Introduction
Liver transplantation is the only efficacious
clinical treatment available for end-stage liver disease, which can
significantly increase rates of survival and improve quality of
life. However a shortage of donors, high surgical costs and risks
of immune rejection limit the application of liver transplantation,
and novel alternative therapies are urgently required (1).
Previously, stem cell-based liver regeneration has
been suggested as a potential technique in the treatment of
end-stage liver disease. Several types of exogenous stem cells can
be differentiated into hepatocytes, including induced pluripotent
stem cells (2), embryonic stem
cells (3), bone marrow mesenchymal
stem cells (4) and hematopoietic
stem cells. Therefore, these exogenous cells types may offer
potential for use as seed cells for liver regeneration. In
addition, candidate endogenous adult stem/progenitor cells can be
recruited from the terminal bile ductules and activated, to
proliferate and differentiate into hepatocytes and promote liver
regeneration (5). The liver oval
cells of the liver also possess stemness potential, which can give
rise to hepatocytes and express the hepatocyte marker (6).
Various strategies have been investigated to induce
hepatocyte differentiation in stem cells, including the use of
growth factors (7) and hepatic
stem cell niches (8), and there is
increasing interest in the use of potential of traditional Chinese
medicines to alter the proliferation and differentiation of stem
cells. In previous years, the effects of several traditional
Chinese medicines on stem cell behavior have been characterized.
For example, β-Elemene, derived from Curcumae Radix, has
been reported to inhibit angiogenesis by targeting Notch-1 in
cancer stem-like cells (9). The
Chinese herbal medicine, Yin-Chen-Hao-Tang, has also been
implicated in the inhibition of fatty liver progression through
increasing adiponectin and promoting endothelial progenitor cell
survival (10).
Matrine, an alkaloid extracted from Sophora
flavescens AIT, is reported to possess pharmacological
properties, and has been found induce a series of therapeutic
effects, including anti-fibrotic activity and the induction of
cancer cell apoptosis (11).
Matrine has been applied in the treatment of liver fibrosis
(12) and it has been found to
protect the liver from hepatic ischemia/reperfusion injury
(13). In our previous study, it
was demonstrated that the in vivo administration of matrine
promoted oval cell-mediated liver regeneration through
down-regulation of the recombination signal-binding protein
(RBP)-JK hairy and enhancer of split-1 (HES1) signaling
pathway, suggesting that this compound may affect the
differentiation of hepatic progenitor cells (14). The aim of the present study was to
characterize the mechanisms underlying these previous observations.
The present study aimed to investigate whether exposure to matrine
serum can affect the proliferation and differentiation of hepatic
progenitor cells in vitro, and to investigate the mechanism
by which matrine affects the differentiation of hepatic progenitor
cells, in order to provide novel insights regarding
matrine-promoted liver regeneration in vivo.
Materials and methods
Reagents
Matrine (cat. no. 110805-200306) was purchased from
the Chinese National Institute of Pharmaceutical and Biological
Products (Beijing, China). Fetal bovine serum and RPMI 1640 medium
were purchased from Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Polyacrylamide, sodium dodecyl sulfate (SDS),
3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT)
and L-glutamine were purchased from Sigma-Aldrich (St. Louis, MO,
USA). TRIzol reagent was purchased from Invitrogen; Thermo Fisher
Scientific, Inc.). Parathyroid hormone (PTH) was obtained from
Bachem (Bubendorf, Switzerland), the BCA™ Protein Assay kit was
purchased from Thermo Fisher Scientific, Inc. and the RNAprep pure
Cell/Bacteria kit was purchased from Qiagen (Hilden, Germany).
Antibodies against ALB, Jagged 1, HES1 and β-actin were obtained
from Santa Cruz Biotechnology, Inc. (Danvers, MA, USA).
Preparation of matrine serum
A total of 40 male Sprague-Dawley rats weighing
~300–400 g (age, 2.5 months) were obtained from the Animal Center
of Chinese Academy of Medical Sciences (Beijing China). Rats were
housed in an air-conditioned room under a 12 h light/dark cycle and
had ad libitum access to water and food. The room
temperature was 23–25°C. These rats were divided into an
experimental group and a control group, each containing 20 rats.
The rats received esophageal infusion of either 2.5 g/l matrine in
physiological saline (experimental group) or physiological saline
(control group) twice each day for 7 days. The matrine infusion was
administered at a dose of ~1 ml/100 g. Subsequently, 1 h
following the final infusion, blood from the inferior vena cava was
collected and maintained at 4°C for 4 h, following which the blood
was centrifuged at 600 × g for 10 min to obtain the matrine drug or
control serum samples, which were sterilized by filtering through a
0.22 μm millipore filtration membrane and stored at −20°C
for further use. The animal use and care protocol for the animal
experiments in the present study was approved by the Institutional
Animal Care and Use Committee of Capital Medical University
(Beijing, China), and the study was approved by the ethics
committee of Capital Medical University.
Cell culture and exposure to matrine
serum
Rat hepatic progenitor cells (WB-F344) were
purchased from the Drug Research Institute, Chinese Academy of
Medical Sciences, and were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum at 37°C in 5%
CO2. The WB-F344 medium was supplemented with 5, 10, 20
and 40% serum from the rats administered with saline (negative
control) or matrine (matrine serum) for 24 or 72 h at 37°C, as
indicated.
Evaluation of cell viability and
inhibition
The WB-F344 cells were seeded into flat plates
(Costar 3524; Corning Inc., Corning, NY, USA) at a density of
5×104/cm2 and the culture medium was
supplemented with 5–40% matrine serum. After 24 h at 37°C, cell
viability was determined using acridine orange/propidium iodide
(AO/PI) staining (Sigma-Aldrich), using a standard protocol. After
24, 48 and 72 h at 37°C, the proliferation rates of the WB-F344
cells were evaluated using an MTT assay. Proliferation inhibition
was calculated by comparison with cells incubated with culture
medium only.
MTT assay
The WB-F344 cells were seeded into 96-well plates at
a density of 2×104/cm2 and incubated
overnight. The medium was replaced with fresh RPMI 1640 medium
supplemented with 5, 10 or 20% matrine serum. After 48 h, MTT (5
mg/ml; 20 ml) was added to each well. After 4 h at 37°C, the medium
was replaced with 150 ml dimethyl sulfoxide and incubated for 20
min. The optical density (OD) at 490 nm was measured using an
Evolution™ 300 spectrophotometer (Thermo Fisher Scientific, Inc.).
The inhibition ratio was calculated according to the following
formula: Inhibition ratio = (1 - experimental OD) / control
* 100%. The experiments were repeated three times.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis of the expression of Jagged 1, HES1 and
ALP
To determine the effects of matrine on the
differentiation of WB-F344 cells, RNA was extracted from the cells
following incubation in the presence or absence of 5, 10 and 20%
matrine serum for 24 or 48 h, and RT-PCR was performed to assess
the expression levels of Jagged 1, HES1 and ALP. To further
establish the mechanism of action, WB-F344 cells were incubated at
a density of 2×104/cm2 in the presence or
absence of 20% matrine serum for 48 h, following which the cultures
were supplemented with 0.1 mol/l PTH, an activator of the Notch
signaling pathway, and incubated for a further 24 h. The WB-F344
cells were collected and total RNA was extracted using an RNAprep
Pure Cell/Bacteria kit (Tiangen Biotech Co., Ltd., Beijing China).
Standard procedures were followed to obtain first-strand cDNA using
a one-step RT-PCR kit (Qiagen GmbH, Hilden, Germany) according to
the manufacturer's protocol. The total volume of the PCR reaction
system was 20 μl, including 1 μl cDNA sample, 1
μl forward primers and 1 μl reverse primers, 10
μl 2X Power Taq PCR MasterMix (BioTeke Corporation,
Beijing, China) and 7 μl double-distilled H2O.
The PCR cycling conditions were as follows: Initial denaturation at
94°C for 5 min, followed by 25 cycles, which consisted of
denaturation at 94°C for 30 sec, renaturation at 58°C for 30 sec
and annealing/extension at 72°C for 45 sec. The primers used were
follows: JAGGED 1, forward 5′-ATGCGGTCCCCACGGACGCG-3′ and reverse
5′-ACACTCAGGACCCATCCAGC-3′; HES1, forward
5′-CAACACGACACCGGACAAACC-3′ and reverse
5′-AGTGCGCACCTCGGTGTTAAC-3′; β-actin, forward
5′-GCCATGTACGTAGCCATCCA-3′ and reverse 5′-GAACCGCTCATTGCCGATAG-3′.
ALP, forward 5′-TGTCCCCAAAGAGTTTAAAGCTG-3′ and reverse
5′-TCTTTATCTGCTTCTCCTTGTCTGG-3′. PCR products were detected by 2%
agarose gel electrophoresis and were stained by ethidium bromide.
The band intensity was quantified using ImageJ 1.48u software
(National Institutes of Health, Bethesda, MD, USA). Signal
intensity of the amplified product was normalized to its respective
β-actin signal intensity.
Immunohistochemistry
The WB-F344 cells incubated in the presence or
absence of 20% matrine serum for 48 h were fixed in 4%
paraformaldehyde, and immunohistochemistry against Jagged1 and ALB
was performed to examine the expression of ALB and Jagged 1 using
conventional methods (15). The
immunostained samples were observed using an Olympus BX51
microscope (Olympus Deutshland GmbH, Hamburg, Germany).
Immunohistochemical staining was quantified with Image-Pro Plus 6.0
for Windows software (Media Cybernetics, Inc., Rockville, MD, USA)
using its measurement function. The positively stained area was
labeled and calculated according to the software guidelines. The
intensity of immunostaining was expressed as positive cell area /
total cell area × 100%.
Western blotting
The WB-F344 cells incubated in the presence or
absence of 20% matrine serum for 48 h were lysed using protein
lysate buffer. Following the removal of debris through
centrifugation at 12,000 × g for 20 min at 4°C, the protein content
was determined using a BCA™ Protein Assay kit (Thermo Fisher
Scientific, Inc.). Total protein (30 μg) was loaded onto 12%
polyacrylamide-SDS gels and electrophoresed, followed by transfer
onto polyvinylidene difluoride membranes under a constant
electronic current of 300 mA for 2 h. Following blocking in 5%
fat-free milk, the membranes were incubated with rabbit polyclonal
anti-ALB (1:2,000; cat. no. sc-50536), goat polyclonal anti-Jagged
1 (1:2,000; cat. no. sc-34473) and rabbit polyclonal anti-HES1
(1:2,000; cat. no. sc-25392) antibodies at 4°C overnight, and were
subsequently incubated with horseradish peroxidase-conjugated goat
anti-rabbit and rabbit anti-goat immunoglobulin G secondary
antibodies (1:10,000; cat. nos. ZB-5301 and ZB-2306, respectively;
ZSGB-Bio, Beijing, China) at room temperature for 1 h. Protein
separation was detected via enhanced chemiluminescence (Applygen
Technologies, Inc., Beijing, China). The signals for ALB, Jagged 1
and HES1 were normalized to that of goat polyclonal anti-β-actin
(1:2,000; cat. no. sc-1616).
Statistical analysis
All data are expressed as the mean ± standard
deviation of experiments repeated at least three times. Data were
analyzed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). The
statistical significance of differences in quantitative data were
analyzed using one-way analysis of variance and
Student-Newman-Keuls test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of matrine treatment on the
viability of WB-F344 hepatic progenitor cells
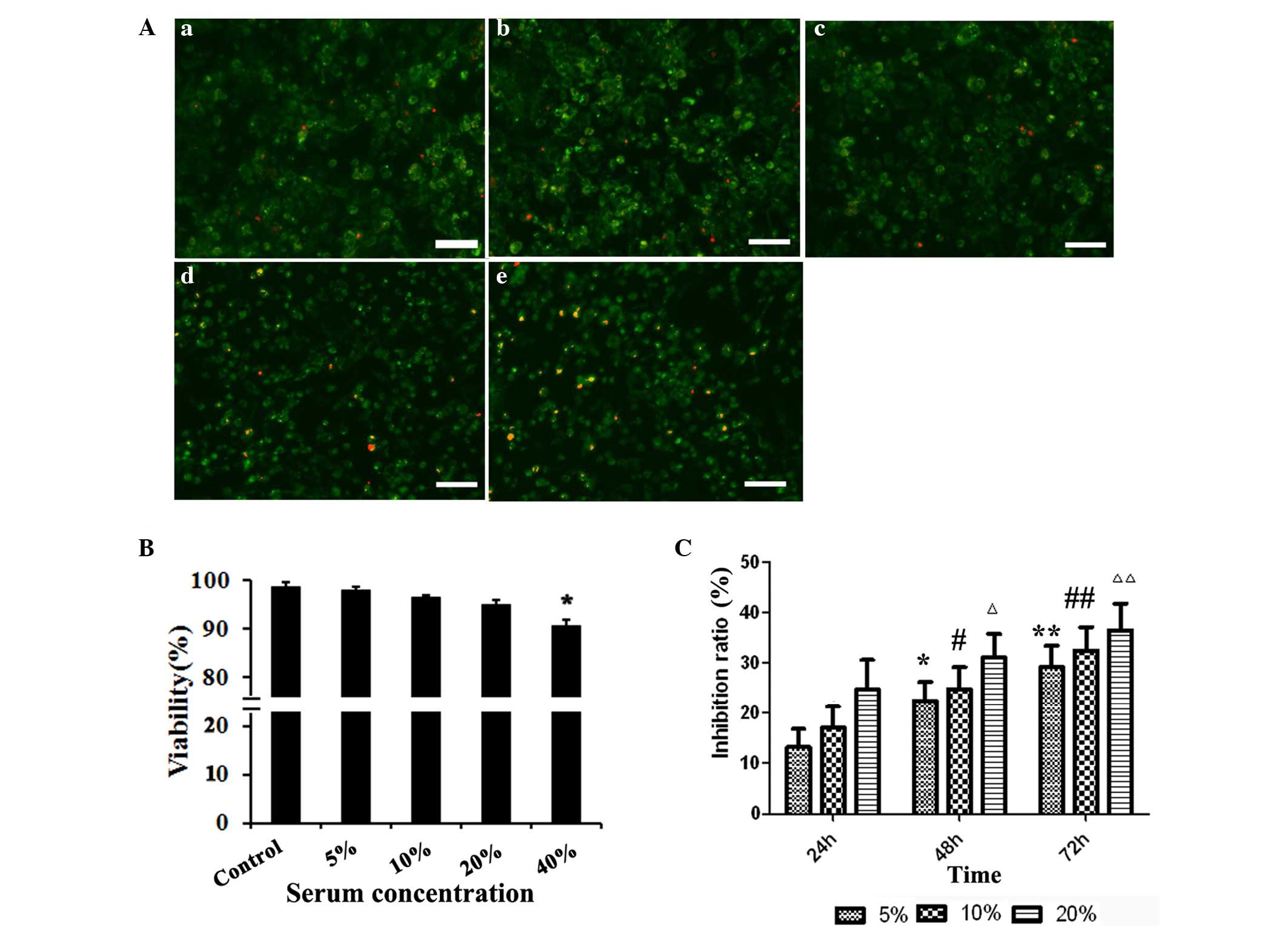
To determine the effects of matrine on the viability
of hepatic progenitor cells in the present study, WB-F344 cells
were incubated in medium supplemented with the serum of animals
administered with saline or matrine (matrine serum) for 24 h, and
cell viability was measured using AO/PI staining. Supplementation
of the culture medium with control serum or with 5–20% matrine
serum had no significant effect on the viability of the WB-F344
cells (P>0.05), however, when the culture medium contained 40%
matrine serum, cell viability was reduced (P<0.05; Fig. 1A and B). Therefore, only 5, 10 and
20% drug serum were used in the subsequent experiments.
Matrine inhibits the proliferation of
WB-F344 cells
To evaluate the effect of matrine on the
proliferation of WB-F344 cells, the present study measured the
proliferation of WB-F344 cells incubated in the presence of absence
of matrine serum for 24, 48 and 72 h using an MTT assay. The
inhibition of matrine serum inhibited the proliferation of the
WB-F344 cells in a concentration- and time-dependent manner
(Fig. 1C), whereas control serum
had no effect on cell proliferation (data not shown).
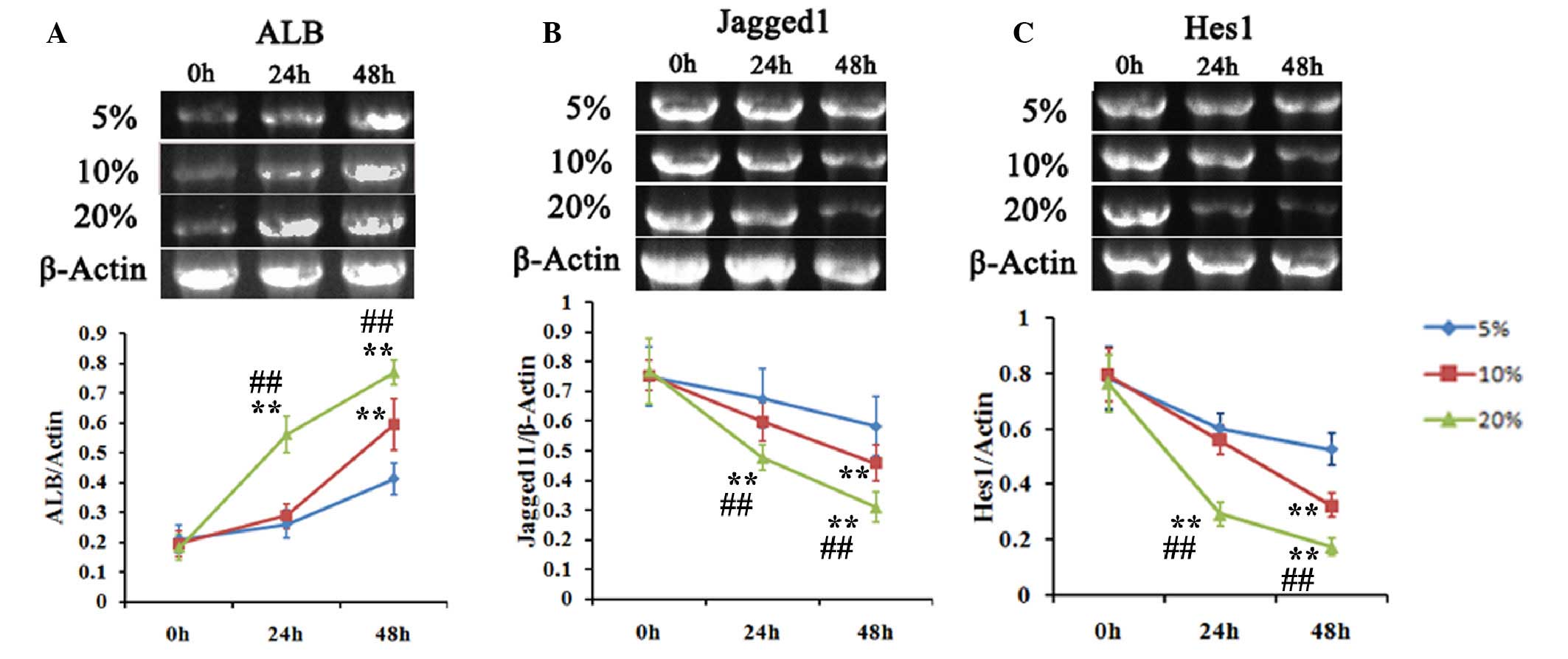
Matrine promotes the hepatic
differentiation of WB-F344 cells
The differentiation of WB-F344 hepatic progenitor
cells into hepatic cells is characterized by the expression of ALB,
a biomarker of mature hepatic cells. To evaluate the effect of
matrine on the differentiation of hepatic progenitor cells, the
transcription and distribution of ALB in WB-F344 cells incubated
with 5–20% matrine serum were determined using RT-PCR analysis. The
transcription of ALB was enhanced by incubation with matrine serum
in a time- and concentration-dependent manner (Fig. 2A).
In liver regeneration, several cell signaling
pathways affect repair of the injured liver. Notch signaling has
been implicated in the differentiation of stem cells (16). The present study used RT-PCR
analysis to measure the expression levels of the Notch signaling
pathway ligands, Jagged 1 and HES1, in WB-F344 cells incubated with
matrine serum. The results demonstrated concentration- and
time-dependent reductions in the expression levels of Jagged 1 and
HES1 (Fig. 2A and B).
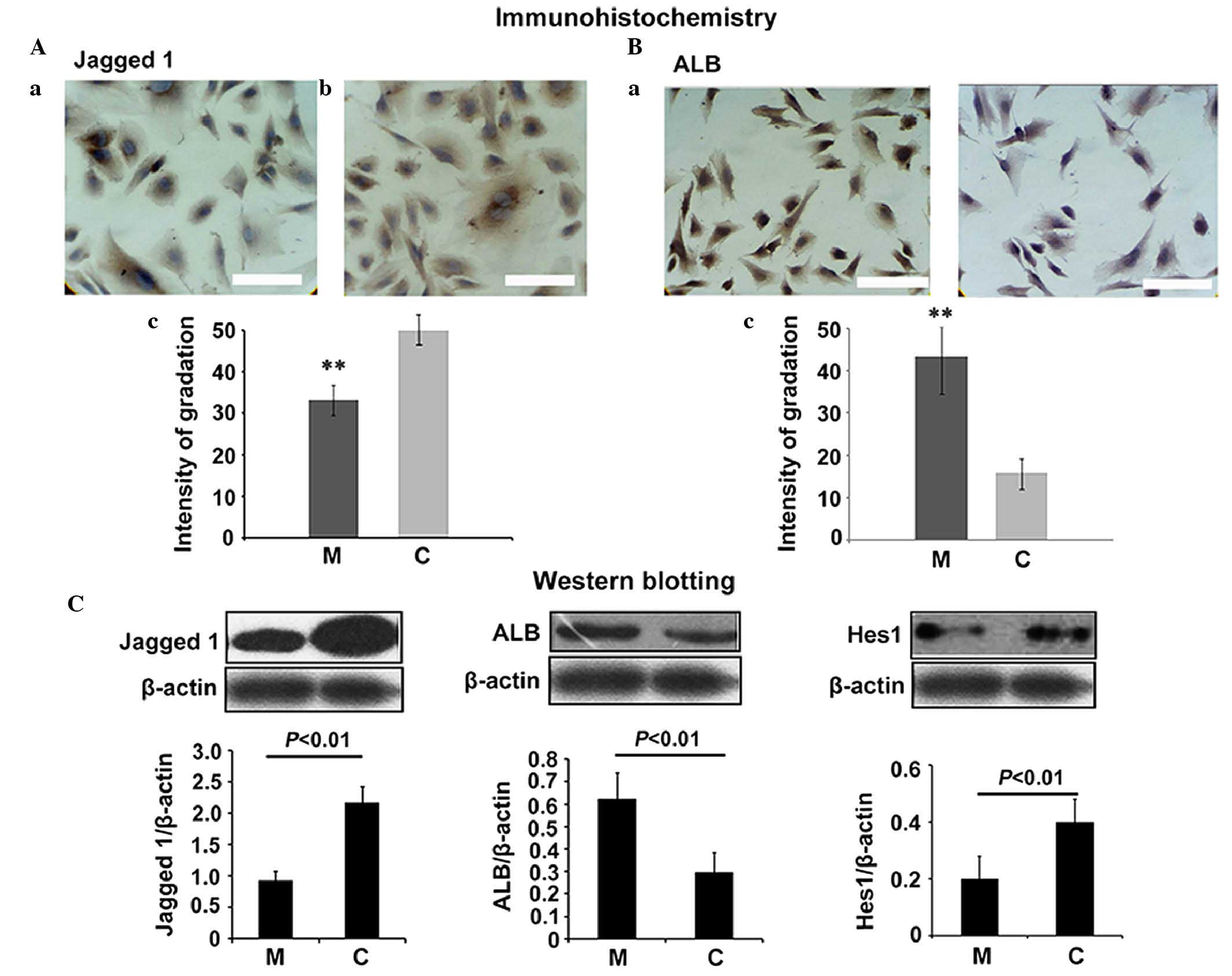
To further evaluate the effects of matrine on
hepatic progenitor cell differentiation, the present study used
immunohistochemistry to assess the levels of ALB and Jagged 1 in
the WB-F344 cells incubated with matrine serum. The results
revealed that incubation with 20% matrine serum significantly
increased the content of ALB in the cytoplasm and plasma membrane,
and reduced the content of Jagged 1 (Fig. 3A and B; P<0.01). The western
blotting confirmed these observed differences in the contents of
ALB, Jagged 1 and HES1 in the WB-F344 incubated with matrine serum.
A 20% concentration of matrine serum significantly promoted the
accumulation of ALB, and reduced the accumulation of Jagged 1
(Fig. 3C; P<0.01). These
results suggested that matrine promoted the differentiation of
WB-F344 cells and downregulated the Notch signaling pathway.
Matrine promotes the hepatic
differentiation of WB-F344 cells by inhibiting the Notch signaling
pathway
To further investigate whether the Notch signaling
pathway was involved in the hepatic differentiation of WB-F344
cells induced by matrine, the present study pre-treated the WB-F344
cells with 20% matrine serum, and then supplemented the medium with
PTH, which is an activator of the Notch signaling pathway (17). The transcription levels of Jagged 1
and HES1 were then determined using RT-PCR analysis. It was
observed that the addition of 0.1 mol/l PTH ameliorated the changes
in the expression levels of Jagged 1, ALB and HES1 induced by
matrine (Fig. 4). The expression
levels of Jagged 1, ALB and HES1 in the WB-F344 cells
simultaneously exposed to matrine serum and PTH were comparable
with those in the control group (P>0.05).
The promotion of Notch by PTH likely enhanced the
transcription of Jagged 1 and HES1 in WB-F344 exposed to matrine,
and was accompanied with a significant decrease in the expression
of ALB (P<0.01). These results provided further support for the
hypothesis that matrine promotes the hepatic differentiation of
WB-F344 by inhibiting the Notch signaling pathway.
Discussion
Our previous study demonstrated that the
administration of the traditional Chinese medicine, matrine,
promotes oval cell-mediated liver regeneration, suggesting that
this compound affected hepatic progenitor cell differentiation
(14). In the present study, the
effect of matrine on the differentiation of the WB-F344 rat hepatic
progenitor cell line were investigated. It was found that matrine
affected the proliferation and hepatic differentiation of the
WB-F344 cells in a concentration- and time-dependent manner. It was
demonstrated that matrine inhibited the expression of the notch
signaling ligands, Jagged 1 and HES1, in the WB-F344, and induced
the expression of ALB, a biomarker of mature hepatocytes.
Furthermore, the exogenous activation of notch signaling by PTH
prevented the effects of matrine, reducing the expression of ALB,
and recovering the expression of Jagged 1 and HES1.
Matrine, a compound extracted from Sophora
flavescens Ait, has been used as clinically in China for a wide
range of conditions, particularly in protecting the liver and
inhibiting cancer, and matrine has been reported to reduce
inflammation, viral replication and fibrosis (11–14,18).
It has also been reported that matrine can reduce the severity of
acute liver injury through its anti-inflammatory and anti-oxidative
activities (13). Matrine has also
been reported to modulate signaling pathways to inhibit the
proliferation and promote the apoptosis of hepatoma cells (18).
The present study reported for the first time, to
the best of out knowledge, that matrine can affect the
proliferation and differentiation of hepatoma stem cells. When the
liver is severely injured, hepatocytes may be lost through
apoptosis, necrosis or reduced proliferation. Previous
investigations have suggested that a hepatic progenitor cell
population of oval cells are recruited to repair the damaged liver
(19). Hepatic oval cells are
considered to represent a stem-like cell lineage, originating from
the intrahepatic bile ducts or bone marrow cells (20,21).
In a rat model of partial hepatectomy, oval cells were recruited
for involvement in liver regeneration. Therefore, oval cells may
represent good candidate seeding cells for liver tissue
engineering.
Liver regeneration is markedly affected by the local
hepatic microenvironment, which is composed of non-parenchymal
cells, the extracellular matrix and growth factors, which act
through paracrine or autocrine pathways to modulate the
proliferation and differentiation of oval cells (22,23).
This is mediated intracellularly through the phosphoinositide
3-kinase/AKT-nuclear factor-κB signaling pathways (24,25).
The Notch signaling pathway is a highly conserved
signal transduction pathway, which is essential for the
differentiation and proliferation of stem cells (26). In mammals, notch receptors,
ligands, including Jagged 1 and RBP-Jk/CBF1 in the nucleus, and
downstream target genes, including HES1, have been implicated in
the regulation of stem cell differentiation and proliferation
(27). Activation of Notch
signaling is reported to restrict oligodendrocyte differentiation
and promoteastrogliogenesis (28).
Notch signaling has been implicated in mammary stem cell and
luminal cell commitment, and activation of Notch enhances
self-renewal and transformation (29). In liver development, Notch has been
reported to regulate liver stem cell differentiation into
hepatocytes (30). In the present
study, it was observed that high levels of Jagged 1 and HES1 may
favor self-renewal of WB-F344 cells. Exposure to matrine inhibited
the expression of Jagged 1 and HES1, and promoted the expression of
ALB, a biomarker of mature hepatocytes. Furthermore, the effects of
matrine were ameliorated by the addition of PTH, an activator of
the Notch signaling pathway. These results indicated that matrine
can stimulate the differentiation of WB-F344 into hepatocytes
through inhibition of the Notch-Jagged 1-HES1 signaling
pathway.
The present study represents the first report, to
the best of our knowledge, of the effects of matrine on the hepatic
differentiation of WB-F344 cells. The present study demonstrated
that matrine likely induced the hepatic differentiation of WB-F344
cells through a mechanism involving down-regulation of the
Notch-Jagged 1-HES1 signaling pathway. The precise molecular
mechanisms underlying the effect of matrine on the Notch-Jagged
1-HES1 pathway remain to be elucidated, however, the present study
highlights an important physiological component of stem cell
differentiation, and suggest that matrine may be important in the
stimulation of hepatic stem cell differentiation.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 30873423), the Beijing
Natural Science Foundation of China (grant no. 142081) and the
Capital Health Research and Development of Special (grant no.
2016-271-21).
References
|
1
|
Zhu Y, Miao Z, Gong L and Chen W:
Transplantation of mesenchymal stem cells expressing TIMP-1-shRNA
improves hepatic fibrosis in CCl4-treated rats. Int J
Clin Exp Pathol. 8:8912–8920. 2015.
|
|
2
|
Tessier S, Karczewski P, Krause EG,
Pansard Y, Acar C, Lang-Lazdunski M, Mercadier JJ and Hatem SN:
Regulation of the transient outward K(+) current by
Ca(2+)/calmodulin-dependent protein kinases II in human atrial
myocytes. Circ Res. 85:810–819. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bodart V, Bouchard JF, McNicoll N, Escher
E, Carrière P, Ghigo E, Sejlitz T, Sirois MG, Lamontagne D and Ong
H: Identification and characterization of a new growth
hormone-releasing peptide receptor in the heart. Circ Res.
85:796–802. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Knight B, Akhurst B, Matthews VB, Ruddell
RG, Ramm GA, Abraham LJ, Olynyk JK and Yeoh GC: Attenuated liver
progenitor (oval) cell and fibrogenic responses to the choline
deficient, ethionine supplemented diet in the BALB/c inbred strain
of mice. J Hepatol. 46:134–141. 2007. View Article : Google Scholar
|
|
5
|
Roskams TA, Theise ND, Balabaud C, Bhagat
G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA,
Desmet V, et al: Nomenclature of the finer branches of the biliary
tree: Canals, ductules, and ductular reactions in human livers.
Hepatology. 39:1739–1745. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duncan AW, Dorrell C and Grompe M: Stem
cells and liver regeneration. Gastroenterology. 137:466–481. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Newsome PN, Hussain MA and Theise ND:
Hepatic oval cells: Helping redefine a paradigm in stem cell
biology. Curr Top Dev Biol. 61:1–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanimizu N, Tsujimura T, Takahide K,
Kodama T, Nakamura K and Miyajima A: Expression of Dlk/Pref-1
defines a subpopulation in the oval cell compartment of rat liver.
Gene Expr Patterns. 5:209–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yano Y, Hayashi Y, Teramoto T, Nakaji M,
Nagy P, Ninomiya T, Wada A, Hirai M, Kim SR, Seo Y, et al:
Apoptotic pathway related to oval cell proliferation. J
Gastroenterol Hepatol. 19:866–872. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee TY, Chang HH, Lo WC and Lin HC:
Alleviation of hepatic oxidative stress by Chinese herbal medicine
Yin-Chen-Hao-Tang in obese mice with steatosis. Int J Mol Med.
25:837–844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu T, Song Y, Chen H, Pan S and Sun X:
Matrine inhibits proliferation and induces apoptosis of pancreatic
cancer cells in vitro and in vivo. Biol Pharm Bull. 33:1740–1745.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao HY, Li GY, Lou MM, Li XY, Wei XY and
Wang JH: Hepatoprotective effect of Matrine salvianolic acid B salt
on carbon tetrachloride-induced hepatic fibrosis. J Inflamm (Lond).
9:162012. View Article : Google Scholar
|
|
13
|
Zhang F, Wang X, Tong L, Qiao H, Li X, You
L, Jiang H and Sun X: Matrine attenuates endotoxin-induced acute
liver injury after hepatic ischemia/reperfusion in rats. Surg
Today. 41:1075–1084. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang ZY, Wang L, Hou YX and Wang XB:
Effects of matrine on oval cell-mediated liver regeneration and
expression of RBP-Jκ and HES1. Mol Med Rep. 7:1533–1538.
2013.PubMed/NCBI
|
|
15
|
Jubb AM, Browning L, Campo L, Turley H,
Steers G, Thurston G, Harris AL and Ansorge O: Expression of
vascular Notch ligands Delta-like 4 and Jagged-1 in glioblastoma.
Histopathology. 60:740–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Calvi LM, Adams GB, Weibrecht KW, Weber
JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P,
Bringhurst FR, et al: Osteoblastic cells regulate the
haematopoietic stem cell niche. Nature. 425:841–846. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ke Z, Mao X, Li S, Wang R, Wang L and Zhao
G: Dynamic expression characteristics of Notch signal in bone
marrow-derived mesenchymal stem cells during the process of
differentiation into hepatocytes. Tissue Cell. 45:95–100. 2013.
View Article : Google Scholar
|
|
18
|
Zhang JQ, Li YM, Liu T, He WT, Chen YT,
Chen XH, Li X, Zhou WC, Yi JF and Ren ZJ: Antitumor effect of
matrine in human hepatoma G2 cells by inducing apoptosis and
autophagy. World J Gastroenterol. 16:4281–4290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Assimakopoulos SF, Tsamandas AC,
Alexandris IH, Georgiou C, Vagianos CE and Scopa CD: Stimulation of
oval cell and hepatocyte proliferation by exogenous bombesin and
neurotensin in partially hepatectomized rats. World J Gastrointest
Pathophysiol. 2:146–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lowes KN, Croager EJ, Olynyk JK, Abraham
LJ and Yeoh GC: Oval cell-mediated liver regeneration: Role of
cytokines and growth factors. J Gastroenterol Hepatol. 18:4–12.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oh SH, Witek RP, Bae SH, Zheng D, Jung Y,
Piscaglia AC and Petersen BE: Bone marrow-derived hepatic oval
cells differentiate into hepatocytes in
2-acetylaminofluorene/partial hepatectomy-induced liver
regeneration. Gastroenterology. 132:1077–1087. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mavier P, Martin N, Couchie D, Préaux AM,
Laperche Y and Zafrani ES: Expression of stromal cell-derived
factor-1 and of its receptor CXCR4 in liver regeneration from oval
cells in rat. Am J Pathol. 165:1969–1977. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Chen XP, Zhang WG, Zhang F, Xiang
S, Dong HH and Zhang L: Hepatic non-parenchymal cells and
extracellular matrix participate in oval cell-mediated liver
regeneration. World J Gastroenterol. 15:552–560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Malato Y, Ehedego H, Al-Masaoudi M, Cubero
FJ, Bornemann J, Gassler N, Liedtke C, Beraza N and Trautwein C:
NF-κB essential modifier is required for hepatocyte proliferation
and the oval cell reaction after partial hepatectomy in mice.
Gastroenterology. 143:1597–1608.e11. 2012. View Article : Google Scholar
|
|
25
|
Okano J, Shiota G, Matsumoto K, Yasui S,
Kurimasa A, Hisatome I, Steinberg P and Murawaki Y: Hepatocyte
growth factor exerts a proliferative effect on oval cells through
the PI3 K/AKT signaling pathway. Biochem Biophys Res Commun.
309:298–304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiba S: Notch signaling in stem cell
systems. Stem Cells. 24:2437–2447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ge W, Martinowich K, Wu X, He F, Miyamoto
A, Fan G, Weinmaster G and Sun YE: Notch signaling promotes
astrogliogenesis via direct CSL-mediated glial gene activation. J
Neurosci Res. 69:848–860. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang S, Sdrulla AD, diSibio G, Bush G,
Nofziger D, Hicks C, Weinmaster G and Barres BA: Notch receptor
activation inhibits oligodendrocyte differentiation. Neuron.
21:63–75. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bouras T, Pal B, Vaillant F, Harburg G,
Asselin-Labat ML, Oakes SR, Lindeman GJ and Visvader JE: Notch
signaling regulates mammary stem cell function and luminal
cell-fate commitment. Cell Stem Cell. 3:429–441. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang T, You N, Tao K, Wang X, Zhao G, Xia
N, Li N, Tang L, Liu W and Dou K: Notch is the key factor in the
process of fetal liver stem/progenitor cells differentiation into
hepatocytes. Dev Growth Differ. 54:605–617. 2012. View Article : Google Scholar : PubMed/NCBI
|