Introduction
Retinal pigment epithelium (RPE) cells, which have a
critical role in the neural retina, are associated with the
maintenance of functional and healthy photoreceptors (1–3).
These cells may be exposed to various extracellular stimuli that
can promote their survival or their death through highly regulated
cellular signals. Exposure to solar ultraviolet (UV) radiation is
an external stressor, which induces DNA breakdown and reactive
oxygen species (ROS) production (4). Under normal physiological conditions,
ocular tissues express several intrinsic antioxidant enzymes, as a
consequence of normal metabolism. However, during ocular injury,
ROS and free radicals may be overproduced leading to the induction
of pathological conditions (5).
Notably, oxidative damage is associated with the pathogenesis of
several degenerative ocular pathologies, including cataracts,
age-related macular degeneration (AMD), glaucoma and diabetic
retinopathy (6).
Exposure to solar radiation has been implicated in
numerous ocular pathologies, particularly macular degeneration
(7). Briefly, UV radiation can
induce the production of ROS, mitochondrial dysfunction, DNA
damage, and can increase apoptotic activity (8–10).
The mechanism underlying UV-mediated RPE cell death is
controversial and remains unclear. At present two hypotheses are
the most accredited: i) Exposure to UV radiation induces the
production of ROS, which may damage RPE cells through the
activation of apoptotic mechanisms (11); ii) UV radiation stimulates an
elevated production of ROS, which can lead to irreversible cellular
necrosis (12).
Within this framework, to better understand the
molecular basis and temporal sequence of the degenerative processes
underlying RPE cell death following exposure to UV radiation, the
present study investigated cell viability, ROS production and the
expression of principal apoptotic genes in ARPE-19 cells following
UV-A radiation for 5 or 6 consecutive hours.
Materials and methods
Cell culture conditions and UV
exposure
The human RPE cell line (ARPE-19) was provided as a
gift by Professor Stefano Cacchione (Department of Biology and
Biotechnology 'Charles Darwin', Sapienza University of Rome, Rome,
Italy). The cell line (CRL-2302) was originally obtained from
American Type Culture Collection (Manassas, VA, USA). The ARPE-19
cell line was verified by the BMR Genomics S.r.l. Cell Line
Authentication Service (Padova, Italy; ref. Nr 130264) using short
tandem repeat analysis and amelogenin gender determination, as
presented in Table I. As shown,
the percentage match between the submitted sample and the database
profile was 100%. Cells were used between passages 5 and 8.
 | Table IShort tandem repeat genotyping and
amelogenin (AMEL) gender determination of the ARPE-19 cells used in
the present study. |
Table I
Short tandem repeat genotyping and
amelogenin (AMEL) gender determination of the ARPE-19 cells used in
the present study.
| Locus | Test results for
submitted sample
| ATCC reference
database profile
|
|---|
| Query profile:
A504-ARPE-19 | Database profile:
ARPE-19 |
|---|
| AMEL | X | Y | X | Y |
| D3S1358 | 14 | 15 | | |
| D1S1656 | 11 | 15 | | |
| D2S441 | 10 | 15 | | |
| D10S1248 | 13 | 15 | | |
| D13S317 | 11 | 12 | 11 | 12 |
| Penta E | 7 | 11 | | |
| D16S539 | 9 | 11 | 9 | 11 |
| D18S51 | 12 | 16 | | |
| D2S1338 | 19 | | | |
| CSF1PO | 11 | | 11 | |
| Penta D | 11 | 13 | | |
| TH01 | 6 | 9.3 | 6 | 9.3 |
| vWA | 16 | 19 | 16 | 19 |
| D21S11 | 28 | 29 | | |
| D7S820 | 9 | 11 | 9 | 11 |
| D5S818 | 13 | | 13 | |
| TPOX | 9 | 11 | 9 | 11 |
| DYS391 | 10 | | | |
| D8S1179 | 13 | | | |
| D12S391 | 21 | 22 | | |
| D19S433 | 12 | 13 | | |
| FGA | 23 | | | |
| D22S1045 | 11 | 16 | | |
The cells were cultured in 50/50 Ham's
F12/Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), containing 15% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin/streptomycin at 37°C in a humidified environment
containing 5% CO2. After 1 week, the cells were passaged
and plated into 6 cm cell culture dishes (1×106 cells)
for further experiments. UV exposure was produced using a UV lamp
(Vilber Lourmat VL-62C Power 6W; Vilber Lourmat Deutschland GmbH,
Eberhardzell, Germany) in a custom-designed UV irradiation unit at
37°C and 5% CO2. UV-A exposure of the cells (at 365 nm)
was conducted 10 cm from the source for 30 min, and 1, 2, 3, 4, 5
and 6 h at an intensity of ~0.06 J/cm2/sec. For
minimizing absorption of radiation by the medium, a thin layer of
medium was retained above the cells throughout the UV-A
exposure.
Total cell count and cell viability
Following treatment of the cells, floating and
attached cells were collected for analysis. A NucleoCounter
(ChemoMetec A/S, Allerod, Denmark) was used to determine the total
concentration of cells and viability, according to the
manufacturer's protocol. The NucleoCounter uses a technique based
on propidium iodide (PI) uptake and fluorescence microscopy, which
is able to quantify non-viable cells and total cell concentration
(13). Briefly, a mixture
containing cell suspension (200 µl), lysis buffer (200
µl) and stabilizing buffer (200 µl) was loaded into
the NucleoCassette, which contains the fluorescent dye PI. The
cassette was then introduced into the NucleoCounter chamber for
measurement of the total cell concentration. Another NucleoCassette
was loaded with a sample of untreated cells to count the number of
non-viable cells (based on counting PI-stained membrane-damaged
cells). When the concentration of non-viable cells and the total
concentration of cells were known it was possible to calculate the
viability percentage of the cells, according to a formula provided
by the manufacturer.
Bright-field images of the cells following UV-A
exposure for various durations were obtained using a TE300 Eclipse
microscope (Nikon Corporation, Tokyo, Japan) in a blind manner, and
the images were digitalized using a Cool SNAP professional digital
camera (Nikon Corporation) and LUCIA-G/F imaging software (Nikon
Corporation). All images were acquired during one session using the
same brightness setting.
Detection of ROS
ROS detection was performed after staining the cells
using a 2′,7′-dichlorofluorescin diacetate (DCFDA) Cellular ROS
Detection Assay kit (Abcam, Cambridge, UK). Briefly, after
irradiation, cells were grown in a 96-well microplate (25,000
cells/well) and were treated successively with DCFDA for 30 min at
37°C, which is initially non-fluorescent and is converted to the
fluorescent molecular DCF by oxidation. DCF was subsequently
quantified using a CytoFluor Multi-well Plate Reader (Applied
Biosystems; Thermo Fisher Scientific, Inc.), with 485 nm excitation
and 538 nm emission filters. ROS production was expressed as
fluorescence intensity.
RNA isolation and semiquantitative
polymerase chain reaction (PCR)
Total RNA was isolated from the cells using the
PureLink® RNA Mini kit (Thermo Fisher Scientific, Inc.).
The elimination of any genomic DNA was performed using on-column
DNase treatment. RNA concentration was spectrophotometrically
evaluated at 280 and 260 nm. Total RNA was used for first-strand
cDNA synthesis using SCRIPT cDNA Synthesis kit and Oligo-dT as
random primers (Jena Bioscience GmbH, Jena, Germany) according to
the manufacturer's protocol. The PCR was performed with ~200 ng
cDNA using Hot Start Master (Jena Bioscience GmbH) according to the
manufacturer's protocol. The following primer sequences
(Invitrogen; Thermo Fisher Scientific, Inc.) were used for
amplification: Glyceraldehyde 3-phosphate dehydrogenase, forward
5′-AACGGATTTGGTCGTATTG-3′, reverse 5′-GGAAGATGGTGATGGGATT-3′ (208
bp); B-cell lymphoma 2 (Bcl-2), 5′-CGACGACTTCTCCCGCCGCTACCGC-3′,
reverse 5′-CCGCATGCTGGGGCCGTACAGTTCC-3′ (319 bp); Bcl-2-associated
X protein (Bax), forward 5′-ACCAAGAAGCTGAGCGAGTGTC-3′, reverse
5′-ACAAAGATGGTCACGGTCTGCC (365 bp); and caspase-3, forward
5′-AGAAGATCACAGCAAAAGGAG-3′, and reverse 5′-TCAAGCTTGTCGGCATACTG-3′
(378 bp). The experimental protocols for PCR were: Denaturation,
94°C for 1 min; followed by 40 cycles of denaturation at 94°C for 1
min; annealing for 40 sec at 58°C (GAPDH), 68°C (Bcl-2), 63°C (Bax)
or 60°C (caspase-3); and elongation at 72°C for 1 min; and a final
elongation step at 72°C for 5 min. PCR products were analyzed by
1.8% agarose gel electrophoresis with ethidium bromide (1
µg/ml) in TBE 1X buffer (Tris 40 mM, EDTA 1 mM, boric acid
44 mM) for 2 h at 80 V (constant voltage); a 100 bp ladder was used
as a molecular weight marker. Gel images were acquired (Bio-Rad Gel
Doc 2000) and were scanned (Bio-Rad GS800) using Bio-Rad Quantity
One software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
density of the PCR bands were divided by that of the housekeeping
gene and expressed as a percentage of the control band density.
Statistical analysis
Data were analyzed by one-way analysis of variance
followed by post-hoc Dunnett's test, to compare the treatment
groups with the control. Data were analyzed using Prism software,
version 5.01 (GraphPad, Inc., La Jolla, CA, USA). Comparison of the
mean survival rates of cells exposed to UV-A radiation was made
using the unpaired, Student's t-test. All results are presented as
the mean ± standard error of the mean of at least three independent
experiments performed in duplicate, unless otherwise specified.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The present study examined a sequence of molecular
and biological events induced by prolonged and continuous treatment
of ARPE-19 cells with UV-A. Preliminary experiments were conducted
to investigate cellular viability following exposure to UV
radiation for various durations (0.50, 1, 2, 3, 4, 5 and 6 h).
Treatment with UV-A radiation resulted in a time-dependent decrease
in cellular viability from 2 h onward, compared with the
non-irradiated controls (70, 20 and 10% viability at 3, 4 and 5 h,
respectively) (Fig. 1A). Cell
death was detected morphologically after 5 h irradiation, as
follows: i) A marked reduction in the number of living cells; ii)
cells were obviously swollen and loss of plasma membrane integrity
was detected (Fig. 1C).
A second series of experiments were performed to
ascertain whether the reduction in cellular viability observed
post-irradiation was associated with oxidative stress, since
various studies have suggested that ROS generation may be an
underlying cause of RPE cell death (4–6). A
significant and consistent increase in ROS levels (200% increase)
was observed after 1 h UV-A irradiation in the experimental
paradigm. These levels remained constant throughout the subsequent
time-points (Fig. 2).
By this stage of the present study, the involvement
of ROS in the irradiation-induced reduction of cellular viability
had been confirmed; therefore, additional experiments were
conducted to determine the underlying molecular mechanism(s) by
which ROS caused this effect. In this regard, the expression levels
of principle apoptotic (Bax and caspase-3) and anti-apoptotic
(Bcl-2) genes were detected in our experimental model after 1, 3 or
5 h of UV-A exposure.
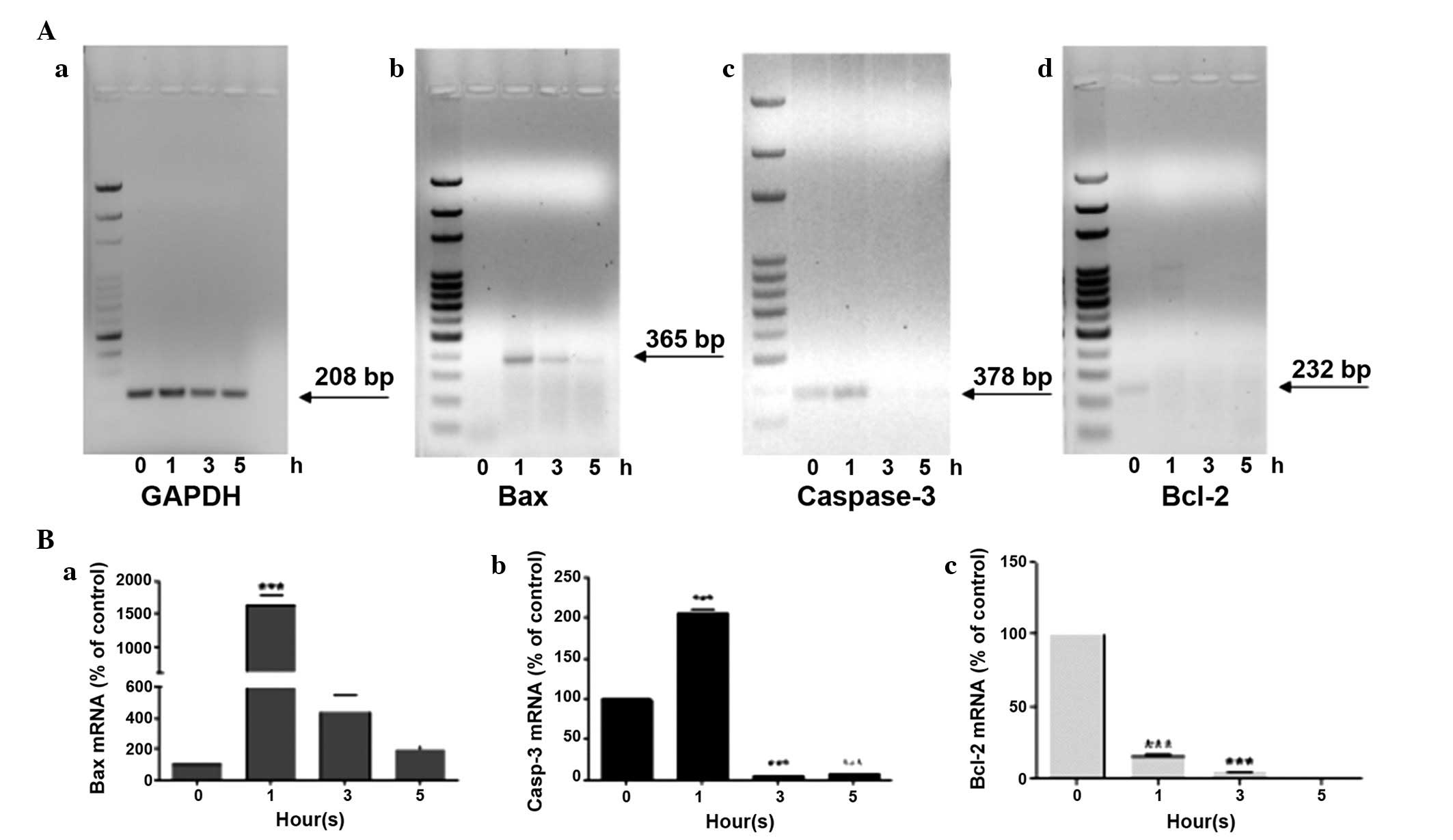
After 1 h of treatment an overexpression of Bax was
detected, which dropped drastically at 3 h, until reaching values
similar to those of the untreated controls after 5 h (Fig. 3Ab and Ba). An analogous trend was
also observed in the gene expression levels of caspase-3 (Fig. 3Ac and Bb); after 1 h of UV-A
treatment its expression doubled, compared with at 0 h; however,
the expression almost disappeared from 3 h onward. Notably, ARPE-19
cells exhibited caspase-3 gene expression at 0 h. Finally, the
expression levels of the anti-apoptotic gene Bcl-2 were detected.
Bcl-2 was exclusively expressed in untreated cells at 0 h, whereas
it was expressed at low levels in cells following exposure to UV-A
radiation for various durations(Fig.
3Ad and Bc).
Discussion
Knowledge of the numerous functions performed by RPE
cells has improved the understanding of several diseases that lead
to blindness. The RPE layer is one of the major ocular tissues
known to be metabolically active and sensitive to oxidative stress.
The balance between oxidative stress and cellular death may be
responsible for the progression of several diseases of the
underlying retina, including AMD. However the molecular events that
correlate with ROS generation and cellular death in RPE cells
remain partially unclear. Notably, contrasting evidence has been
reported in the literature in recent years. In particular, some
studies have reported that RPE cells exposed to UV undergo
apoptotic cell death (11,14), whereas other studies have claimed
that apoptosis is not the main mechanism of RPE cell death
(12,15), thus indicating that necrosis may be
responsible for RPE cell death in response to oxidative stress.
The present study aimed to elucidate these
mechanisms by describing the sequence of molecular events that
occur after continued exposure to UV-A radiation. UV-A exposure for
5 consecutive hours induced cytotoxic effects due to the production
of high levels of ROS. After 1 h irradiation, ROS levels were
markedly increased and induced an increase in the expression of
apoptotic genes (Bax and caspase-3), and decreased the expression
of the anti-apoptotic gene, Bcl-2. Subsequent hours were
characterized by a marked decrease in Bax and caspase-3 expression,
whereas ROS levels remained high and constant. Notably, during this
time range cell viability continued to rapidly decline, and in the
last hour the observed morphological features of the cells
suggested that they were undergoing necrosis.
The present study hypothesized an intermediary role
for oxidative stress in the induction of apoptosis, since molecular
events observed leading to Bax and caspase-3 activation were
induced by initially increasing levels of ROS. Conversely, when ROS
levels remained consistently high, apoptotic gene expression was
suppressed and RPE death appeared to occur by necrosis. Consistent
with this hypothesis, previous studies have reported that low
levels of ROS upregulate apoptotic genes and downregulate
anti-apoptotic genes in cells, leading to apoptotic cell death
(16,17), whereas high levels of ROS can lead
to lipid peroxidation, cellular membrane damage, inactivation of
caspases and necrotic cell death (18).
To the best of our knowledge, the present study is
the first to describe the sequence of molecular events that occur
following continuous exposure to UV-A radiation. UV-A induced an
increase in ROS levels, which initially led to activation of
apoptotic events, followed by the induction of irreversible
cellular necrosis. These findings, even if preliminary, may be
useful for improving understanding regarding the oxidative damage
that underlies UV-induced ocular cell damage.
In conclusion, the results of the present study are
the first, to the best of our knowledge, to describe the sequence
of cellular events starting from the first 30 min of UV-A
treatment. UV-A radiation induced the activation of apoptotic
events and subsequently led to irreversible cellular necrosis.
These important findings may have future pharmacological
applications in protecting RPE cells from the initiation and
progression of UV-A-induced ocular disorders.
Acknowledgments
The authors of the present study would like to thank
Professor Stefano Cacchione and Dr Sara Luzzi (Department of
Biology and Biotechnology 'Charles Darwin', Sapienza University of
Rome, Rome, Italy) for providing the ARPE-19 cells. They would also
like to thank Dr Lucia Lisi for her contribution to the
experimental work described. The present study was financed by the
Italian National Research Council (CNR) and UCSC internal funding
(Fondi di Ateneo 2013 to G.T.).
References
|
1
|
Kuznetsova AV, Kurinov AM and Aleksandrova
MA: Cell models to study regulation of cell transformation in
pathologies of retinal pigment epithelium. J Ophthalmol.
2014:8017872014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rizzolo LJ: Barrier properties of cultured
retinal pigment epithelium. Exp Eye Res. 126:16–26. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pfeffer BA and Philp NJ: Cell culture of
retinal pigment epithelium: Special Issue. Exp Eye Res. 126:1–4.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glickman RD: Ultraviolet phototoxicity to
the retina. Eye Contact Lens. 37:196–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blasiak J, Petrovski G, Veréb Z, Facskó A
and Kaarniranta K: Oxidative stress, hypoxia, and autophagy in the
neovascular processes of age-related macular degeneration. Biomed
Res Int. 2014:7680262014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tu G, Zhang YF, Wei W, Li L, Zhang Y, Yang
J and Xing Y: Allicin attenuates H2O2-induced
cytotoxicity in retinal pigmented epithelial cells by regulating
the levels of reactive oxygen species. Mol Med Rep. 13:2320–2326.
2016.PubMed/NCBI
|
|
7
|
Plestina-Borjan I and Klinger-Lasić M:
Long-term exposure to solar ultraviolet radiation as a risk factor
for age-related macular degeneration. Coll Antropol. 31(Suppl 1):
33–38. 2007.PubMed/NCBI
|
|
8
|
Li X, Zhao H, Wang Q, Liang H and Jiang X:
Fucoidan protects ARPE-19 cells from oxidative stress via
normalization of reactive oxygen species generation through the
Ca2+-dependent ERK signaling pathway. Mol Med
Rep. 11:3746–3752. 2015.PubMed/NCBI
|
|
9
|
Øsnes-Ringen O, Azqueta AO, Moe MC,
Zetterström C, Røger M, Nicolaissen B and Collins AR: DNA damage in
lens epithelium of cataract patients in vivo and ex vivo. Acta
Ophthalmol. 91:652–656. 2013. View Article : Google Scholar
|
|
10
|
Ji Y, Cai L, Zheng T, Ye H, Rong X, Rao J
and Lu Y: The mechanism of UVB irradiation induced-apoptosis in
cataract. Mol Cell Biochem. 401:87–95. 2015. View Article : Google Scholar
|
|
11
|
Roduit R and Schorderet DF: MAP kinase
pathways in UV-induced apoptosis of retinal pigment epithelium
ARPE19 cells. Apoptosis. 13:343–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanus J, Zhang H, Wang Z, Liu Q, Zhou Q
and Wang S: Induction of necrotic cell death by oxidative stress in
retinal pigment epithelial cells. Cell Death Dis. 4:e9652013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shah D, Naciri M, Clee P and Al-Rubeai M:
NucleoCounter-An efficient technique for the determination of cell
number and viability in animal cell culture processes.
Cytotechnology. 51:39–44. 2006. View Article : Google Scholar
|
|
14
|
Chan CM, Huang CH, Li HJ, Hsiao CY, Su CC,
Lee PL and Hung CF: Protective effects of resveratrol against
UVA-induced damage in ARPE19 cells. Int J Mol Sci. 16:5789–5802.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murakami Y, Matsumoto H, Roh M, Giani A,
Kataoka K, Morizane Y, Kayama M, Thanos A, Nakatake S, Notomi S, et
al: Programmed necrosis, not apoptosis, is a key mediator of cell
loss and DAMP-mediated inflammation in dsRNA-induced retinal
degeneration. Cell Death Differ. 21:270–277. 2014. View Article : Google Scholar :
|
|
16
|
Hildeman DA, Mitchell T, Aronow B,
Wojciechowski S, Kappler J and Marrack P: Control of Bcl-2
expression by reactive oxygen species. Proc Natl Acad Sci USA.
100:15035–15040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han S, Lu Q and Wang N: Apr3 accelerates
the senescence of human retinal pigment epithelial cells. Mol Med
Rep. 13:3121–3126. 2016.PubMed/NCBI
|
|
18
|
Kannan K and Jain SK: Oxidative stress and
apoptosis. Pathophysiology. 7:153–163. 2000. View Article : Google Scholar : PubMed/NCBI
|