated using
radioimmunoprecipitation assay buffer with the addition of protease
and phosphatase inhibitors (all Sigma-Aldrich). Western blot
analysis was conducted after protein separation using 60 μg
sample loaded onto a 12% sodium dodecyl sulfate-polyacrylamide gel
(Sigma-Aldrich). Proteins were transferred to polyvinlydene
fluoride membranes and blocked in 0.1% milk in Tris-buffered
saline. The membranes were incubated overnight at 4°C in the
following primary antibodies: Rabbit anti-Phospho-p44/42 MAPK
(Erk1/2) (Thr202/Tyr204) (1:2,000; 9101; Cell Signaling Technology,
Inc., Beverly, MA, USA), and rabbit anti-p44/42 MAPK (Erk1/2)
(1:2,000; 9102; Cell Signaling Technology, Inc.). Subsequently,
membranes were incubated for 1 h at room temperature with
horseradish peroxidase-conjugated goat anti-rabbit antibody
(1:2,000; AP132P; EMD Millipore, Billerica, MA, USA). Detection was
conducted using the Supersignal West Pico Chemiluminescent
substrate (Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression levels of Collagen (Col) I, Col II
and Col X were assessed by RT-qPCR. RT-qPCR was performed in the
Biorad IQ5 multicolor Real Time PCR detection system, using
Sybergreen Supermix (Bio-Rad, Hercules, CA, USA). RNA extracts were
obtained using TRIzol reagent (Thermo Fisher Scientific, Inc.).
Each RT reaction was conducted using 1.5 mg RNA mixed with 10
mmol/l dNTPs (New England Biolabs, Ipswich, MA, USA), 0.5
μg/ml oligo dTs (Thermo Fisher Scientific, Inc.) and 3 mg/ml
random hexamer primers (Thermo Fisher Scientific, Inc.), it was
heated at 65°C for 5 min and quick-chilled on ice. The RT Mix
containing 200 U/μl M-MuLV ProtoScript II Reverse
Transcriptase, 5X ProtoScript II RT Reaction buffer, 0.1 mol/l DTT
and murine RNAse inhibitor (40 U/μl) (New England Biolabs)
was then added, and the reactants were incubated at 42°C for 50 min
and 70°C for 20 min. Subsequently, each reaction was obtained in 20
μl using 10 μl KAPA SYBR FAST qPCR Master mix (Kapa
Biosystems Inc., Wilmington, MA, USA), 2 μl cDNA and 0.3
μmol/l primers (23,24).
The sequences for the primers used were as follows: Forward:
5′-AGGGCTCCAACGAGATCGAGA-3′ and reverse: 5′-AGGAAGCAGACAGGGCCA-3′
for Col I; forward: 5′-CTATCTGGACGAAGCAGCTGGCA-3′ and reverse:
5′-ATGGGTGCAATGTCAATGATGG-3′ for Col II; forward:
5′-GCTAAGGGTGAAAGGGGTTC-3′ and reverse: 5′-CTCCAGGATCACCTTTTGGA-3′
for Col X; and forward: 5′-CCTCGCCTTTGCCGA-3′ and reverse:
5′-TGGTGCCTGGGGCG-3′ for β-actin (Eurofins Genomics GmbH,
Ebersberg, Germany).
The PCR conditions were the same in all three cases:
95°C for 3 min for 1 cycle, 94°C for 3 sec, then 60°C for 30 sec
for 40 cycles, and 70°C for 15 sec for 50 cycles (increasing 0.5°C
after every cycle) to determine the melt curve. Each reaction was
performed in triplicate and values were normalized to β-actin. mRNA
levels were quantified using iQ5 Optical System Software (Bio-Rad)
and the ΔΔCq method (32).
Alcian blue
Cartilage matrix deposition in cells cultured in
six-well dishes was quantified by Alcian blue staining. Cells were
stained with Alcian blue (1% in 3% acetic acid; Sigma-Aldrich) for
30 min, washed three times for 2 min in 3% acetic acid, rinsed once
with 1X PBS, and solubilized in 1% SDS. The staining was separately
repeated without the final solubilization step. The absorbance at
605 nm was determined using a VersaMax microplate reader for
triplicate samples (33)
(Molecular Devices, LLC, Sunnyvale, CA, USA). To avoid random
differentiation, the treatments were conducted in cells at 60–70%
confluency.
Migration/invasion assay
Cell migration and invasion were analyzed using the
Cultrex cell invasion assay (Trevigen Inc., Gaithersburg, MD, USA)
according to the manufacturer's instructions. Briefly, for the
invasion assay, the membrane in the upper chamber of the 96-well
plate was coated with 0.5X basement membrane matrix/Matrigel. For
the migration assay, the membrane was left uncoated. hMSCs were
starved in serum-free medium for 8 h prior to the assay, then
seeded at a density of 5×104 cells/well. After seeding
(24 h), the cells on the lower surface were dissociated by
aspiration and counted using a hemocytometer.
Wound healing assay
Isolated MSCs were cultured in DMEM supplemented
with 10% FBS and then seeded into 24-well tissue culture plate
wells at a density of 4×106 cells/well, so that after 24
h of growth, they should reach 90–95% confluency as a monolayer.
The monolayers were scratched with a sterilized 200 μl
pipette tip vertically across the center of the well. After
scratching, the wells were washed twice with 1X PBS to remove the
detached cells and replenished with fresh medium containing either
TGF-β1 (13 nM), PEc (17 nM) or TGF-β1 (13 nM)+PEc (17 nM).
Scratched monolayers replenished with just fresh medium were used
as control samples. Images were collected at 0, 8, 16, and 24 h
using a Olympus BX40 microscope. Afterwards, the tscratch software,
version 7.8 (Computational Science and Engineering Laboratory,
Swiss Federal Institute of Technology, Zurich, Switzerland) was
implemented in order to perform image analysis to measure the gap
areas.
Statistical analysis
Changes in gene expression levels and cell numbers
were assessed using one-way analysis of variance (SPSS v. 11
statistical package; SPSS Inc., Chicago, IL, USA). Where
significant F ratios were found (P<0.05), the means were
compared using Tukey's post-hoc tests. All data are presented as
the mean ± standard error of the mean. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of PEc on MSC stimulation
Exogenous administration of the synthetic human PEc
peptide stimulates the MSCs, as was determined by western blot
analysis to determine the expression of p-ERK 1/2. This was also
demonstrated for TGF-β1. Further analysis prior to determining the
optimum concentration of exogenous PEc, suggested that
administration of 17 nM PEc led to a prolonged phosphorylation time
of ERK 1/2 compared with the higher concentrations used in this
study (Fig. 1B).
Effect of PEc on MSC proliferation
Since PEc has previously been demonstrated to affect
cellular proliferation in various cell lines, the effect of PEc on
MSC proliferation was also examined. Exogenous administration of
PEc on hMSCs was not associated with a significant increase in cell
proliferation at any of the concentrations used (results not
shown).
Expression of collagen
Since ERK 1/2 phosphorylation is associated with
hMSC differentiation towards a chondrocytic fate, hMSCs treated
with the optimum concentrations of PEc and with PEc+TGF-β1 were
examined for their expression of Col I, which is mainly expressed
in tendons, ligaments and brittle cartilage, Col II that is the
basis of articular and hyaline cartilage, and Col X that is
expressed by hypertrophic chondrocytes during endochondral
ossification (34). It was
determined that the administration of PEc and PEc+TGF-β1 were
associated with a significantly lower expression of Col I at the
15th day compared with the effects of TGF-β1 alone, and they
presented similar levels of expression as the untreated hMSCs
(Fig. 2A).
 | Figure 2Quantitative analysis of the mRNA of
Col I, Col II and Col X in hMSCs as assessed by reverse
transcription-quantitative polymerase chain reaction, after the
administration of TGF-β1, PEc and the combination of both at
different time points (2, 4, 6, 8, 10 and 15 days). (A)
Administration of PEc (17 nM) and of PEc+TGF-β1 lead to
significantly lower expression of Col I compared with that obtained
by TGF-β1 treatment at day 15 (P<0.001). (B) PEc and PEc+TGF-β1
administration were also associated with significant elevation of
thew expression of Col II compared with that obtained by TGF-β1
(P=0.002). (C) No significant difference was observed in any of the
three treatments in respect to Col X expression.
*P<0.05, **P<0.005. hMSCs, human
mesenchymal stem cells; Col, collagen; TGF-β1, transforming growth
factor β1; PEc, Ec peptide. |
Col II expression was significantly higher in all
the conditions examined compared with the untreated hMSCs, through
the course of the study. Although PEc appears to be associated with
a significant increase in Col II at days 4, 6 and 8 compared with
the Col II levels obtained by TGF-β1 and PEc+TGF-β1 administration,
at day 10 and 15 no significant difference was identified in the
Col II expression stimulated by PEc, TGF-β1 and PEc+TGF-β1
treatments (Fig. 2B).
None of the factors examined were associated with a
significant increase in Col X through the course of the study
(Fig. 2C).
Differentiation of hMSCs into a
chondrocyte lineage
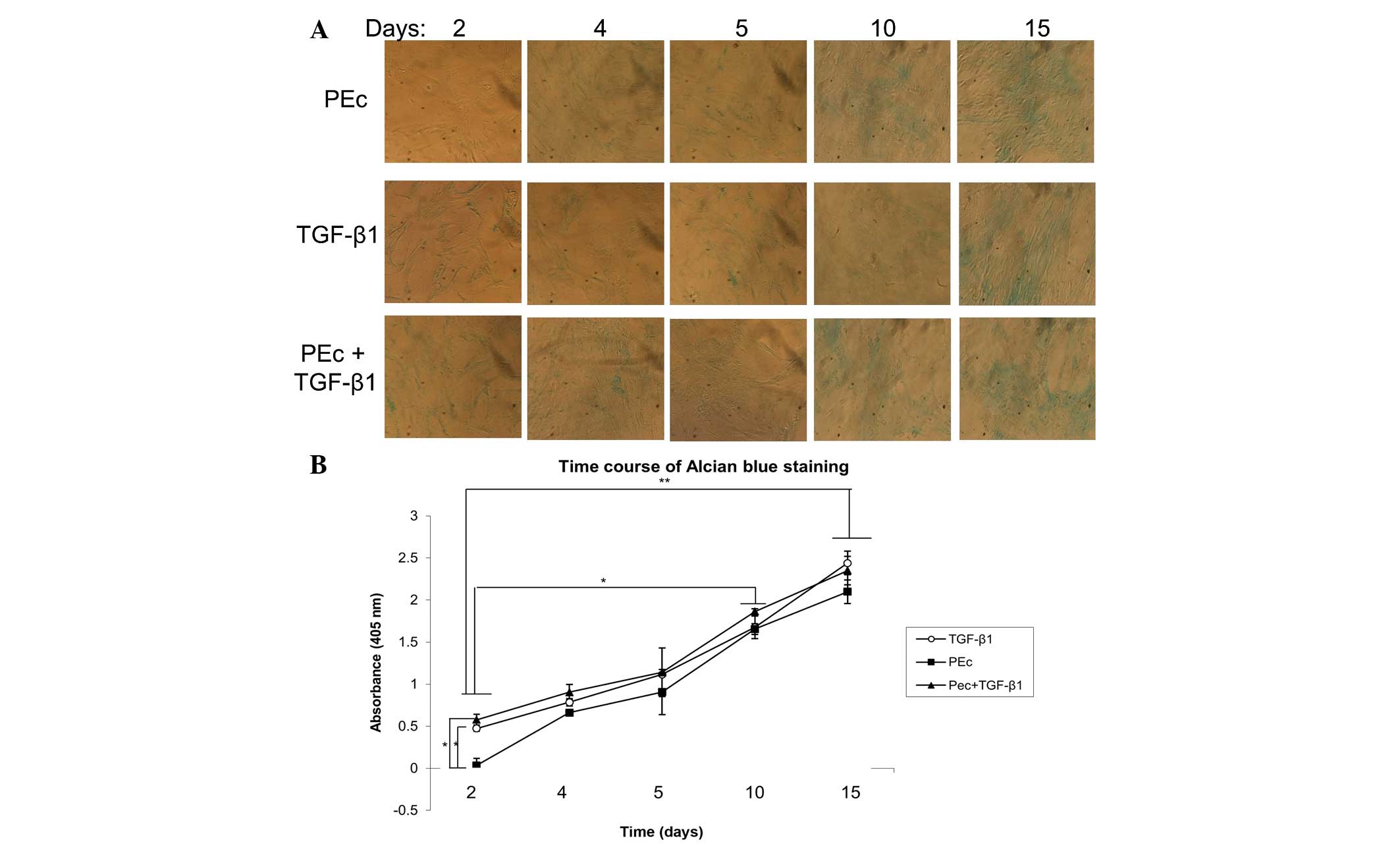
The stimulation of hMSCs towards chondrocytes was
also examined by Alcian blue quantitation, where it was determined
that PEc and PEc+TGF-β1 presented similar levels of Alcian blue
staining to that obtained by TGF-β1 treatment alone, through the
course of the study (15 days) (Fig. 3A
and B). Spontaneous differentiation towards chondrocytes was
examined in untreated hMSCs incubated in tissue culture conditions
for 15 days. No significant transformation was observed of the
untreated hMSC towards chondrocytes by Alcian blue staining after
15 days in tissue culture conditions (results not shown).
Recent evidence suggests that synthetic PEc can
induce mesenchymal cell mobilization in vitro. Prior to
that, the ability of PEc and of PEc in combination with TGF-β1 to
attract mesenchymal cells, was examined using a migration/invasion
assay. PEc presented a significantly higher rate of mesenchymal
cell migration and invasion capacity compared with TGF-β1
(P<0.05), and the combination of the two factors was associated
with a significantly higher mesenchymal cell migration and invasion
capacity than PEc alone (Fig.
4A).
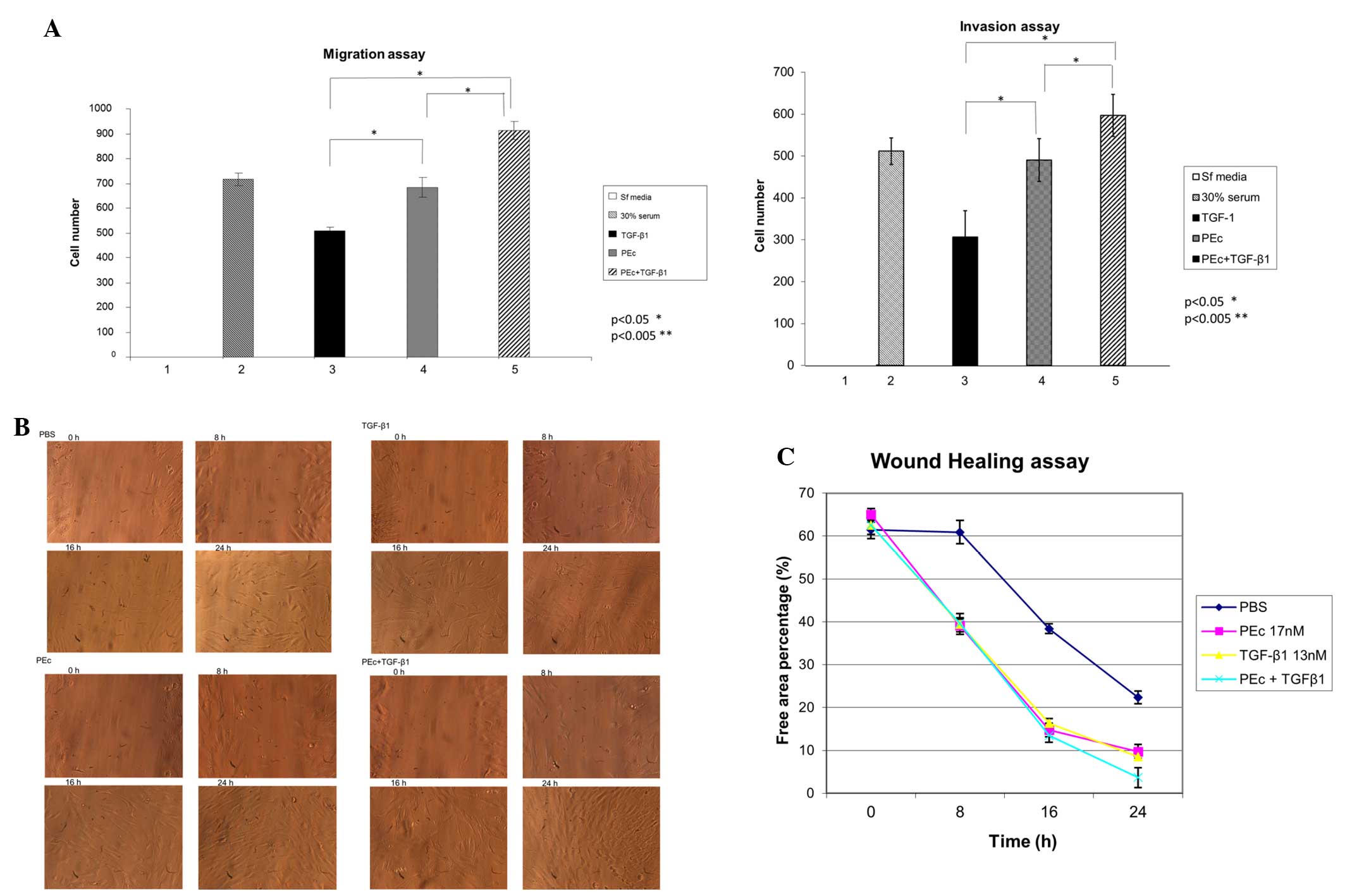 | Figure 4(A) Determination of the effects of
PEc, TGF-β1 and the combination of both, in the hMSC attraction as
assessed by migration/invasion assays (Transwell system) at 24 h.
The top well always contained the hMSCs. PEc and PEc+TGF-β1 were
associated with a significant increase in hMSC migration and
invasion compared with TGF-β1 alone (migration assay PEc vs.
TGF-β1: P=0.04, PEc+TGF-β1: P<0.001; invasion assay, PEc vs. TGF
β1 and PEc vs. PEc+TGF β1: P<0.002). (B) Wound healing assay of
the effects of PEc, TGF-β1 and TGF-β1+PEc on the HMC mobilization.
(C) Quantification of the wound healing assay. The combination
treatment presented a significant increase in migration of hMSCs
compared with the PEc alone (P=0.004). HMC treated with PEc in
combination with TGF-β1 presented a much greater mobilization
activity in 24 h compared to the HMC treated with TGF-β1 alone
(one-way analysis of variance, P=0.008, n=3).
*P<0.05, **P<0.005. PEc, Ec peptide;
TGF-β1, transforming growth factor β1; hMSCs, human mesenchymal
stem cells. |
To further examine the effect of these factors on
hMSC mobilization, a wound healing assay was conducted. It was
determined that hMSCs treated with PEc+TGF-β1 exhibited a
significantly elevated migration level at 24 h compared with TGF-β1
or PEc alone, similar to that obtained by PEc treatment (Fig. 4B and C).
Discussion
MSCs are able to differentiate into osteoblasts,
chondrocytes, and adipocytes and expand to numbers relevant for
clinical application. Growth factors are essential in the
chondrogenic differentiation of hMSCs. Previous studies have
demonstrated that supplementation of TGF-β1 initiated and improved
chondrogenic differentiation of MSCs in a time- and dose-dependent
manner (35).
In this study, the effects of PEc (the last 24 aa of
the COO− terminal of the IGF-1Ec isoform, not included
in the mature IGF-1) on human mesenchymal cell differentiation and
migration, alone and in combination with TGF-β1 were examined.
Recent evidence suggests that PEc attracts mesenchymal cells prior
to the repair of myocardial cells and of muscle cells (26–28),
whereas it was also found to inhibit HMSC osteogenic
differentiation and induce differentiation towards adipocytes
(28). TGF-β1 is a known factior
to cause HMSC cells to differentiate towards chondrocytes that has
been introduced in clinical practise (12). The aim of the present study was to
examine the possibility of using a combination of these factors in
clinical practice. It was observed that PEc activates ERK 1/2 in a
similar manner to TGF-β1. Since TGF-β1 induces mesenchymal
differentiation through ERK 1/2 activation, the effect of PEc on
mesenchymal cell differentiation was examined. The results obtained
suggest that PEc, apart from inducing mesenchymal cell
mobilization, interestingly and in contrast to a previous study
(28), can also induce the
differentiation towards chondrocytes, similar to TGF-β1. The
combination of both factors exhibited a greater effect as shown by
significantly higher production of Col II and decreased production
of Col I compared with the treatment with the two factors
separately, whilst Col X was unaffected.
The effect of PEc, TGF-β1, and the combination of
both on the migration and invasion capacities of hMSCs was also
investigated. PEc induced hMSC migration and invasion capacities
more than TGF-β1, whereas the combination of both factors was
associated with a significant increase of the hMSC migration and
invasion ability compared with PEc alone. The results from the
wound healing assay were similar as it was determined that the
combination of these two factors was associated with increased
mesenchymal cell mobilization compared with TGF-β1 alone and was
similar to that induced by PEc.
These results suggest that the two factors do not
negatively interfere with each other in respect to their effect on
the differentiation and migration capacity of hMSCs into
chondrocytes. The combination of these factors appears to have a
synergistic effect in causing hMSC differentiation towards
chondrocytes. Furthermore, the ability of PEc to attract hMSCs is
not diminished when it is combined with TGF-β1.
Further studies in in vivo models are
required in order to better assess the effects of the introduction
of PEc and of PEc together with TGF-β1 on cartilage formation.
Acknowledgments
This study was supported by the National and
Kapodistrian University of Athens, as well as the Department of
Physiology, Athens Medical School, Greece.
References
|
1
|
Clegg TE, Caborn D and Mauffrey C:
Viscosupplementation with hyaluronic acid in the treatment for
cartilage lesions: A review of current evidence and future
directions. Eur J Orthop Surg Traumatol. 23:119–124. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nicolini AP, Carvalho RT, Dragone B, Lenza
M, Cohen M and Ferretti M: Updates in biological therapies for knee
injuries: Full thickness cartilage defect. Curr Rev Musculoskelet
Med. 7:256–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sofat N and Kuttapitiya A: Future
directions for the management of pain in osteoarthritis. Int J Clin
Rheumtol. 9:197–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Safran MR and Seiber K: The evidence for
surgical repair of articular cartilage in the knee. J Am Acad
Orthop Surg. 18:259–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sterodimas A, de Faria J, Correa WE and
Pitanguy I: Tissue engineering and auricular reconstruction: A
review. J Plast Reconstr Aesthet Surg. 62:447–452. 2009. View Article : Google Scholar
|
|
6
|
Huckle J, Dootson G, Medcalf N, McTaggart
S, Wright E, Carter A, Schreiber R, Kirby B, Dunkelman N, Stevenson
S, et al: Differentiated chondrocytes for cartilage tissue
engineering. Novartis Found Symp. 249:103–112; discussion 112–117,
170–174, 239–241. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baghaban Eslaminejad M and Malakooty Poor
E: Mesenchymal stem cells as a potent cell source for articular
cartilage regeneration. World J Stem Cells. 6:344–354. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Florine EM, Miller RE, Porter RM, Evans
CH, Kurz B and Grodzinsky AJ: Effects of dexamethasone on
mesenchymal stromal cell chondrogenesis and aggrecanase activity:
Comparison of agarose and self-assembling peptide scaffolds.
Cartilage. 4:63–74. 2013. View Article : Google Scholar
|
|
9
|
Haleem-Smith H, Calderon R, Song Y, Tuan
RS and Chen FH: Cartilage oligomeric matrix protein enhances matrix
assembly during chondrogenesis of human mesenchymal stem cells. J
Cell Biochem. 113:1245–1252. 2012. View Article : Google Scholar :
|
|
10
|
Seo S and Na K: Mesenchymal stem
cell-based tissue engineering for chondrogenesis. J Biomed
Biotechnol. 2011:8068912011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhardwaj N and Kundu SC: Chondrogenic
differentiation of rat MSCs on porous scaffolds of silk
fibroin/chitosan blends. Biomaterials. 33:2848–2857. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Re'em T, Kaminer-Israeli Y, Ruvinov E and
Cohen S: Chondrogenesis of hMSC in affinity-bound TGF-beta
scaffolds. Biomaterials. 33:751–761. 2012. View Article : Google Scholar
|
|
13
|
Chen CC, Liao CH, Wang YH, Hsu YM, Huang
SH, Chang CH and Fang HW: Cartilage fragments from osteoarthritic
knee promote chondrogenesis of mesenchymal stem cells without
exogenous growth factor induction. J Orthop Res. 30:393–400. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JI, Sato M, Kim HW and Mochida J:
Transplantatation of scaffold-free spheroids composed of
synovium-derived cells and chondrocytes for the treatment of
cartilage defects of the knee. Eur Cell Mater. 22:275–290;
discussion 290. 2011.PubMed/NCBI
|
|
15
|
Nakamura T, Sekiya I, Muneta T, Hatsushika
D, Horie M, Tsuji K, Kawarasaki T, Watanabe A, Hishikawa S,
Fujimoto Y, et al: Arthroscopic, histological and MRI analyses of
cartilage repair after a minimally invasive method of
transplantation of allogeneic synovial mesenchymal stromal cells
into cartilage defects in pigs. Cytotherapy. 14:327–338. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato M, Uchida K, Nakajima H, Miyazaki T,
Guerrero AR, Watanabe S, Roberts S and Baba H: Direct
transplantation of mesenchymal stem cells into the knee joints of
Hartley strain guinea pigs with spontaneous osteoarthritis.
Arthritis Res Ther. 14:R312012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vanlauwe J, Huylebroek J, Van Der Bauwhede
J, Saris D, Veeckman G, Bobic V, Victor J, Almqvist KF, Verdonk P,
Fortems Y, et al: Clinical outcomes of characterized chondrocyte
implantation. Cartilage. 3:173–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tuli R, Tuli S, Nandi S, Huang X, Manner
PA, Hozack WJ, Danielson KG, Hall DJ and Tuan RS: Transforming
growth factor-beta-mediated chondrogenesis of human mesenchymal
progenitor cells involves N-cadherin and mitogen-activated protein
kinase and Wnt signaling cross-talk. J Biol Chem. 278:41227–41236.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Zhao Z, Liu J, Huang N, Long D, Wang
J, Li X and Liu Y: MEK/ERK and p38 MAPK regulate chondrogenesis of
rat bone marrow mesenchymal stem cells through delicate interaction
with TGF-beta1/Smads pathway. Cell Prolif. 43:333–343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zaslav K, McAdams T, Scopp J, Theosadakis
J, Mahajan V and Gobbi A: New frontiers for cartilage repair and
protection. Cartilage. 3(Suppl 1): S77–S86. 2012. View Article : Google Scholar
|
|
21
|
Achar RA, Silva TC, Achar E, Martines RB
and Machado JL: Use of insulin-like growth factor in the healing of
open wounds in diabetic and non-diabetic rats. Acta Cir Bras.
29:125–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stavropoulou A, Halapas A, Sourla A,
Philippou A, Papageorgiou E, Papalois A and Koutsilieris M: IGF-1
expression in infarcted myocardium and MGF E peptide actions in rat
cardiomyocytes in vitro. Mol Med. 15:127–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Armakolas A, Philippou A, Panteleakou Z,
Nezos A, Sourla A, Petraki C and Koutsilieris M: Preferential
expression of IGF-1Ec (MGF) transcript in cancerous tissues of
human prostate: Evidence for a novel and autonomous growth factor
activity of MGF E peptide in human prostate cancer cells. Prostate.
70:1233–1242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Philippou A, Armakolas A, Panteleakou Z,
Pissimissis N, Nezos A, Theos A, Kaparelou M, Armakolas N,
Pneumaticos SG and Koutsilieris M: IGF1Ec expression in MG-63 human
osteoblast-like osteosarcoma cells. Anticancer Res. 31:4259–4265.
2011.PubMed/NCBI
|
|
25
|
Milingos DS, Philippou A, Armakolas A,
Papageorgiou E, Sourla A, Protopapas A, Liapi A, Antsaklis A,
Mastrominas M and Koutsilieris M: Insulinlike growth factor-1Ec
(MGF) expression in eutopic and ectopic endometrium:
Characterization of the MGF E-peptide actions in vitro. Mol Med.
17:21–28. 2011. View Article : Google Scholar :
|
|
26
|
Wu J, Wu K, Lin F, Luo Q, Yang L, Shi Y,
Song G and Sung KL: Mechano-growth factor induces migration of rat
mesenchymal stem cells by altering its mechanical properties and
activating ERK pathway. Biochem Biophys Res Commun. 441:202–207.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hill M, Wernig A and Goldspink G: Muscle
satellite (stem) cell activation during local tissue injury and
repair. J Anat. 203:89–99. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui H, Yi Q, Feng J, Yang L and Tang L:
Mechano growth factor E peptide regulates migration and
differentiation of bone marrow mesenchymal stem cells. J Mol
Endocrinol. 52:111–120. 2014. View Article : Google Scholar
|
|
29
|
Armakolas A, Kaparelou M, Dimakakos A,
Papageorgiou E, Armakolas N, Antonopoulos A, Petraki C, Lekarakou
M, Lelovas P, Stathaki M, et al: Oncogenic role of the Ec peptide
of the IGF-1Ec isoform in prostate cancer. Mol Med. 21:167–179.
2015.PubMed/NCBI
|
|
30
|
Matheny RW Jr, Nindl BC and Adamo ML:
Minireview: Mechano-growth factor: A putative product of IGF-I gene
expression involved in tissue repair and regeneration.
Endocrinology. 151:865–875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soleimani M and Nadri S: A protocol for
isolation and culture of mesenchymal stem cells from mouse bone
marrow. Nat Protoc. 4:102–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). Method Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Lunstrum GP, Keene DR, Weksler NB, Cho YJ,
Cornwall M and Horton WA: Chondrocyte differentiation in a rat
mesenchymal cell line. J Histochem Cytochem. 47:1–6. 1999.
View Article : Google Scholar
|
|
34
|
Pelttari K, Steck E and Richter W: The use
of mesenchymal stem cells for chondrogenesis. Injury. 39(Suppl 1):
S58–S65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pei M, He F and Vunjak-Novakovic G:
Synovium-derived stem cell-based chondrogenesis. Differentiation.
76:1044–1056. 2008. View Article : Google Scholar : PubMed/NCBI
|