Introduction
Neural stem cell (NSC) implant therapy offers
considerable potential for the regeneration or self-repair of
injury or degeneration of the brain or spinal cord. However, the
transplantation of exogenous NSCs is limited due to low survival
rates and differentiation of the implanted NSCs. The leading cause
of implanted cell death is associated with the microenvironment of
the injured tissue, including inflammatory responses, oxidative
stress and the development of pro-apoptotic factors. Several
attempts have aimed to enhance stem cell survival, proliferation
and differentiation following cell therapy (1–3). For
example, neurotrophin-3, released from
poly(ε-caprolactone)-block-poly(l-lactic acid-co-ε-caprolactone
scaffolds, has been shown to promote the survival and neuronal
differentiation of transplanted NSCs in a rat spinal cord injury
model (4). Hydrogel scaffolding
also increases the survival of engrafted NSCs (5). Therefore, there is substantial
interest in the identification of drugs and other methods for the
protection of implanted cells from the adverse environment of the
injured tissue for successful cell therapy.
Resveratrol
(trans-3,5,4′-trihydroxystilebene), a naturally occurring
polyphenolic phytoalexin, is predominantly found in dietary
sources, including grapes, red wine, peanuts, plums and other
plants (6). Studies have shown
that resveratrol has anti-inflammatory, anti-oxidant, anticancer
and chemopreventive properties (7,8). In
addition, it has been reported that resveratrol has neuroprotective
effects in ischemic cerebral stroke, Alzheimer disease and
Parkinson's disease (9–11). Resveratrol can decrease neuronal
apoptosis by upregulating the expression of B cell lymphoma-2
(Bcl-2) and down-regulating the expression of Bcl-2-associated X
protein in the hippocampus following focal cerebral ischemia in
rats (12). Resveratrol also
enhances hippocampal neurogenesis and alleviates hippocampal
atrophy in prenatally stressed rats and mice with chronic fatigue
(13,14). Previously, it was observed that
resveratrol pretreatment can attenuate cerebral ischemic injury by
upregulating the expression levels of the nuclear factor erythroid
2-related factor 2 (Nrf-2) and heme oxygenase 1 (HO-1)
transcription factors to improve neurological function (15). Gorbunov et al (16) also found that resveratrol can
modify cardiac stem cells to enhance their survival and
differentiation, and to improve cardiac function. However, whether
resveratrol can decrease injury and promote proliferation of NSCs
following cerebral ischemic damage remain to be fully
elucidated.
Nrf2, a cap'n'Collar (CNC) transcription factor, is
important in antioxidative stress (17). When cells or organisms are exposed
to oxidative stress, electrophiles or chemopreventive agents, the
Nrf2 pathway is activated to trigger the expression of antioxidant
response element (ARE)-dependent genes, including HO-1 and NAD(P)
H:quinone oxidoreductase 1 (NQO1), which attenuate cellular
oxidative stress (18,19).
Therefore, the present study investigated whether
and how resveratrol pretreatment decreases injury and promotes the
proliferation of NSCs following oxygen-glucose
deprivation/reoxygenation (OGD/R) in vitro. The results
showed that resveratrol markedly and dose-dependently enhanced NSC
survival and proliferation, decreased NSC apoptosis and the levels
of malondiadehyde (MDA), increased SOD activity and glutathione
(GSH) content, and upregulated the protein expression levels of
Nrf2, HO-1 and NQO1 following OGD/R injury of NSCS in
vitro.
Materials and methods
NSC culture
The NSCs were obtained from eight neonatal 1–2
day-old male (n=5) and female (n=3) Sprague-Dawley rats, provided
by the Department of Animal Experiments, Chongqing Medical
University (Chongqing, China), and used for primary NSC
cultivation, as described previously with modifications (20–22).
The rats were maintained at 20±2°C, humidity 60±5% under a 12-h
light/dark cycle with access to food and water ad libitum.
The rats were sacrificed by immersion in 75% ethanol for 5 min
prior to cervical dislocation. The cerebellum was dissected from
the brains. Briefly, the cerebral cortices were dissected, washed
with Dulbecco's modified Eagle's medium/F12 (DMEM/F12) medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
digested with 0.125% trypsin at 37°C for 30 min. The tissue was
then dissociated into a single cell suspension by mechanical
titration with a sterile, fire-polished glass Pasteur pipette. The
cells were counted and suspended at a density of
5×105/ml in a 25-cm2 glass culture flask in
DMEM/F12 medium supplemented with 2% B27 (Gibco; Thermo Fisher
Scientific, Inc.), 20 ng/ml recombinant human epidermal growth
factor, 20 ng/ml recombinant human basic fibroblast growth factor
(Peprotech, Inc., Rocky Hill, NJ, USA), 100 mg/ml streptomycin and
100 U/ml penicillin (GE Healthcare Life Sciences Hyclone
Laboratories), Logan, UT, USA). The cells were maintained at 37°C
in an incubator with a humidified atmosphere of 5% carbon dioxide,
and half the medium was replaced every other day. The cells were
passaged approximately every 7 days. When passaging, the
neurospheres were incubated in 0.125% trypsin for 5 min and
dissociated mechanically into a single-cell suspension using a
sterile, fire-polished glass Pasteur pipette. Following
centrifugation at 79 × g for 5 min at room temperature, the cells
were reseeded in new glass culture flasks at the same density as in
the primary culture. The third passage NSCs were inoculated at a
density of 5×105/ml into plastic culture flasks coated
with poly-d-lysine (Beyotime Institute of Biotechnology, Jiangsu,
China) and laminin (Sigma-Aldrich, St. Louis, MO, USA) for adherent
culture.
All experimental procedures were performed with the
approval of the Animal Experimental Committee of Chongqing Medical
University, and submitted to relevant laws.
OGD/R model
According to previously described methods, OGD/R of
the cortical NSCs was performed to mimic cerebral artery occlusion
and reperfusion injury (23,24).
Briefly, following washing twice with D-Hanks solution (GE
Healthcare Life Sciences Hyclone Laboratories), The NSCs were
incubated with D-Hanks solution at 37°C for 150 min in an incubator
with a humidified atmosphere containing 95% N2 and 5%
CO2 (Thermo 3111; Thermo Fisher Scientific, Inc.). When
reoxygenated, the D-Hanks solution was replaced with NSC medium,
and the NSCs were maintained in a humidified normoxic atmosphere
containing 5% CO2 for 24 h at 37°C.
Drug treatment
To determine whether resveratrol enhanced NSC
viability following OGD/R injury in vitro, four treatment
groups were used for comparison: i) Normal group, NSCs were
cultured in NSC culture medium without OGD; ii) model group, NSCs
were treated with OGD only; iii) ethanol group, NSCs were treated
with NSC medium containing ethanol (volume fraction 1.3%) for 24 h
prior to OGD; iv) resveratrol pretreatment groups, NSCs were
maintained in complete medium containing different concentrations
(1, 5, 10, 20, 50 and 100 μmol/l) of resveratrol (purity
99%; Sigma-Aldrich) for 24 h prior to OGD/R. The optimal effect was
observed at a concentration of 5 μmol/l resveratrol, and
thus 1, 5 and 20 μmol/l resveratrol were used for the
subsequent experiments.
To investigate whether resveratrol promoted NSC
proliferation, and decreased NSC apoptosis and anti-oxidative
stress, three treatment groups were used for comparison: i) Normal
group, NSCs were cultured in NSC culture medium without OGD; ii)
model group, NSCs were treated with OGD only; iii) resveratrol
pretreatment groups, NSCs were maintained in complete medium
containing different concentrations (1, 5 and 20 μmol/l) of
resveratrol for 24 h prior to OGD/R.
To examine the effects of resveratrol on the protein
expression levels of Nrf-2, NQO-1 and HO-1 in the NSCs following
OGD/R, three groups were used for comparison: i) Normal group, NSCs
were cultured in NSC culture medium without OGD; ii) model group,
NSCs were treated with OGD only; iii) 5 μmol/l resveratrol
pretreatment group, NSCs were maintained in complete medium
containing 5 μmol/l resveratrol for 24 h prior to OGD/R.
MTT cell viability assay
The MTT assay (Beyotime Institute of Biotechnology)
was used to quantitatively measured cell viability. Briefly, the
third passage NSCs (~5,000 cells/well) were seeded into
poly-L-lysine-coated 96-well plates, with six replicates in each
group, and subjected to the various treatments described above.
Following treatment, MTT solution (5 mg/ml) was added to each
culture well, and the NSCs were incubated for 3 h at 37°C. The
medium was carefully removed, and the blue formazan products were
dissolved with 200 μl DMSO per well. The absorbance was then
measured on a microplate reader (Thermo Labsystems, Vantaa,
Finland) at 570 nm, with cell survival rates expressed as a
percentage of absorbance value of cells without any treatment. Each
experiment was repeated three times.
Flow cytometric analysis of NSC
proliferation
Cell cycle analysis was performed using a BD FACS
Vantage SE flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA). The NSCs in the different culture conditions were harvested
synchronously, dissociated into single cells, resuspended in 70%
ethanol and stored at 4°C overnight. The cells were then washed
with PBS, centrifuged at 124 × g for 5 min at room temperature and
resuspended in 0.5 μl buffer containing propidium iodide (50
μg/ml; Sigma-Aldrich) and RNase (100 μg/ml;
Sigma-Aldrich) for 30 min. Flow cytometry was then performed to
detect cell cycle. Each experiment was repeated three times.
Measurements of SOD activity, MDA levels
and GSH content
To measure the activity of SOD, the NSCs were lysed
with RIPA lysis buffer and centrifuged at 124 × g for 5 min at room
temperature, according to the manufacturer's protocol at 4°C
(Beyotime Institute of Biotechnology). This method uses xanthine
and xanthine oxidase to generate superoxide radicals, which react
with p-iodonitrotetrazolium violet to form a red formazan dye. The
absorbances of each standard and sample were read at 450 nm using a
96-well microplate reader, and the background absorbance from
culture medium, which was not used for any cell cultures, was
determined. SOD activity was expressed as U/mg protein. Each
experiment was repeated three times.
The levels of MDA were measured using the
thiobarbituric acid method. Briefly, the NSCs were harvested and
homogenized. An Enhanced BCA Protein Assay kit (Beyotime Institute
of Biotechnology) was used to determined the quantity of total
protein in the supernatant of the NSC homogenate. The absorbance
was measured at 532 nm by spectrometry. Each experiment was
repeated three times.
For the GSH assay, the cells were lysed with RIPA
lysis buffer and the cell suspension was centrifuged at 124 × g for
5 min at room temperature, according to the manufacturer's
protocol, at 4°C (Beyotime Institute of Biotechnology), and the
supernatant was used for further analyses. The content of GSH was
measured on the principle that reduced GSH can be quantified by
colorimetry at 405 nm when it reacts with 5,5′-Dithiobis
(2-nitrobenzoic acid). The absorbance of GSH was measured at 405
nm. Cellular GSH content was calculated using a concurrently run
GSH standard curve and expressed as nmol of GSH per milligram of
cellular protein (nmol/mg protein). Each experiment was repeated
three times.
Hoechst 33258 staining of nuclei for
analysis of apoptotic cells
Hoechst 33258 staining of nuclei was used to detect
apoptotic cells. Hoechst 33258, a blue fluorescent dye, readily
permeates the cell membrane for the determination and
semi-quantification of cells with fragmented and condensed
chromatin. Following the 24 h re-oxygenation period, the NSCs were
washed three times with PBS and fixed in 4% formaldehyde for 10
min. The fixed cells were then washed with PBS and incubated with
Hoechst 33258 (Sigma-Aldrich) for 5 min in a humidified chamber,
protected from light, at 37°C. The cells were washed three times
with PBS and mounted with one drop of mounting solution. The cell
nuclei were observed and images were captured using a fluorescence
microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan).
Western blot analysis
Following the various treatments described above,
the cellular proteins were extracted and the protein levels were
analyzed. Briefly, the cells were rinsed with ice-cold PBS and
lysed using a nuclear and cytoplasmic protein extraction kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. The concentrations of nuclear and
cytoplasmic protein were measured using a BCA protein assay kit.
The sample lysates (50 μg) were loaded onto a 10% SDS-PAGE
gel for electrophoresis, following which the protein was
transferred onto a 0.45 μm PVDF membrane (EMD Millipore,
Billerica, MA, USA), blocked in 5% skim milk powder-TBST buffer,
and incubated with primary antibodies and horseradish peroxidase
(HRP)-conjugated secondary antibodies. The membranes were incubated
with the appropriate primary antibodies at 4°C overnight. The
primary antibodies used were as follows: Polyclonal rabbit
anti-Nrf-2 (1:1,000; cat. no. ab62352), polyclonal goat anti-NQO-1
(1:1,000; cat. no. ab2346), monoclonal rabbit anti-HO-1 (1:1,000;
cat. no. ab68477) and anti-β-actin (1:1,000; cat. no. ab8229) all
obtained from Abcam (Hong Kong, China). The membranes were then
treated with HRP-conjugated goat anti-rabbit (cat. no. ZDR-5306)
and rabbit anti-goat (cat. no. ZDR-5308) secondary antibodies
(1:100; Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd.,
Beijing, China) for 1 h at 37°C. The antigen-antibody complexes
were then detected with an ECL reagent kit (Beyotime Institute of
Biotechnology), and visualized and analyzed using the ChemiDoc™
XRS+ imaging system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The results were quantified using QuantityOne 1-D analysis
software (Bio-Rad Laboratories, Inc.). The quantities of each
product were normalized by dividing the average gray level of the
signal by that of the corresponding β-actin amplicon. Each
experiment was repeated three times.
Cell counts
The numbers of positively-stained cells were counted
in 10 randomly-selected visual fields (magnification, ×200) for
each sample using a BX51 fluorescence microscope and analysis using
Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
Statistical analysis was performed using the SPSS
19.0 statistical software package (IBM SPSS, Armonk, NY, USA). All
values are expressed as the mean ± standard deviation. Every
experiment was repeated at least three times. Data were
statistically analyzed by one-way analysis of variance and Tukey's
post-hoc test. P<0.05 were considered to indicate a
statistically significant difference.
Results
Resveratrol pretreatment decreases injury
and promotes proliferation of NSCs in a concentration-dependent
manner following OGD/R in vitro
An MTT assay was used to determine the optimal
concentration of resveratrol pretreatment on NSC viability
following OGD/R in vitro. As shown in Fig. 1, NSC viability was significantly
reduced in the model, ethanol and resveratrol groups compared with
the normal group. No differences were found between the model,
ethanol and 100 μmol/l resveratrol groups. However, NSC
viability in the resveratrol groups (1, 5, 10 and 50 μmol/l)
was significantly higher, compared with that of the model group,
and the highest viability was observed in the 5 μmol/l
resveratrol group. These results showed that resveratrol decreased
the OGD/R-induced injury of the NSCs and the most effective
concentration of resveratrol was 5 μmol/l. Therefore, 1, 5
and 20 μmol/l resveratrol were selected for further
experiments.
 | Figure 1Resveratrol promotes NSC viability in
a concentration-dependent manner following OGD/R injury. The MTT
assay revealed that NSC viability was significantly reduced in the
Mod, Eth and Res groups, compared with the Nor group following
OGD/R. No significant differences was found between the Mod, Eth
and 100 μmol/l resveratrol (Res100) groups. However, NSC
viability was significantly higher in the 1, 5, 10 and 50
μmol/l resveratrol (Res1, 5, 10 and 50) groups, compared
with the Mod group, with the highest viability in the 5
μmol/l resveratrol (Res5) group. ▲P<0.05, vs.
Nor group; ΔP<0.05, vs. Mod group;
*P<0.05, vs. Res1, Res10, Res20, Res50 and Res100
groups; #P<0.05, vs. Res20 group. Data are presented
as the mean ± standard deviation and were compared using one-way
analysis of variance (n=3 for each group). NSC, neural stem cell;
OGD/R, oxygen-glucose deprivation/reoxygenation; Nor, normal; Mod,
model; Eth, ethanol; Res, resveratrol. |
To investigate whether resveratrol decreases the
apoptosis of NSCs following OGD/R injury in vitro, apoptotic
NSCs were labeled with Hoechst 33258, a blue fluorochrome, which
penetrates the cell membrane. Hoechst 33258-positive cells under
the fluorescence microscope were indicative of apoptotic cells. As
shown in Fig. 2A, few Hoechst
33258-positive cells were observed in the normal group; there were
significantly higher numbers in the control and resveratrol groups
(Fig. 2B–F). The numbers of
Hoechst 33258-positive cells in the resveratrol groups were
significantly lower, compared with that in the control group, and
were lowest in the 5 μmol/l resveratrol group. These results
showed that resveratrol decreased the apoptosis of NSCs following
OGD/R injury in vitro.
 | Figure 2Resveratrol decreases apoptosis of
NSCs in a concentration-dependent manner following OGD/R injury.
Apoptosis was determined using Hoechst 33258 staining
(magnification, ×400). Few Hoechst 33258-positive cells were
identified in the (A) Nor group, whereas there were significantly
higher numbers in the (B) Mod and the (C–E) Res1, 5 and 20 groups.
The numbers of Hoechst 33258-positive cells in the Res groups were
significantly lower, compared with that in the Mod group, and were
lowest in the 5 μmol/l resveratrol (Res5) group. (F) Graph
showing the numbers of Hoechst 33258-positive NSCs in each group.
These results suggested that Res reduced apoptosis of the NSCs
following OGD/R injury in vitro. ▲P<0.05, vs.
Nor group; ΔP<0.05, vs. Mod group;
*P<0.05, vs. Res1 and Res20 groups. Data are
presented as the mean ± standard deviation and were compared using
one-way analysis of variance (n=3 for each group). NSCs, neural
stem cells; OGD/R, oxygen-glucose deprivation/reoxygenation; Nor,
normal; Mod, model; Res, resveratrol. |
To investigate whether resveratrol can promote the
proliferation of NSCs following OGD/R injury in vitro,
proliferated NSCs were examined using flow cytometry. As shown in
Fig. 3, the ratio of NSCs in the S
and G2/M phase was significantly higher in the normal group
(Fig. 3A), compared with the model
group (Fig. 3B), and in the
resveratrol groups, compared with the model group, and was highest
in the 5 μmol/l resveratrol group (Fig. 3C–F). These result suggested that
resveratrol promoted proliferation of the NSCs following OGD/R
in vitro.
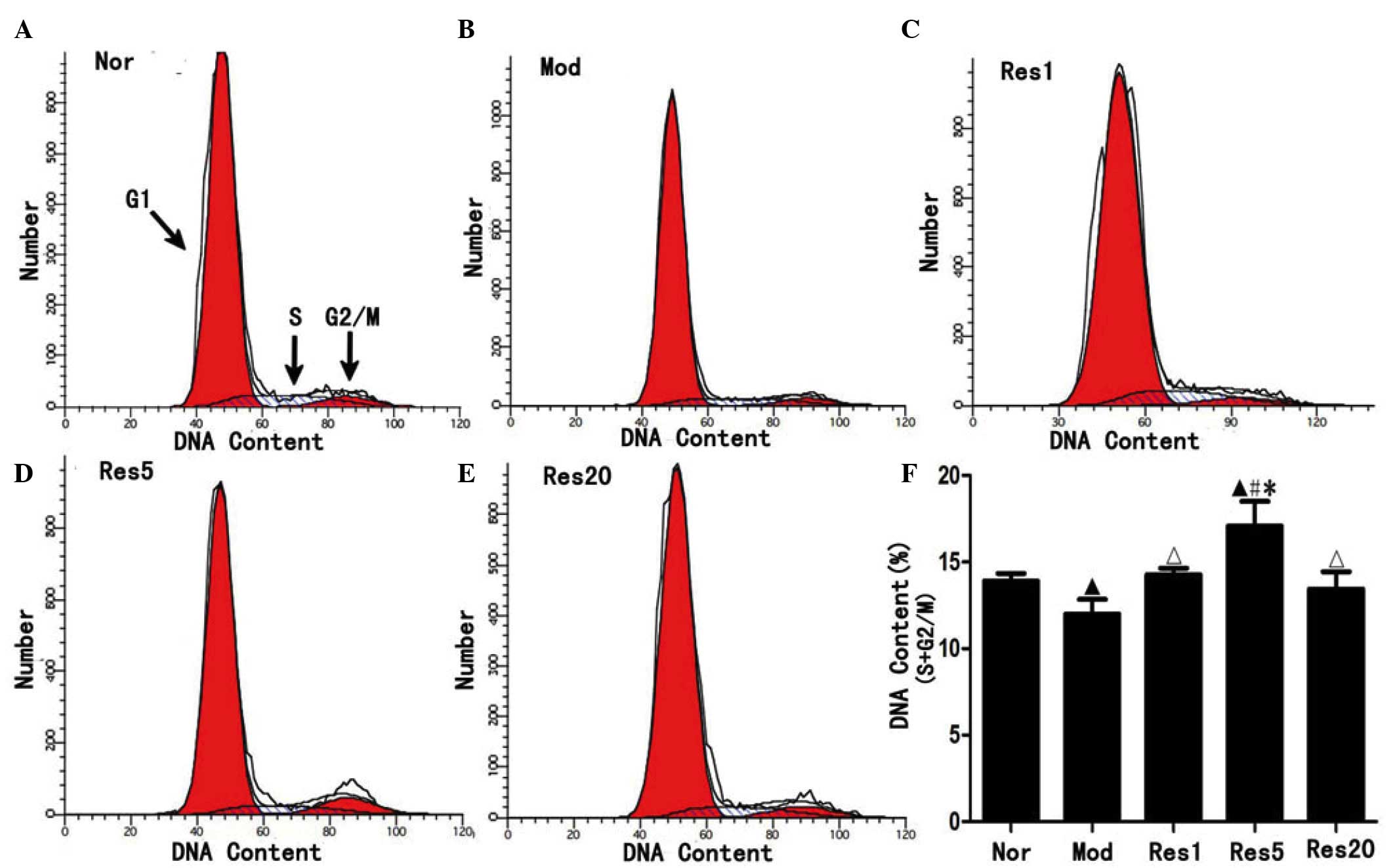 | Figure 3Resveratrol promotes the
proliferation of NSCs in a concentration-dependent manner following
oxygen-glucose deprivation/reoxygenation injury. Flow cytometry
revealed that, compared with the (A) Nor group, the ratio of NSCs
in the S and G2/M phase were significantly decreased in the (B) Mod
group and, compared with the Mod group, increased in the (C–E) Res
groups and, compared with the (C and E) Res1 and Res20 groups,
increased in the (D) Res5 group, peaking in the 5 μmol/l
(Res5) group. ▲P<0.05, vs. Nor group;
ΔP<0.05 and #P<0.01, vs. Mod group;
*P<0.05, vs. Res1 and Res20 groups. Data are
presented as the mean ± standard deviation and were compared using
one-way analysis of variance (n=3 for each group). NSCs, neural
stem cells; Nor, normal; Mod, model; Res, resveratrol. |
Taken together, the above results showed that
resveratrol decreased injury and promoted the proliferation of NSCs
in a concentration-dependent manner following OGD/R injury in
vitro. Furthermore, the most effective concentration of
resveratrol was 5 μmol/l.
Resveratrol pretreatment attenuates
oxidative damage and upregulates protein expression levels of
Nrf-2, NQO-1 and HO-1 in NSCs following OGD/R injury in vitro
Oxidative stress was assessed by evaluating the
products of lipid peroxidation with the levels of MDA, enzymatic
activity of SOD and content of GSH. As shown in Fig. 4A, the levels of MDA in the control
and resveratrol groups were significantly higher, compared with
that in the normal group. However, the levels of MDA in the
resveratrol groups were significantly lower, compared with that in
the control group, and was lowest in the 5 μmol/l
resveratrol group (Fig. 4A). The
activity of SOD and content of GSH were significantly lower in the
control group, compared with the normal group (P<0.05), and were
significantly higher in the resveratrol pretreatment groups,
compared with the normal and control groups, with the highest
levels observed in the 5 μmol/l resveratrol groups (Fig. 4B and C). These results indicated
that resveratrol pretreatment ameliorated oxidative damage of the
NSCs following OGD/R in vitro.
 | Figure 4Resveratrol ameliorates oxidative
damage of neural stem cells in a concentration-dependent manner
following oxygen-glucose deprivation/reoxygenation in vitro.
(A) Levels of MDA were significantly higher in the Mod group,
compared with the Nor group, and significantly lower in the Res
groups, compared with the Mod group, and was lowest in the 5
μmol/l resveratrol (Res5) group. (B) SOD activity and (C)
GSH content were significantly lower in the Mod group, compared
with the Nor group, and significantly higher in the Res groups,
compared with the Nor and Mod groups, being highest in the 5
μmol/l resveratrol (Res5) group. ▲P<0.05, vs.
Nor group; ΔP<0.05, vs. Mod group;
#P<0.01 and *P<0.05, vs. Res1 and Res20
groups Data are presented as the mean ± standard deviation and were
compared using one-way analysis of variance (n=3 for each group).
Nor, normal; Mod, model; Res, resveratrol; MDA, malondiadehyde;
SOD, superoxide dismutase; GSH, glutathione. |
Nrf2, a CNC transcription factor, regulates the
expression of numerous reactive oxygen species (ROS), detoxifying
agents and antioxidants. The components of the Nrf2 signaling
pathway in mammals include Nrf2, NQO-1 and HO-1. Therefore, the
present study examined whether resveratrol upregulated the
expression levels of Nrf2, NQO-1 and HO-1.
Western blot analysis (Fig. 5A) showed that the protein
expression levels of Nrf2 in the nuclei, and NQO-1 and HO-1 in the
cytoplasm of NSCs were significantly upregulated in the model and
resveratrol groups, compared with those in the normal group. The
highest levels were observed in the 5 μmol/l resveratrol
group (P<0.05; Fig. 5B–D).
These results indicated that resveratrol pretreatment upregulated
the protein expression levels of Nrf2, NQO-1 and HO-1 of the NSCs
following OGD/R in vitro.
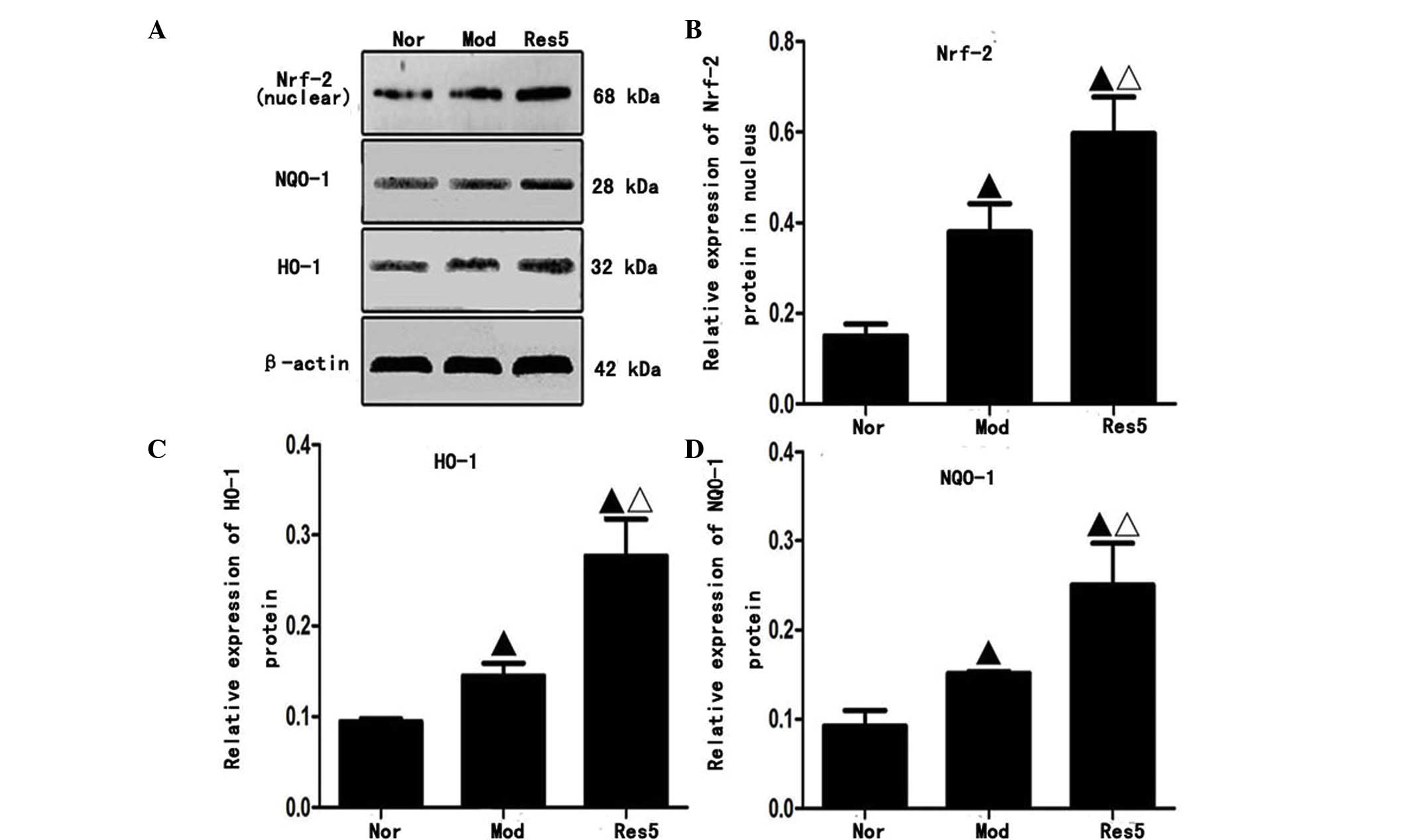 | Figure 5Resveratrol upregulates the protein
expression levels of Nrf-2 in the nuclei, and NQO-1 and HO-1 in the
cytoplasm of neural stem cells following oxygen-glucose
deprivation/reoxygenation injury in vitro. (A) Protein
expression of Nrf2 in nuclei and NQO-1 and HO-1 in cytoplasm.
Quantification of data for (B) Nrf2, (C) NQO-1 and (D) HO-1.
Western blot analysis revealed that the protein expression levels
of Nrf2 in the nuclei, and NQO-1 and HO-1 in the cytoplasm were
significantly upregulated in the Mod and Res groups, compared with
those in the Nor group. The levels were highest in the 5
μmol/l resveratrol (Res5) group suggesting resveratrol
increased expression of Nrf2 in the nuclei and NQO-1 and HO-1 in
the cytoplasm. ▲P<0.05, vs. Nor group;
ΔP<0.05, vs. Mod group. Data are presented as the
mean ± standard deviation and were compared using one-way analysis
of variance (n=3 for each group). Nor, normal; Mod, model; Res,
resveratrol; Nfr-2, nuclear factor erythroid 2-related factor 2;
NQO-1 NAD(P)H:quinone oxidoreductase 1; HO-1, heme oxygenase 1. |
Discussion
The present study showed that resveratrol
pretreatment increased the viability, ameliorated the apoptosis and
promoted the proliferation of NSCs following OGD/R injury in
vitro. This occurred in a concentration-dependent manner, and
the most effective concentration of resveratrol was 5
μmol/l. An increasing number of reports have shown that
resveratrol has a general range of activities, which depend on
several factors, including the concentration of resveratrol, the
cell or organism type being examined, and the physiological or
pathological state of the cells or organisms. Moriya et al
(14) reported that resveratrol
(40 mg/kg body weight) promoted hippocampal neurogenesis and
ameliorated hippocampal atrophy in mice with chronic fatigue.
Harada et al (25) found
that resveratrol enhanced hippocampal neurogenesis and improved
cognitive function in wild-type mice. Madhyastha et al
(13) also reported that
resveratrol enhanced postnatal hippocampal neurogenesis in
prenatally stressed rats. However, Park et al (26) reported that resveratrol inhibited
the proliferation of neural progenitor cells in culture (20 and 50
μmol/l) and hippocampal neurogenesis (1–10 mg/kg body
weight). Leong et al (27)
also found that 30–120 μmol/l resveratrol inhibited the
proliferation of embryonic cardiomyoblast. In the present study, it
was observed that 1–50 μmol/l resveratrol promoted NSC
viability and 100 μmol/l resveratrol decreased NSC viability
following OGD/R injury in vitro. Therefore, further
investigations are required to determine whether resveratrol has a
dual concentration effect on neurogenesis in different
physiological or pathological states.
Oxidative stress generated by ROS is one of the
important pathological mechanisms of OGD/R injury (28–30).
Following OGD/R injury, excess ROS are generated. As NSCs have low
levels of antioxidative enzyme activity, they are vulnerable to
ROS, which causes oxidative damage and leads to cell death.
Therefore, antioxidant treatment is important in OGD/R injury.
MDA, a product of lipid peroxidation, is an
important marker for oxidative stress. In addition, all cells or
organisms have defense mechanisms, which involve GSH reductase,
SOD, catalase and GSH peroxidase, to protect cells against the
damaging effects of ROS (31). SOD
and GSH, two important endogenous free radical scavengers, are
crucial in maintaining the oxidative and antioxidative balances of
the body, as they scavenge free radicals to protect cells from
oxidative damage. Increased activity of SOD and GSH increases the
ability of cells to eliminate oxygen free radicals. In the present
study, the levels of MDA were significantly higher in the control
group, compared with the normal group. The results suggested that
OGD/R-induced injury of the NSCs involves oxidative stress.
Following resveratrol pretreatment, the levels of MDA were
significantly decreased. By contrast, the activity of SOD and
content of GSH were increased significantly in the resveratrol
pretreatment groups, compared with the normal and control groups.
Konyalioglu et al (32)
reported that resveratrol protects embryonic neural stem cells
against hydrogen peroxide-induced oxidative stress (32). Taken together, these findings
suggested that resveratrol pretreatment attenuated oxidative
damage, and had neuroprotective and neurogenetic effects on the
NSCs following OGD/R injury in vitro. However, the exact
protective mechanisms underlying the effect of resveratrol on NSCs
remains to be fully elucidated.
Nrf2 is vital in antioxidative stress (30,33).
Under basal conditions, Nrf2-dependent transcription is repressed
by a negative regulator, Kelch-like ECH-associated protein 1
(Keap1). When the cells are exposed to oxidative stress,
electrophiles or chemopreventive agents, Nrf2 evades Keap1-mediated
repression and activates the expression of antioxidant response
element (ARE)-dependent genes, including HO-1 and NQO1, to maintain
cellular redox homeostasis and attenuate cellular oxidative stress
(18,19,34).
Yang et al (35) reported that the expression levels
of Nrf2 and HO-1 were upregulated at 3 h and peaked 24 h following
cerebral ischemia, prior to gradually decreasing at 48 and 72 h
(35). In our previous study, the
expression levels of Nrf2 and HO-1 were upregulated at 2 h and
peaked 24 h following cerebral I/R in rats (15). The present study showed that the
protein expression levels of Nrf2 in the nuclei, and NQO-1 and HO-1
in the cytoplasm of the NSCs were significantly dysregulated
following OGD/R injury in vitro. These findings suggested
that the Nrf2/ARE signaling pathway may be important in vivo
and in vitro following stroke or OGD/R injury.
Sakata et al (36) reported that resveratrol upregulates
the expression of HO-1 in a time- and dose-dependent manner, and
protects against free-radical or excitotoxic damage of cortical
neuronal cells in mice in vitro. Resveratrol also protects
dopaminergic SH-SY5Y cells against rotenone-induced neurotoxicity
by inducing HO-1-dependent autophagy, and upregulating the
expression levels of Nrf-2 and NQO-1, which suppresses the
oxidative and inflammatory stress responses of a high-fat,
high-carbohydrate meal (37,38).
In addition, Gorbunov et al (16) and Gurusamy et al (39) reported that resveratrol
pretreatment upregulated the expression of Nrf-2, and enhanced
implanted multi-potent clonogenic cardiac stem cell survival and
proliferation. Our previous study demonstrated that resveratrol
pretreatment for 7 days significantly decreased cerebral ischemic
injury, improved neurological function, upregulated the expression
levels of the Nrf2 and HO-1 transcription factors, and ameliorated
oxidative damage in rats with middle cerebral artery occlusion
(15). In addition, the present
study demonstrated that resveratrol pretreatment significantly
upregulated the expression levels of Nrf2, NQO-1 and HO-1,
decreased injury and promoted the proliferation of NSCs in a
concentration-dependent manner following OGD/R in vitro.
Therefore, these findings suggested that the Nrf2/ARE signaling
pathway is vital in the neuroprotection by resveratrol of NSCs
against OGD/R injury.
Taken together, the present study demonstrated that
resveratrol pretreatment had a neuroprotective effect on NSCs
following OGD/R injury. This neuroprotective effect was likely
caused, at least in part, by upregulated expression levels of Nrf2,
HO-1 and NQO1 to decrease oxidative damage, ameliorate apoptosis
and promote neurogenesis. The findings of the present study are
important for understanding the mechanism underlying the
resveratrol-induced decreased injury and increased proliferation of
NSCs. In the future, investigations are required to determine
whether Nrf2 signaling mediates resveratrol-induced
neurorestorative processes, including neurogenesis, axonal
remodeling and oligodendrogenesis, following stroke in
vivo.
Acknowledgments
The present study was supported by grants from the
National Key Clinical Specialties Construction Program of China for
Neurology [The First Affiliated Hospital of Chongqing Medical
University; grant no. (2014)27] and the National Natural Science
Foundation of China (grant no. 81071119).
References
|
1
|
Keuters MH, Aswendt M, Tennstaedt A,
Wiedermann D, Pikhovych A, Rotthues S, Fink GR, Schroeter M, Hoehn
M and Rueger MA: Transcranial direct current stimulation promotes
the mobility of engrafted NSCs in the rat brain. NMR Biomed.
28:231–239. 2015. View
Article : Google Scholar
|
|
2
|
Lu P, Graham L, Wang Y, Wu D and Tuszynski
M: Promotion of survival and differentiation of neural stem cells
with fibrin and growth factor cocktails after severe spinal cord
injury. J Vis Exp. e506412014.PubMed/NCBI
|
|
3
|
Sarnowska A, Jablonska A, Jurga M, Dainiak
M, Strojek L, Drela K, Wright K, Tripathi A, Kumar A, Jungvid H, et
al: Encapsulation of mesenchymal stem cells by bioscaffolds
protects cell survival and attenuates neuroinflammatory reaction in
injured brain tissue after transplantation. Cell Transplant.
22(Suppl 1): S67–S82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang S, Liao X, Shi B, Qu Y, Huang Z, Lin
Q, Guo X and Pei F: The effects of controlled release of
neurotrophin-3 from PCLA scaffolds on the survival and neuronal
differentiation of transplanted neural stem cells in a rat spinal
cord injury model. PLoS One. 9:e1075172014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang Y, Walczak P and Bulte JW: The
survival of engrafted neural stem cells within hyaluronic acid
hydrogels. Biomaterials. 34:5521–5529. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walle T: Bioavailability of resveratrol.
Ann N Y Acad Sci. 1215:9–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin HY, Tang HY, Davis FB and Davis PJ:
Resveratrol and apoptosis. Ann N Y Acad Sci. 1215:79–88. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sinha K, Chaudhary G and Gupta YK:
Protective effect of resveratrol against oxidative stress in middle
cerebral artery occlusion model of stroke in rats. Life Sci.
71:655–665. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin F, Wu Q, Lu YF, Gong QH and Shi JS:
Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson's
disease in rats. Eur J Pharmacol. 600:78–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li F, Gong Q, Dong H and Shi J:
Resveratrol, a neuroprotective supplement for Alzheimer's disease.
Curr Pharm Des. 18:27–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Pang L, Fang F, Zhang G, Zhang J,
Xie M and Wang L: Resveratrol attenuates brain damage in a rat
model of focal cerebral ischemia via up-regulation of hippocampal
Bcl-2. Brain Res. 1450:116–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Madhyastha S, Sekhar S and Rao G:
Resveratrol improves postnatal hippocampal neurogenesis and brain
derived neurotrophic factor in prenatally stressed rats. Int J Dev
Neurosci. 31:580–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moriya J, Chen R, Yamakawa J, Sasaki K,
Ishigaki Y and Takahashi T: Resveratrol improves hippocampal
atrophy in chronic fatigue mice by enhancing neurogenesis and
inhibiting apoptosis of granular cells. Biol Pharm Bull.
34:354–359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren JW, Fan C, Chen N, Huang J and Yang Q:
Resveratrol pretreatment attenuates cerebral ischemic injury by
upregulating expression of transcription factor Nrf2 and HO-1 in
rats. Neurochem Res. 36:2352–2362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gorbunov N, Petrovski G, Gurusamy N, Ray
D, Kim doH and Das DK: Regeneration of infarcted myocardium with
resveratrol-modified cardiac stem cells. J Cell Mol Med.
16:174–184. 2012. View Article : Google Scholar
|
|
17
|
Mohagheghi F, Khalaj L, Ahmadiani A and
Rahmani B: Gemfibrozil pretreatment affecting antioxidant defense
system and inflammatory, but not Nrf-2 signaling pathways resulted
in female neuroprotection and male neurotoxicity in the rat models
of global cerebral ischemia-reperfusion. Neurotox Res. 23:225–237.
2013. View Article : Google Scholar
|
|
18
|
Zhang DD: Mechanistic studies of the
Nrf2-Keap1 signaling pathway. Drug metab Rev. 38:769–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ping Z, Liu W, Kang Z, Cai J, Wang Q,
Cheng N, Wang S, Wang S, Zhang JH and Sun X: Sulforaphane protects
brains against hypoxic-ischemic injury through induction of
Nrf2-dependent phase 2 enzyme. Brain Res. 1343:178–185. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reynolds BA, Tetzlaff W and Weiss S: A
multipotent EGF-responsive striatal embryonic progenitor cell
produces neurons and astrocytes. J Neurosci. 12:4565–4574.
1992.PubMed/NCBI
|
|
21
|
Andersen RK, Johansen M, Blaabjerg M,
Zimmer J and Meyer M: Neural tissue-spheres: A microexplant culture
method for propagation of precursor cells from the rat forebrain
subventricular zone. J Neurosci Methods. 165:55–63. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Babu H, Claasen JH, Kannan S, Rünker AE,
Palmer T and Kempermann G: A protocol for isolation and enriched
monolayer cultivation of neural precursor cells from mouse dentate
gyrus. Front Neurosci. 5:892011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen T, Liu W, Chao X, Qu Y, Zhang L, Luo
P, Xie K, Huo J and Fei Z: Neuroprotective effect of osthole
against oxygen and glucose deprivation in rat cortical neurons:
Involvement of mitogen-activated protein kinase pathway.
Neuroscience. 183:203–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu X, Zhao J, Yu S, Chen Y, Wu J and Zhao
Y: Sulforaphane protects primary cultures of cortical neurons
against injury induced by oxygen-glucose deprivation/reoxygenation
via anti-apoptosis. Neurosci Bull. 28:509–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harada N, Zhao J, Kurihara H, Nakagata N
and Okajima K: Resveratrol improves cognitive function in mice by
increasing production of insulin-like growth factor-I in the
hippocampus. J Nutr Biochem. 22:1150–1159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park HR, Kong KH, Yu BP, Mattson MP and
Lee J: Resveratrol inhibits the proliferation of neural progenitor
cells and hippocampal neurogenesis. J Biol Chem. 287:42588–42600.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leong CW, Wong CH, Lao SC, Leong EC, Lao
IF, Law PT, Fung KP, Tsang KS, Waye MM, Tsui SK, et al: Effect of
resveratrol on proliferation and differentiation of embryonic
cardiomyoblasts. Biochem Biophys Res Commun. 360:173–180. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Slemmer JE, Shacka JJ, Sweeney MI and
Weber JT: Antioxidants and free radical scavengers for the
treatment of stroke, traumatic brain injury and aging. Curr Med
Chem. 15:404–414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nassar NN, Abdelsalam RM, Abdel-Rahman AA
and Abdallah DM: Possible involvement of oxidative stress and
inflammatory mediators in the protective effects of the early
preconditioning window against transient global ischemia in rats.
Neurochem Res. 37:614–621. 2012. View Article : Google Scholar
|
|
30
|
Oh YI, Kim JH and Kang CW: Protective
effect of short-term treatment with parathyroid hormone 1-34 on
oxidative stress is involved in insulin-like growth factor-I and
nuclear factor erythroid 2-related factor 2 in rat bone marrow
derived mesenchymal stem cells. Regul Pept. 189:1–10. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De la Lastra CA and Villegas I:
Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and
clinical implications. Biochem Soc Trans. 35:1156–1160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Konyalioglu S, Armagan G, Yalcin A,
Atalayin C and Dagci T: Effects of resveratrol on hydrogen
peroxide-induced oxidative stress in embryonic neural stem cells.
Neural Regen Res. 8:485–495. 2013.PubMed/NCBI
|
|
33
|
Maher J and Yamamoto M: The rise of
antioxidant signaling-the evolution and hormetic actions of Nrf2.
Toxicol Appl Pharmacol. 244:4–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quincozes-Santos A, Bobermin LD, Latini A,
Wajner M, Souza DO, Gonçalves CA and Gottfried C: Resveratrol
protects C6 astrocyte cell line against hydrogen peroxide-induced
oxidative stress through heme oxygenase 1. PloS One. 8:e643722013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang C, Zhang X, Fan H and Liu Y: Curcumin
upregulates transcription factor Nrf2, HO-1 expression and protects
rat brains against focal ischemia. Brain Res. 1282:133–141. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sakata Y, Zhuang H, Kwansa H, Koehler RC
and Doré S: Resveratrol protects against experimental stroke:
Putative neuroprotective role of heme oxygenase 1. Exp Neurol.
224:325–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin TK, Chen SD, Chuang YC, Lin HY, Huang
CR, Chuang JH, Wang PW, Huang ST, Tiao MM, Chen JB and Liou CW:
Resveratrol partially prevents rotenone-induced neurotoxicity in
dopaminergic SH-SY5Y cells through induction of heme oxygenase-1
dependent autophagy. Int J Mol Sci. 15:1625–1646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ghanim H, Sia CL, Korzeniewski K, Lohano
T, Abuaysheh S, Marumganti A, Chaudhuri A and Dandona P: A
resveratrol and polyphenol preparation suppresses oxidative and
inflammatory stress response to a high-fat, high-carbohydrate meal.
J Clin Endocrinol Metab. 96:1409–1414. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gurusamy N, Ray D, Lekli I and Das DK: Red
wine antioxidant resveratrol-modified cardiac stem cells regenerate
infarcted myocardium. J Cell Mol Med. 14:2235–2239. 2010.
View Article : Google Scholar : PubMed/NCBI
|



















