Introduction
Despite the great progress that has been made in
early screening of cervical cancer programs, cervical cancer
remains the leading cause of gynecological cancer-associated
mortalities worldwide (1).
Persistent high-risk human papilloma virus infection is suggested
to be the prerequisite for cervical cancer (2); however, the virus alone is
insufficient for the development of cervical cancer, suggesting
that other molecular events are involved in the initiation and
progression of cervical cancer (3). The expression of molecules associated
with tumor invasion and metastasis indicate an unfavorable
prognosis (4). The present
understanding of the molecular mechanisms contributing to the
invasion-metastasis of cervical cancer remains limited, although
accumulating evidence suggests that the epithelial-mesenchymal
transition (EMT) serves a pivotal role in the initial step of tumor
invasion-metastasis (5).
Consequently, the EMT has become the focus of cervical cancer
metastasis research.
The EMT is a process of switching the epithelial
cell phenotype to a more loosely fibroblast-like phenotype
(6). This is coupled with the loss
of cell polarity and intercellular junctions, remodeling of the
cytoskeleton and alteration of various EMT-related markers,
including downregulation of epithelial markers, including
E-cadherin, and upregulation of the mesenchymal markers N-cadherin
and vimentin (6). These changes
provide cancer cells with elevated invasive/metastatic capacities
and therapeutic resistance (7).
Epidermal growth factor receptor pathway substrate 8
(Eps8) was initially identified as a novel substrate for epidermal
growth factor receptor (EGFR) kinase (8). Overexpression of Eps8 facilitates
enhanced mitogenesis and malignant transduction via EGFR-mediated
activation of downstream signaling pathways (9). Previous studies have revealed that
Eps8 is highly expressed in various human tumor types, including
colorectal, pituitary, oral, esophageal, pancreatic, ovarian, lung,
breast, thyroid and cervical cancer (10–17).
Eps8 overexpression has also been shown to be involved in numerous
signaling pathways associated with tumorigenesis, metastasis and
proliferation of cancer, and represents an unfavorable prognostic
biomarker in patients with cancer (10,12–14,17).
Epithelial growth factor (EGF) is suggested to be
one of the most potent EMT regulatory factors in cervical cancer.
Overexpression of EGFR in cervical cancer indicates poor clinical
prognosis and EGF-mediated EMT may account for this (18–20).
More recent studies have shown that chemoresistance is associated
with the EMT in cancer cells, and the acquired chemotherapeutic
resistance is often accompanied by the transformation from the
epithelial to the mesenchymal phenotype in cancer cells (21–23).
Chen et al (17) found that
Eps8 knockdown contributes to increased sensitivity to
chemotherapy. Based on these findings, it was hypothesized that
Eps8 is associated with the process of EMT in cervical cancer.
In the present study, immunohistochemistry was used
to detect the expression of Eps8 in different cervical samples.
Correlations between Eps8 expression and the clinicopathological
characteristics, as well as EMT associated proteins in cervical
cancer tissues were further analyzed. RNA interference (RNAi) was
applied to inhibit the expression of Eps8 in cervical cancer cells,
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analyses were performed to investigate
the influence of Eps8 knockdown on EMT markers at the mRNA and
protein expression levels. Transwell cell migration and Matrigel
invasion assays were used to investigate the effects of
Eps8-knockdown on the capacity of invasive and migration in HeLa
and SiHa cells.
Materials and methods
Cell culture and clinical specimens
Human HeLa and SiHa cervical cancer cell lines were
purchased from the Cell Bank of China (Shanghai, China) and were
maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin/streptomycin solution (Gibco; Thermo Fisher
Scientific, Inc.). The cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2.
A total of 134 paraffin-embedded cervical specimens,
including 16 normal cervical epithelia (NC), 25 low-grade squamous
intraepithelial lesion (LSIL), 30 high-grade squamous
intraepithelial lesion (HSIL) and 63 squamous cervical cancer (SCC)
specimens, were obtained from the Pathology Department at the
International Peace Maternity and Child Health Hospital of the
China Welfare Institute (Shanghai, China) between 2008 and 2014.
The age of the patients was between 29 and 68 years (mean age, 42
years). The NC specimens were obtained from patients who underwent
hysterectomies for uterine fibroids, the LSIL and HSIL specimens
were obtained from patients who underwent cervical conization. None
of the patients enrolled in the present study received preoperative
chemotherapy, radiotherapy or immunotherapy. The tumor clinical
stages and clinicopathological classifications were based on the
International Federation of Gynecology and Obstetrics (FIGO)
criteria. Approval for the use of these specimens was granted by
the Ethics Committee of the International Peace Maternity and Child
Health Hospital of the China Welfare Institute. The main clinical
characteristics of the patients with cervical cancer are summarized
in Table I.
 | Table ICorrelation between the
clinicopathological characteristics, and the expression levels of
Eps8, E-cadherin and vimentin in patients with cervical cancer. |
Table I
Correlation between the
clinicopathological characteristics, and the expression levels of
Eps8, E-cadherin and vimentin in patients with cervical cancer.
| Characteristic | No. patients | Eps8 expression
| E-cadherin
expression
| Vimentin expression
|
|---|
| Negative | Low | High | P-value | Negative | Low | High | P-value | Negative | Low | High | P-value |
|---|
| Age | | | | | 0.503 | | | | 0.816 | | | | 0.240 |
| ≤45 years | 26 | 2 | 6 | 18 | | 5 | 14 | 7 | | 0 | 9 | 17 | |
| >45 years | 37 | 8 | 5 | 24 | | 11 | 14 | 12 | | 6 | 7 | 24 | |
| FIGO stage | | | | | 0.072 | | | | 0.491 | | | | 0.979 |
| I | 41 | 8 | 9 | 24 | | 8 | 21 | 12 | | 2 | 13 | 26 | |
| II | 22 | 2 | 2 | 18 | | 8 | 7 | 7 | | 4 | 3 | 15 | |
| Histological
grade | | | | | 0.001 | | | | 0.104 | | | | 0.508 |
| Well | 12 | 6 | 3 | 3 | | 2 | 4 | 6 | | 1 | 5 | 6 | |
| Moderate | 41 | 3 | 7 | 31 | | 12 | 21 | 8 | | 4 | 9 | 28 | |
| Poor | 10 | 1 | 1 | 8 | | 2 | 3 | 5 | | 1 | 2 | 7 | |
| Invasive
interstitial depth | | | | | <0.001 | | | | 0.015 | | | | 0.005 |
| <1/2 | 16 | 8 | 4 | 4 | | 2 | 5 | 9 | | 5 | 4 | 7 | |
| ≥1/2 | 47 | 2 | 7 | 38 | | 14 | 23 | 10 | | 1 | 10 | 36 | |
| Lymph node
metastases | | | | | 0.009 | | | | 0.006 | | | | 0.015 |
| Negative | 46 | 9 | 11 | 26 | | 15 | 21 | 10 | | 6 | 15 | 25 | |
| Positive | 17 | 1 | 0 | 16 | | 1 | 7 | 9 | | 0 | 1 | 16 | |
Immunohistochemical analysis
Immunohistochemical staining was performed on 4
µm thick paraffin-embedded tissue sample sections. Briefly,
the sections were dewaxed with xylene twice for 15 min and
rehydrated through a graded alcohol series (100, 90 and 70%) at
room temperature. Antigen retrieval was performed using citrate
buffer (pH 6.0) at 95°C for 15 min and endogenous peroxidase
activity was quenched by immersion in 3% H2O2
for 10 min at room temperature. Subsequently, tissue sections were
blocked by incubation in 1% bovine serum albumin (BSA; Beyotime
Institute of Biotechnology, Beijing, China) for 30 min at room
temperature to prevent non-specific binding. The tissue sections
were then incubated at 4°C overnight with anti-Eps8 (1:200;
ab203272; Abcam, Cambridge, UK), anti-E-cadherin (1:300; 3195S;
Cell Signaling Technology, Inc., Danvers, MA, USA) and
anti-vimentin (1:200; 5741S; Cell Signaling Technology, Inc.)
primary rabbit monoclonal antibodies. Following incubation with the
primary antibodies, the tissues sections were incubated at 37°C for
30 min with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibody (1:500; GTX26721; GeneTex Inc., Irvine, CA,
USA). The 3,3′-diaminobenzidine substrate was applied to visualize
the immunoreactivity prior to counterstaining the slides with 10%
hematoxylin, dehydrating in graded ethanol and cleared in xylene.
For negative controls, phosphate-buffered saline was substituted
for the primary antibodies. The expression levels were evaluated by
two pathologists in a blinded manner. The protein expression levels
of Eps8, E-cadherin and vimentin were scored semi-quantitatively by
multiplying the 'percent positivity score' by the 'staining
intensity score'. The percent positivity was scored according to
the following criteria: 0, <10%; 1, 10–24%; 2, 25–50%; 3,
>50% positive tumor cells. Staining intensity was graded as 0,
no staining; 1, weak staining; 2, moderate staining; or 3, strong
staining. The final score was defined as follows: 0, negative; 1–4,
low expression; 5–12, high expression.
Transient transfection
The short hairpin (sh)RNA sequence targeting Eps8
(Eps8-shRNA: 5′-GCTGTGAGCCTGATTGATTTA-3′) and the negative control
shRNA were synthesized and cloned into the pGPU6/green fluorescent
protein (GFP)/Neo vector (GenePharma, Shanghai, China). The HeLa
(2×105) or SiHa (4×105) cells were grown in
6-well plates at 70–80% confluence prior to transient transfection
with a mixture of Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) and Eps8 shRNA or control shRNA, according to the
manufacturer's instructions. Following incubation for 6 h, the
transfection medium was replaced with regular medium and after 48
h, GFP expression was evaluated by fluorescent microscopy (Zeiss
Axioskop 40; Zeiss, Oberkochen, Germany), for the qualitative
assessment of the transfection efficiency. The cells were harvested
at 48 h post-transfection for RNA analyses and at 72 h
post-transfection for protein analyses to confirm the
downregulation of Eps8 expression.
RNA extraction and RT-qPCR
The total RNA from HeLa and SiHa cells was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
The extracted RNA (2 µg) was reverse-transcribed into cDNA
using the Prime Script RT reagent kit (Takara Bio, Inc., Dalian,
China), according to the manufacturer's protocol. RT-qPCR was then
performed in Eps8-silenced and control cells using the cDNA as a
template and the SYBR Green PCR kit (Takara Bio, Inc.), according
to the manufacturer's protocol. All primers used in this
experiments were designed using Premier 5.0 software and their
sequences are listed in Table II.
The PCR reaction conditions were as follows: 95°C for 3 min,
followed by 40 cycles at 95°C for 10 sec and 60°C for 30 sec. The
target gene expression levels were normalized against that of
GAPDH. Quantification of the relative expression of target genes
was calculated using the comparative 2−ΔΔCq method
(24). All experiments were
performed in triplicate.
 | Table IIQuantitative polymerase chain
reaction primer sequences. |
Table II
Quantitative polymerase chain
reaction primer sequences.
| Gene | Sequence
(5′-3′) |
|---|
| Eps8 | |
| Forward |
TGAATGGCTACGGATCATCACC |
| Reverse: |
CACTGTCCCGTGCATAATTCT |
| E-cadherin | |
| Forward |
CGAGAGCTACACGTTCACGG |
| Reverse |
GGGTGTCGAGGGAAAAATAGG |
| Vimentin | |
| Forward |
GACGCCATCAACACCGAGTT |
| Reverse |
CTTTGTCGTTGGTTAGCTGGT |
| Snail | |
| Forward |
TCGGAAGCCTAACTACAGCGA |
| Reverse |
AGATGAGCATTGGCAGCGAG |
| GAPDH | |
| Forward |
GGAGCGAGATCCCTCCAAAAT |
| Reverse |
GGCTGTTGTCATACTTCTCATGG |
Western blot analysis
HeLa and SiHa cells were harvested and washed twice
using ice-cold PBS. The cells were subsequently lysed on ice for 30
min with radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology, Beijing, China), containing 1 mM
phenylmethanesulfonyl fluoride. The lysates were subsequently
centrifuged at 12,000 × g at 4°C for 15 min. The protein
concentrations were measured using bicinchoninic acid protein
assays (Beyotime Biotechnology). Equivalent quantities (60
µg) of total protein were separated on 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gel and blotted onto a
polyvinylidene membrane (EMD Millipore, Billerica, MA, USA).
Following blocking in 5% BSA at room temperature for 2 h, the
membranes were incubated overnight at 4°C with the following
primary rabbit monoclonal antibodies: Anti-Eps8 (1:1,000; Abcam),
anti-E-cadherin (1:1,000; Cell Signaling Technology, Inc.),
anti-vimentin (1:1,000; Cell Signaling Technology, Inc.) and
anti-GAPDH (1:2,000; Tiangen Biotech Co., Ltd., Beijing, China).
The membranes were washed in Tris-buffered saline containing 0.1%
Tween-20 three times and were subsequently incubated with
horseradish peroxidase-conjugated goat-anti-rabbit secondary
antibody (1:2,000; GeneTex Inc.) for 1 h at room temperature.
Target protein bands were visualized by electrochemical
luminescence using an Enhanced Chemiluminescence Plus kit (Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol. The levels of target proteins were normalized against
that of GAPDH using Image Pro Plus 6.0.
Cell migration and invasion assays
The migration/invasion assay was performed using
24-well Transwell chambers (Corning, Inc., Glendale, AZ, USA)
containing polycarbonate filters (8 µm pore size) that were
uncoated/coated with Matrigel (BD Biosciences, Hercules, CA, USA).
At 48 h after transfection, 2×104 cells were resuspended
in 200 µl serum-free DMEM medium with 0.1% BSA and were
added to the upper chambers, while 500 µl DMEM with 20% FBS
was placed in the lower chambers. Following incubation for 24 h,
the non-migratory/non-invasive cells on the upper chamber surface
were wiped off using a cotton swab and the cells that had
migrated/invaded to the lower membrane surface were fixed for 20
min in 4% paraformaldehyde, and stained for 15 min with 0.1%
crystal violet. A total of 10 random visual fields were selected
under an inverted microscope (magnification, ×200) to capture
images and count the number of migrated/invading cells. Each assay
was performed in triplicate.
Statistical analysis
Each experiment was performed in triplicate. SPSS
17.0 software was used to perform statistical analysis. The data
are presented as the mean ± standard deviation. Kruskal-Wallis
one-way analysis of variance or Student's unpaired t-test was
applied to evaluate significant differences. Correlations were
assessed using the Pearson's correlation coefficient test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression levels of Eps8, E-cadherin and
vimentin in the normal cervix, LSIL, HSIL and SCC
To investigate the expression of Eps8 and its
association with EMT-related proteins, immunohistochemistry was
performed using Eps8, E-cadherin and vimentin antibodies in NC,
LSIL, HSIL and SCC (Fig. 1A). Eps8
and vimentin were predominantly localized in the cytoplasm of
cervical cancer tissues, and were absent from the 16 normal cervix
samples. Weak expression of Eps8 and vimentin was observed in 12/25
(48.0%) LSIL specimens, and 13/30 (43.3%) and 15/30 (50.0%) of the
30 HSIL specimens, respectively. Of the 63 SCC cases, the staining
intensity of Eps8 was weak and strong in 11 (17.5%) and 42 (66.7%)
specimens, respectively. In addition, vimentin was expressed weakly
in 16 cases (25.4%) and strongly in 41 cases (65.1%). E-cadherin
was expressed predominantly in the plasma membrane of epithelial
cells in the NC. Based on the expression scores, the percentage of
high E-cadherin-expressing samples decreased gradually from 87.5%
(14/16) in NC, >72.0% (18/25) in LSIL and >60.0% (18/30) in
HSIL to 30.2% (19/63) in SCC (Fig.
1B).
 | Figure 1Expression levels of Eps8 and EMT
markers in cervical cancer samples. (A) Immunohistochemical
staining for Eps8 and the EMT markers, E-cadherin and Vimentin, in
NC, LSIL, HSIL and SCC tissues (magnification, ×400). (B) The
expression levels of Eps8, E-cadherin and Vimentin were categorized
as negative, low or high expression in the normal cervix, LSIL,
HSIL, and SCC samples. Esp8, epidermal growth factor receptor
kinase substrate 8; EMT, epithelial-mesenchymal transition; NC,
normal cervical epithelia; LSIL, low-grade squamous intraepithelial
lesion; HSIL, high-grade squamous intraepithelial lesion; SCC,
squamous cell carcinoma. |
Association of the expression levels of
Eps8, E-cadherin, vimentin with the clinicopathological
characteristics of patients with SCC
As shown in Table
II, Eps8 expression was significantly associated with
histological grade (P=0.001), invasive interstitial depth
(P<0.001) and lymph node metastases (P=0.009). However, little
association was demonstrated between the expression of Eps8 and age
(P=0.503), and FIGO stage (P=0.072). The expression levels of
E-cadherin and vimentin demonstrated a marked correlation with
invasive interstitial depth (P=0.015 and P=0.005, respectively) and
lymph node metastases (P=0.006 and P=0.015, respectively).
Expression of Eps8 correlates with
EMT-related proteins in cervical cancer samples
The present study further explored correlations
between the expression of Eps8 and EMT marker proteins in 63
samples of cervical SCC tissues. In accordance with the changes in
the expression of proteins during the EMT process, the reduced
expression of E-cadherin was significantly associated with the
increased expression of vimentin (P=0.001; r=−0.398). Furthermore,
a significant negative correlation was observed between the
expression levels of E-cadherin and Eps8 (P=0.004; r=−0.355), and
the expression of Eps8 correlated positively with that of vimentin
(P<0.001; r= 0.562) (Table
III). These analyses suggested that Eps8 is associated with the
expression of the EMT-related proteins and may be an important
indicator of the EMT process in cervical cancer.
 | Table IIIAssociation between the expression
levels of Eps8, E-cadherin and vimentin in patients with cervical
cancer. |
Table III
Association between the expression
levels of Eps8, E-cadherin and vimentin in patients with cervical
cancer.
| Definition | Eps8 expression
| Vimentin expression
|
|---|
| Negative | Low | High | P-value | r | Negative | Low | High | P-value | r |
|---|
| E-cadherin
expression | | | | 0.004 | −0.355 | | | | 0.001 | −0.398 |
| Negative | 2 | 1 | 13 | | | 1 | 2 | 13 | | |
| Low | 2 | 3 | 23 | | | 1 | 5 | 22 | | |
| High | 6 | 7 | 6 | | | 4 | 9 | 6 | | |
| Vimentin
expression | | | | <0.001 | 0.562 | | | | | |
| Negative | 6 | 0 | 0 | | | | | | | |
| Low | 3 | 9 | 4 | | | | | | | |
| High | 1 | 2 | 38 | | | | | | | |
Knockdown of Eps8 led to increased
expression of epithelial markers and reduced expression of
mesenchymal markers
To further characterize the role of Eps8 in the
process of the EMT in cervical cancer, Eps8-targeting shRNA and
control shRNA plasmids were designed and transiently transfected
into HeLa and SiHa cells. To confirm Eps8-knockdown and to
investigate the influence of Eps8 on EMT markers, RT-qPCR and
western blot analyses were performed in Eps8-inhibited cell lines
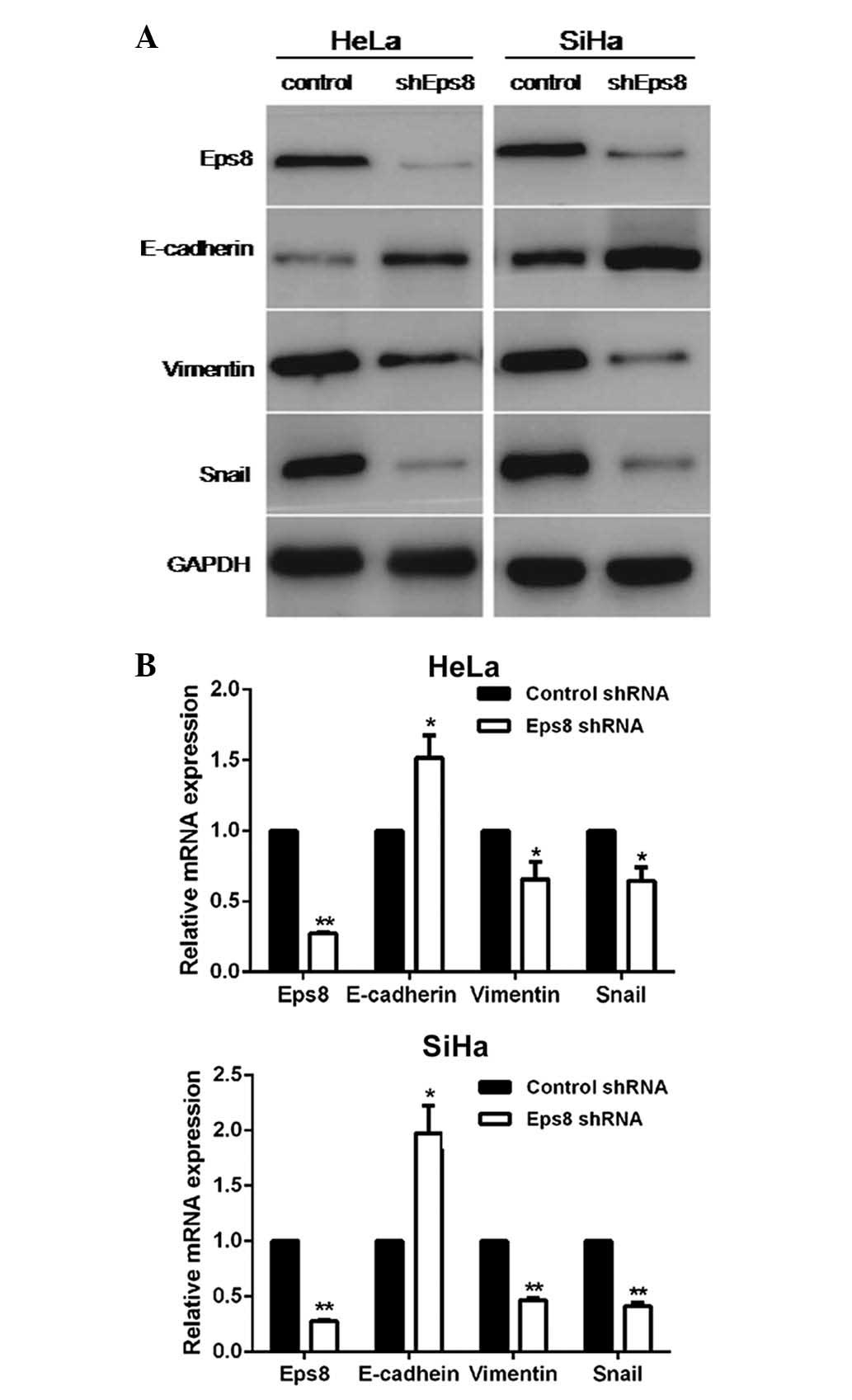
and control cells. As shown in Fig.
2, Eps8 expression in Eps8-inhibited cell lines was
significantly downregulated at both the mRNA and protein expression
levels compared with the control cells. RT-qPCR analysis
demonstrated that knockdown of Eps8 led to increased expression of
the epithelial marker, E-cadherin, and decreased expression of the
mesenchymal marker, vimentin, as well as a key EMT inducer, snail,
at the mRNA level (Fig. 2A).
Additionally, western blot analysis revealed that the inhibition of
Eps8 resulted in downregulated protein expression of vimentin and
snail (Fig. 2B). Taken together,
these results revealed that Eps8 is involved in EMT progression and
that inhibition of Eps8 impairs the process of the EMT in cervical
cancer.
Eps8 silencing represses cell migration
and invasion
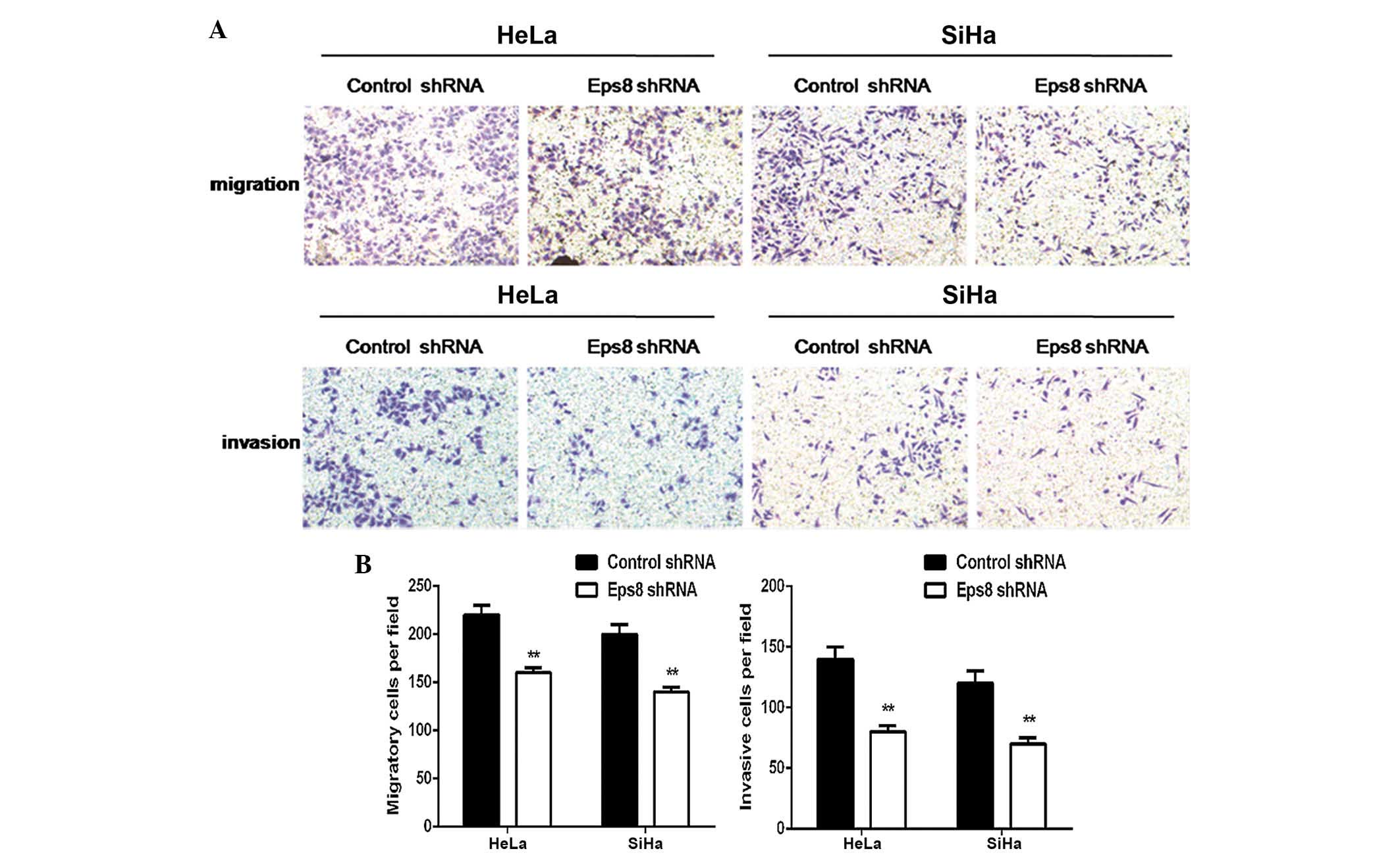
The present study subsequently performed Transwell
cell migration and Matrigel invasion assays to investigate the
effects of Eps8 knockdown on the regulation of cell migration and
invasion in HeLa and SiHa cells. As shown in Fig. 3, the Transwell migration assay
demonstrated that Eps8 knockdown notably inhibited the migration
capacity of the Eps8 shRNA transfected cells compared with that of
the control cells (P<0.01; Fig.
3). Matrigel invasion assays also demonstrated that
transfection with Eps8 shRNA plasmids effectively impaired the
invasive capacity of HeLa and SiHa cells (P<0.01; Fig. 3). These results suggested that Eps8
knockdown decreases the migration and invasiveness of HeLa and SiHa
cell.
Discussion
The progression of malignant tumors is a
sophisticated process involving multiple factors and molecular
events. The EMT, which is believed to be a crucial step in tumor
metastasis (25), allows cancer
cells to invade adjacent tissues and metastasize to distance sites.
It is important to understand the molecular mechanisms underlying
the EMT pathway and identification of ways to prevent or reverse
the process are critical to the development of novel therapeutic
strategies against cancer. Several signaling pathways, including
the Wnt, transforming growth factor-β, EGF, Notch and fibroblast
growth factor pathways, have been shown to be involved in
regulating the process of the EMT by modulating the expression of
certain key transcription factors, including snail, ZEB and twist,
which suppress the expression of E-cadherin and activate the
mesenchymal transcriptomes (26,27).
Eps8 is a novel phosphorylation substrate for EGFR,
which enhances the cellular transformation of EGFR-dependent
mitogenic signals upon overexpression (28). As an actin-capping protein, the
role of Eps8 in cancer metastasis has aroused widespread concern,
resulting in a number of studies to elucidate the mechanisms by
which Eps8 influences tumor migration. Eps8 overexpression is
responsible for enhanced Rac-induced actin cytoskeleton remodeling
and cell migration by formation of Eps8-Abi-1-Sos-1 and Eps8-IRSp53
complexes, which are involved in transducing signals to activate
the Rho-GTPase family members Rac and Cdc42 (29–31).
In addition to the role of Eps8 in tumor metastasis mediated via
Rac-dependent pathways, Eps8 is also involved in tumor migration
mediated via Rac-independent pathways, including the the
Eps8/AKT/FOXM1/matrix metalloproteinase-9 cascade (32). Although considerable efforts have
been made to clarify the functions of Eps8 in the metastasis of
numerous cancer types, the role of Eps8 in the development of
cervical cancer remains to be elucidated. Chen et al
(17) analyzed the expression
levels of Eps8 in 110 cervical cancer samples by
immunohistochemistry and observed that increased Eps8 expression
was associated with shorter disease-free survival and overall
survival (17). These observations
suggested that Eps8 serves a role in the progression of cervical
cancer. Therefore, the present study hypothesized that Eps8 is also
involved in cervical cancer metastasis. The present study focused
predominantly on the EMT-related role of Eps8 in this process.
The present data demonstrated that Eps8 expression
in cervical cancer samples was significantly higher compared with
that in NC, LSIL and HSIL samples; thus, indicating the involvement
of Eps8 in the development of cervical cancer. The analysis with
regard to its clinical significance revealed that high Eps8
expression correlated with histological grade and lymph node
metastases, suggesting that Eps8 serves a pivotal role in cervical
cancer progression, and may be an independent biomarker for an
unfavorable clinical prognosis. This is in agreement with previous
observations that Eps8 overexpression is associated with poor
prognosis in patients with oral squamous cell carcinoma, mixed
lineage leukaemia and ovarian cancer (33–35).
It is generally accepted that decreased expression of E-cadherin,
coupled with increased expression of vimentin, is characteristic of
EMT (36). Therefore, the present
study analyzed the possible association between the expression
levels of Eps8 and EMT-associated proteins (E-cadherin and
vimentin) in cervical carcinoma tissues. Correlation analysis
revealed that high Eps8 expression was negatively correlated with
the expression of E-cadherin and positively correlated with the
expression of vimentin, implying the involvement of Eps8 in the EMT
phenotype. Taken together, it was hypothesized that Eps8 makes a
vital contribution to the progression of cervical cancer, partly
through its influence on the EMT.
Given the results of the immunohistochemical
analysis, the present study further investigated the role of Eps8
in the process of the EMT using shRNA-mediated knockdown of Eps8 in
HeLa and SiHa cells. Eps8 knockdown appeared to cause a reversal of
the EMT process in HeLa and SiHa cells, with changes observed in
the expression of certain EMT markers (Fig. 2), including the E-cadherin
upregulation and vimentin downregulation at both the mRNA and
protein expression levels. E-cadherin is a major cell adhesion
molecule involved in epithelial cell adhesion. Its loss or
decreased expression has been suggested to be a key event in the
EMT process. Reduced expression of E-cadherin serves an pivotal
role in cancer progression and is associated with metastasis
(37–42). E-cadherin is considered to be a
prognostic biomarkers in certain solid malignant tumor types
(43,44). In addition, vimentin, as a
canonical marker of the EMT, is widely expressed in mesenchymal
cells. It has been previously reported that vimentin is
overexpressed in various epithelial-derived tumor cells and tissues
(45), and its overexpression is
associated with enhanced tumor cell invasiveness (46). The present finding that Eps8
knockdown resulted in E-cadherin upregulation and vimentin
downregulation suggested that Eps8 is involved in the EMT in HeLa
and SiHa cells.
The present study also revealed that knockdown of
Eps8 led to the downregulation of the transcription factor snail,
which is hypothesized to be a key regulator of the EMT (47). The expression of snail has been
implicated in suppressing the expression of E-cadherin and is
involved in the initial migratory phenotype (48). Snail is expressed at the invasive
front of tumors and is associated with tumor recurrence and lymph
node metastasis, suggesting a role for snail in tumor progression
(49). Other previous studies have
indicated that Eps8 activates AKT by stimulating
phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K) (16), and that the PI3K/AKT-snail pathway
is involved in the EMT in breast and lung cancer cells (50,51).
Furthermore, it was speculated that downregulation of snail by Eps8
silencing represents a plausible mechanism by which Eps8 is
involved in the process of the EMT in cervical cancer cells.
Furthermore, the present observation that Eps8 depletion in cell
invasion and migration assays significantly impaired the invasive
and migratory capacities of cervical cancer cells indicated that
Eps8 serves a vital role in tumor progression.
In conclusion, the results of the present study
demonstrated that the expression of Eps8 was significantly
associated with the expression of EMT-related proteins in cervical
cancer samples. Furthermore, these results indicated that Eps8
overexpression was correlated with lymphatic and distant metastasis
of cervical cancer. Additionally, decreased expression of Eps8
impaired the EMT process and suppressed the migration and invasion
of HeLa and SiHa cells. These results provided an insight into the
important function of Eps8 in cervical cancer progression by
orchestrating the EMT, and indicated that Eps8 is a novel potential
target for developing therapeutic strategies for cervical cancer.
However, the specific regulatory role of Eps8 in the EMT remains
unknown and further in vitro and in vivo
investigations are required to elucidate the detailed mechanism by
which Eps8 regulates the EMT.
Acknowledgments
The authors would like to thank Dr Huijuan Zhang and
Dr Bei Chen (Department of Pathology of the International Peace
Maternity and Child Health Hospital of the China Welfare Institute)
for their assistance with immunohistochemistry. This research was
supported by grants from the Project of Health and Family Planning
Commission of Shanghai Municipality (no. 201440603), the National
Natural Science Foundation of China (no. 81402134) and the Science
and Technology Commission of Shanghai Municipality (no.
12ZR1451400).
References
|
1
|
Crosbie EJ, Einstein MH, Franceschi S and
Kitchener HC: Human papillomavirus and cervical cancer. Lancet.
382:889–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Partridge EE, Abu-Rustum N, Giuliano A,
Massad S, McClure J, Dwyer M and Hughes M: National comprehensive
cancer network: Cervical cancer screening. J Natl Compr Canc Netw.
12:333–341; quiz 341. 2014.
|
|
3
|
Liu X, Wang D, Liu H, Feng Y, Zhu T, Zhang
L, Zhu B and Zhang Y: Knockdown of astrocyte elevated gene-1
(AEG-1) in cervical cancer cells decreases their invasiveness,
epithelial to mesenchymal transition, and chemoresistance. Cell
Cycle. 13:1702–1707. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim CJ, Jeong JK, Park M, Park TS, Park
TC, Namkoong SE and Park JS: HPV oligonucleotide microarray-based
detection of HPV genotypes in cervical neoplastic lesions. Gynecol
Oncol. 89:210–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jung HY, Fattet L and Yang J: Molecular
pathways: Linking tumor microenvironment to epithelial-mesenchymal
transition in metastasis. Clin Cancer Res. 21:962–968. 2015.
View Article : Google Scholar
|
|
6
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fazioli F, Minichiello L, Matoska V,
Castagnino P, Miki T, Wong WT and Di Fiore PP: Eps8, a substrate
for the epidermal growth factor receptor kinase, enhances
EGF-dependent mitogenic signals. EMBO J. 12:3799–3808.
1993.PubMed/NCBI
|
|
9
|
Matoskova B, Wong WT, Salcini AE, Pelicci
PG and Di Fiore PP: Constitutive phosphorylation of eps8 in tumor
cell lines: Relevance to malignant transformation. Mol Cell Biol.
15:3805–3812. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maa MC, Lee JC, Chen YJ, Chen YJ, Lee YC,
Wang ST, Huang CC, Chow NH and Leu TH: Eps8 facilitates cellular
growth and motility of colon cancer cells by increasing the
expression and activity of focal adhesion kinase. J Biol Chem.
282:19399–19409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu M, Shorts-Cary L, Knox AJ,
Kleinsmidt-DeMasters B, Lillehei K and Wierman ME: Epidermal growth
factor receptor pathway substrate 8 is overexpressed in human
pituitary tumors: Role in proliferation and survival.
Endocrinology. 150:2064–2071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yap LF, Jenei V, Robinson CM, Moutasim K,
Benn TM, Threadgold SP, Lopes V, Wei W, Thomas GJ and Paterson IC:
Upregulation of Eps8 in oral squamous cell carcinoma promotes cell
migration and invasion through integrin-dependent Rac1 activation.
Oncogene. 28:2524–2534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bashir M, Kirmani D, Bhat HF, Baba RA,
Hamza R, Naqash S, Wani NA, Andrabi KI, Zargar MA and Khanday FA:
P66shc and its downstream Eps8 and Rac1 proteins are upregulated in
esophageal cancers. Cell Commun Signal. 8:132010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Welsch T, Endlich K, Giese T, Büchler MW
and Schmidt J: Eps8 is increased in pancreatic cancer and required
for dynamic actin-based cell protrusions and intercellular
cytoskeletal organization. Cancer Lett. 255:205–218. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu PS, Jong TH, Maa MC and Leu TH: The
interplay between Eps8 and IRSp53 contributes to Src-mediated
transformation. Oncogene. 29:3977–3989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Patel V, Miyazaki H, Gutkind JS
and Yeudall WA: Role for EPS8 in squamous carcinogenesis.
Carcinogenesis. 30:165–174. 2009. View Article : Google Scholar
|
|
17
|
Chen YJ, Shen MR, Chen YJ, Maa MC and Leu
TH: Eps8 decreases chemosensitivity and affects survival of
cervical cancer patients. Mol Cancer Ther. 7:1376–1385. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ackland ML, Newgreen DF, Fridman M,
Waltham MC, Arvanitis A, Minichiello J, Price JT and Thompson EW:
Epidermal growth factor-induced epithelia-mesenchymal transition in
human breast carcinoma cells. Lab Invest. 83:435–448. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hagemann T, Bozanovic T, Hooper S, Ljubic
A, Slettenaar VI, Wilson JL, Singh N, Gayther SA, Shepherd JH and
Van Trappen PO: Molecular profiling of cervical cancer progression.
Br J Cancer. 96:321–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen MR, Hsu YM, Hsu KF, Chen YF, Tang MJ
and Chou CY: Insulin-like growth factor 1 is a potent stimulator of
cervical cancer cell invasiveness and proliferation that is
modulated by alphavbeta3 integrin signaling. Carcinogenesis.
27:962–971. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adam L, Zhong M, Choi W, Qi W, Nicoloso M,
Arora A, Calin G, Wang H, Siefker-Radtke A, McConkey D, et al:
MiR-200 expression regulates epithelial-to-mesenchymal transition
in bladder cancer cells and reverses resistance to epidermal growth
factor receptor therapy. Clin Cancer Res. 15:5060–5072. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cannito S, Novo E, di Bonzo LV, Busletta
C, Colombatto S and Parola M: Epithelial-mesenchymal transition:
From molecular mechanisms, redox regulation to implications in
human health and disease. Antioxid Redox Signal. 12:1383–1430.
2010. View Article : Google Scholar
|
|
23
|
Wang Z, Li Y, Ahmad A, Azmi AS, Kong D,
Banerjee S and Sarkar FH: Targeting miRNAs involved in cancer stem
cell and EMT regulation: An emerging concept in overcoming drug
resistance. Drug Resist Updat. 13:109–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
25
|
Qureshi R, Arora H and Rizvi MA: EMT in
cervical cancer: Its role in tumour progression and response to
therapy. Cancer Lett. 356:321–331. 2015. View Article : Google Scholar
|
|
26
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castagnino P, Biesova Z, Wong WT, Fazioli
F, Gill GN and Di Fiore PP: Direct binding of eps8 to the
juxtamembrane domain of EGFR is phosphotyrosine- and
SH2-independent. Oncogene. 10:723–729. 1995.PubMed/NCBI
|
|
29
|
Disanza A, Mantoani S, Hertzog M, Gerboth
S, Frittoli E, Steffen A, Berhoerster K, Kreienkamp HJ, Milanesi F,
Di Fiore PP, et al: Regulation of cell shape by Cdc42 is mediated
by the synergic actin-bundling activity of the Eps8-IRSp53 complex.
Nat Cell Biol. 8:1337–1347. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goicoechea S, Arneman D, Disanza A,
Garcia-Mata R, Scita G and Otey CA: Palladin binds to Eps8 and
enhances the formation of dorsal ruffles and podosomes in vascular
smooth muscle cells. J Cell Sci. 119:3316–3324. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Innocenti M, Frittoli E, Ponzanelli I,
Falck JR, Brachmann SM, Di Fiore PP and Scita G: Phosphoinositide
3-kinase activates Rac by entering in a complex with Eps8, Abi1,
and Sos-1. J Cell Biol. 160:17–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Teh MT, Ji Y, Patel V,
Firouzabadian S, Patel AA, Gutkind JS and Yeudall WA: EPS8
upregulates FOXM1 expression, enhancing cell growth and motility.
Carcinogenesis. 31:1132–1141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chu PY, Liou JH, Lin YM, Chen CJ, Chen MK,
Lin SH, Yeh CM, Wang HK, Maa MC, Leu TH, et al: Expression of Eps8
correlates with poor survival in oral squamous cell carcinoma. Asia
Pac J Clin Oncol. 8:e77–e81. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang H, Wilson CS, Harvey RC, Chen IM,
Murphy MH, Atlas SR, Bedrick EJ, Devidas M, Carroll AJ, Robinson
BW, et al: Gene expression profiles predictive of outcome and age
in infant acute lymphoblastic leukemia: A children's oncology group
study. Blood. 119:1872–1881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen H, Wu X, Pan ZK and Huang S:
Integrity of SOS1/EPS8/ABI1 tri-complex determines ovarian cancer
metastasis. Cancer Res. 70:9979–9990. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lim J and Thiery JP:
Epithelial-mesenchymal transitions: Insights from development.
Development. 139:3471–3486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schipper JH, Frixen UH, Behrens J, Unger
A, Jahnke K and Birchmeier W: E-cadherin expression in squamous
cell carcinomas of head and neck: Inverse correlation with tumor
dedifferentiation and lymph node metastasis. Cancer Res.
51:6328–6337. 1991.PubMed/NCBI
|
|
38
|
Shiozaki H, Tahara H, Oka H, Miyata M,
Kobayashi K, Tamura S, Iihara K, Doki Y, Hirano S, Takeichi M, et
al: Expression of immunoreactive E-cadherin adhesion molecules in
human cancers. Am J Pathol. 139:17–23. 1991.PubMed/NCBI
|
|
39
|
Kadowaki T, Shiozaki H, Inoue M, Tamura S,
Oka H, Doki Y, Iihara K, Matsui S, Iwazawa T, Nagafuchi A, et al:
E-cadherin and alpha-catenin expression in human esophageal cancer.
Cancer Res. 54:291–296. 1994.PubMed/NCBI
|
|
40
|
Bowie GL, Caslin AW, Roland NJ, Field JK,
Jones AS and Kinsella AR: Expression of the cell-cell adhesion
molecule E-cadherin in squamous cell carcinoma of the head and
neck. Clin Otolaryngol Allied Sci. 18:196–201. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koseki S, Aoki T, Ansai S, Hozumi Y,
Mitsuhashi Y and Kondo S: An immunohistochemical study of
E-cadherin expression in human squamous cell carcinoma of the skin:
Relationship between decreased expression of E-cadherin in the
primary lesion and regional lymph node metastasis. J Dermatol.
26:416–422. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu H, Lotan R, Menter D, Lippman SM and Xu
XC: Expression of E-cadherin is associated with squamous
differentiation in squamous cell carcinomas. Anticancer Res.
20:1385–1390. 2000.PubMed/NCBI
|
|
43
|
Berx G, Staes K, van Hengel J, Molemans F,
Bussemakers MJ, van Bokhoven A and van Roy F: Cloning and
characterization of the human invasion suppressor gene E-cadherin
(CDH1). Genomics. 26:281–289. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Prognostic significance of CyclinD1 and
E-Cadherin in patients with esophageal squamous cell carcinoma:
Multiinstitutional retrospective analysis. Research committee on
malignancy of esophageal cancer, Japanese society for esophageal
diseases. J Am Coll Surg. 192:708–718. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Helfand BT, Mendez MG, Murthy SN, Shumaker
DK, Grin B, Mahammad S, Aebi U, Wedig T, Wu YI, Hahn KM, et al:
Vimentin organization modulates the formation of lamellipodia. Mol
Biol CellMol Biol Cell. 22:1274–1289. 2011. View Article : Google Scholar
|
|
46
|
Adam SA and Gerace L: Cytosolic proteins
that specifically bind nuclear location signals are receptors for
nuclear import. Cell. 66:837–847. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moody SE, Perez D, Pan TC, Sarkisian CJ,
Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD and
Chodosh LA: The transcriptional repressor Snail promotes mammary
tumor recurrence. Cancer Cell. 8:197–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu Y and Zhou BP: New insights of
epithelial-mesenchymal transition in cancer metastasis. Acta
Biochim Biophys Sin (Shanghai). 40:643–650. 2008. View Article : Google Scholar
|
|
49
|
Guarino M: Epithelial-mesenchymal
transition and tumour invasion. Int J Biochem Cell Biol.
39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen KC, Chen CY, Lin CR, Yang TY, Chen
TH, Wu LC and Wu CC: Luteolin attenuates TGF-β1-induced
epithelial-mesenchymal transition of lung cancer cells by
interfering in the PI3K/Akt-NF-κB Snail pathway. Life Sci.
93:924–933. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang B, Yin C, Li H, Shi L, Liu N, Sun Y,
Lu S, Liu Y, Sun L, Li X, et al: Nir1 promotes invasion of breast
cancer cells by binding to chemokine (C-C motif) ligand 18 through
the PI3K/Akt/GSK3β/Snail signalling pathway. Eur J Cancer.
49:3900–3913. 2013. View Article : Google Scholar : PubMed/NCBI
|