Introduction
Autogenous microfat grafting is a popular and
relatively simple procedure, which is widely used to augment
depressed deformities or for other cosmetic purposes. Various
temporary and permanent fillers have been used as alternatives with
varying degrees of success (1);
however, the autogenous microfat grafting technique is most
commonly selected by surgeons because it is easy to perform,
produces no ill-effects associated with foreign body reactions, and
can compensate for large volume deficits.
Despite numerous advantages associated with the use
of microfat grafting for soft tissue recontouring, some problems
must be considered with regards to this procedure, particularly
with regards to the unpredictability of the final survival rate due
to absorption and calcification. In addition, microfat retouching
must be frequently performed a few months after the initial
procedure using previously cryopreserved fat tissues. However,
cryopreserved fat tissues tend to exhibit greater absorption with
re-grafting compared with the initial procedure, and increased
survival of the cryopreserved fat tissues is indispensable to the
final result. To prevent unintended absorption, grafted fat tissues
should be revascularized within a short time period (2).
Sphingosylphosphorylcholine (SPC) is a
lysophospholipid with a role in several cellular responses,
including migration, wound healing and differentiation, which is
known to stimulate DNA synthesis and proliferation (3). A strong mitogenic effect of SPC has
also been observed in numerous types of cells, including
endothelial cells from different vascular beds (4).
It has previously been demonstrated that endothelial
progenitor cells (EPCs) can become incorporated into active sites
of angiogenesis, and augment collateral vessel growth to ischemic
tissues (5). In a previous study,
EPCs were reported to enhance the survival rate of transplanted fat
tissues, possibly due to the induction of angiogenesis (6). However, the use of EPCs in the fat
graft domain is limited because it requires human donors and an
in vitro culture period of >7 days. Therefore, the
present study aimed to determine the effects of SPC on EPCs in
vitro, in order to confirm the usefulness of employing SPC in
cryopreserved fat tissues to improve post-transplantation fat graft
survival rates. To verify the effects of SPC on fat tissues in
vivo, cryopreserved human fat tissues were mixed with various
concentrations of SPC and the effects were analyzed.
Materials and methods
Reagents
SPC (99% purity, as verified by thin-layer
chromatography) was purchased from Avanti Polar Lipids, Inc.
(Alabaster, AL, USA). Pertussis toxin (PTX) and VPC23019 were
obtained from Enzo Life Sciences, Inc. (Farmingdale, NY, USA).
3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), dimethyl sulfoxide (DMSO), and modified Hanks' balanced salt
solution were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Endothelial cell basal growth medium-2 (EBM-2) was purchased from
Lonza Group AG (Basel, Switzerland), and fetal bovine serum (FBS)
was obtained from HyClone; GE Healthcare Life Sciences (Logan, UT,
USA). Unless otherwise specified, all other reagents were purchased
from Sigma-Aldrich.
Cell culture
The EPCs were obtained from the Korean Cell Line
Bank (Seoul, South Korea) and were maintained at 37°C in an
atmosphere containing 5% CO2, in EBM-2 (CC-3162; Lonza
Group AG) supplemented with EGM-2 Bullet kit (CC3156 and CC-4176;
Lonza Group AG) and 5% FBS. There cells were then sub-cultured to
90% confluencey for 3–5 days using 0.05% trypsin EDTA. In the
experiments of the present study, the cells used were sub-cultured
less than eight times.
MTT proliferation assay
EPC proliferation was evaluated using an MTT assay.
The cells were plated at a density of 1×104 cells/well
in 24-well plates, were serum-starved for 24 h, and were then
treated with or without various reagents [SPC (0.1, 0.5, 1, 3, 5,
10 and 15 µM), PTX (1, 5, 10, 50 and 100 nM) or VPC23019 (1,
2, 3, 5, 7 and 10 µM)] for the indicated durations (1, 2, 3
and 4 days). After washing, culture medium containing 0.5 mg/ml MTT
was added to each well. The cells were then incubated for 2 h at
37°C, after which the supernatant was removed and the formazan
crystals that had formed in the viable cells were solubilized using
200 µl DMSO. A 100 µl aliquot of each sample was then
transferred to a well in a 96-well plate, and the absorbance was
measured at 560 nm using a microplate reader and XFLUOR4 software
version 4.51 (Tecan Group, Männedorf, Swizerland). This experiment
was repeated four times.
In vitro EPC tube formation
The in vitro angiogenic potential of SPC was
assessed by the ability of the EPCs to form tubes on a basement
membrane matrix (BD Matrigel™ Matrix; BD Biosciences, Franklin
Lakes, NJ, USA). The EPCs were plated at a density of
1×102 cells/well in a 96-well plate and were treated
with 1, 3, 5, 10 and 15 µM SPC. The EPCs were evaluated
using a light microscope (Olympus, Tokyo, Japan) under ×200
magnification after 12 h of culture (7).
Human fat tissue
Adipose tissue was obtained from an elective
surgery. The patient provided written informed consent, and the
study was approved by the Institutional Review Board of Pusan
National University Hospital (Busan, South Korea). Adipose tissue
was obtained through suction-assisted lipectomy from a 22-year-old
healthy woman undergoing suction-assisted lipectomy under general
anesthesia. The areas were injected with a local anesthetic
solution containing lidocaine (0.05%) and adrenaline (1:1,000,000)
prior to the start of the procedure. The fat was aspirated using a
sterile 10 ml syringe and a 14-gauge cannula with a blunt tip. The
aspirated fat was processed under sterile conditions by two-step
centrifugation (4 min each; 1,200 × g). The aspirated fat tissue
was frozen at −20°C for 8 weeks until being used in the
transplantation experiments. Prior to transplantation, the fat was
thawed for 1 h in a 37°C water bath.
Animal model and fat transplantation
The animal experiment used 48 male Balb/C nude mice
(Biogenomics, Inc., Seoul, Korea; age, 6 weeks; weight, 20–30 g).
During the study, mice were housed under constant laminar airflow
and were fed standard laboratory chow and water. They were kept
under an artificial 12 h light/dark cycle at a constant temperature
range (24±2°C) and relative humidity (55±10%). Nude mice have
previously been used to study fat grafts and enable the observation
of the use of human fat in an animal model (8,9). The
mice were divided into 1, 3, 5, 10 and 15 µM SPC-treated
groups, and the control group (n=8 mice/group). Mice in the
SPC-treated groups received a combination of 0.4 ml cryopreserved
fat and 0.02 ml SPC (1, 3, 5, 10 or 15 µM). Mice in the
control group received a combination of 0.4 ml fat and 0.02 ml
normal saline. Fat was injected subcutaneously into the back of
each mouse using a 16-G sharp needle (Coleman injection cannula;
Mentor Worldwide LLC, Santa Barbara, CA, USA). The Animal Care and
Experiment Committee of Pusan National University (Busan, South
Korea) approved the experimental protocol.
Follow-up and data collection
The mice were humanely sacrificed by CO2
asphyxiation 8 weeks after fat transplantation. The grafted fat was
carefully dissected from their backs, and its volume and weight
were measured. The volume was determined using the liquid overflow
method (10). Fat samples from the
six mouse groups [untreated control group, and SPC-treated groups
(1, 3, 5, 10 and 15 µM)] were fixed in 10% formalin and
embedded in paraffin. Tissue sections were acquired from the center
of the dissected fat biopsy. For CD31 staining, 5-mm sections were
permeabilized with 0.3% Triton X-100 for 10 min, then blocked for 1
h with 8% bovine serum albumin (BSA) at room temperature. Primary
rabbit anti-CD31 antibodies (cat. no. Ab28364, Abcam Cambridge, MA,
USA) were diluted to 1:500 in phosphate-buffered saline (PBS) with
2% BSA, and were incubated with the sections overnight at 4°C. The
biotinylated goat anti-rabbit immunoglobulin G (cat. no. BA-1000,
Vector Laboratories: Burlingame, CA, USA) were diluted to 1:100 in
PBS and incubated for 1 h at room temperature. Staining was
visualized using biotin-avidin-peroxidase complexes (Vector
Laboratories) and diaminobenzidine (Vector Laboratories). Images
were collected using a Leica TCL-SP2 confocal microscope system at
×200 magnification (Leica Microsystems Heidelberg GmbH, Heidelberg,
Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was isolated from the EPCs or
homogenized fat using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Waltham, MA, USA). The total RNA (2
µg) was then reverse transcribed into cDNA with the Reverse
Transcriptase M-MLV (Promega Corporation, Madison, WI, USA)
according to the manufacturer's protocol. The primers used in the
experiment were as follows: β-actin, forward 5′-CTGGTGCCTGGGGCG-3′,
reverse 5′-AGCCTCGCCTTTGCCGA-3′; human growth factor (HGF), forward
5′-CCTATGCAGAGGGACAAAGG-3′, reverse 5′-TGCTATTGAAGGGGAACCAG-3′;
interleukin-6 (IL-6), forward 5′-AAAGAGGCACTGGCAGAAAA-3′, reverse
5′-CAGGGGTGGTTATTGCATCT3′; tumor necrosis factor-α (TNF-α), forward
5′-GACAAGCCTGTAGCCCATGT-3′, reverse 5′TTGATGGCAGAGAGGAGGTT-3′;
matrix metallopeptidase (MMP)-2, forward 5′-CAGGTGATCTTGACCAGAAT-3,
reverse 5′-CATCATGGATTCGAGAAAAC-3′; MMP-9, forward
5′-ACCTCGAACTTTGACAGCGACA-3′, reverse 5′-GATGCCATTCACGTCGTCCTTA-3′;
and vascular endothelial growth factor (VEGF), forward
5′-AAGGAGGAGGGCAGAATCAT-3′, and reverse 5′-ATCTGCATGGTGATGTTGGA-3′
(Bioneer Corporation, Deajeon, Korea). All of the primer sequences
were determined according to established GenBank sequences
(http://www.ncbi.nlm.nih.gov/genbank/). RT-qPCR was
conducted using a Power SYBR Green PCR Master mix (Applied
Biosystems, Warrington, UK) on the ABI 7500 Instrument (Applied
Biosystems). The reaction mixture contained 2 µl of 10
ng/µl cDNA, 10 µl SYBR (qPCR master mix), 0.5
µl of 10 pmol forward primer, 0.5 µl of 10 pmol
reverse primer and 7 µl nuclease-free water to produce a
final volume of 20 µl. The amplification program consisted
of one cycle at 95°C with a 60 sec hold (ʻhot startʼ) followed by
40 cycles at 95°C with a 0 sec hold, a 60°C annealing step with a 5
sec hold, 72°C with a 12 sec hold, and a 60°C acquisition step with
a 2 sec hold. All experiments were conducted three times, and
negative and positive controls were included in all experiments.
β-actin mRNA was amplified as an internal control. LightCycler
software version 3.3 (Roche Diagnostics) was used to analyze the
PCR kinetics and calculate the quantitative data. For each sample,
copy numbers of target gene mRNA were divided by those of β-actin
mRNA to normalize for target gene mRNA expression and avoid
inter-sample differences in RNA quantity.
Statistical analysis
The results are presented as the mean ± standard
error of the mean. Comparisons between groups were analyzed using
the Student's t-test or one-way analysis of variance for multiple
groups. Tukey's adjustment post-hoc test was conducted to determine
which means were significantly different. The statistical analysis
of differences in fat graft weight and volume among the six groups
was performed using Mann Whitney U-test. SPSS for Windows (version
17.0; SPSS, Inc., Chicago, IL, USA) was used to analyze the data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
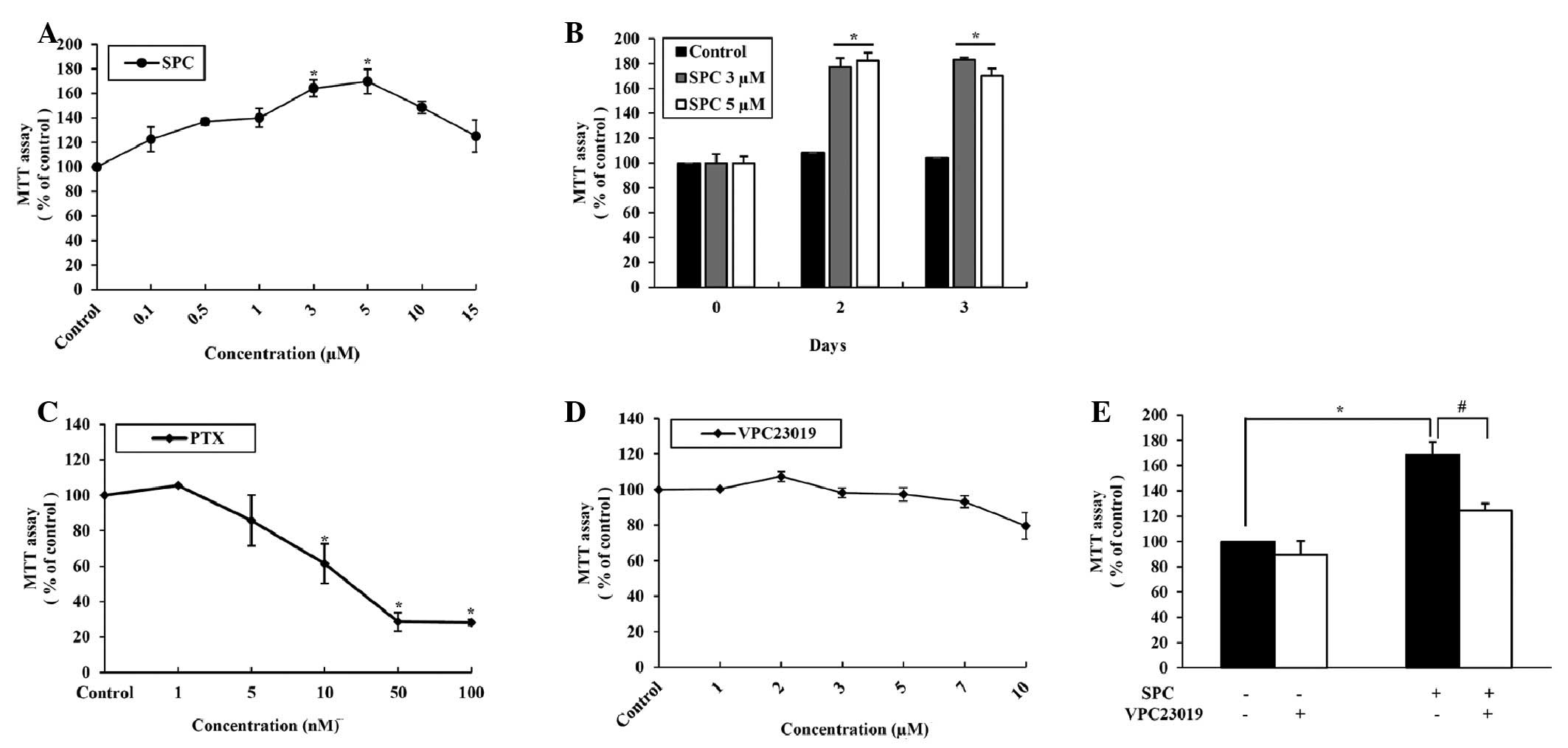
Effects of SPC on EPC proliferation
SPC exhibited a maximal effect on EPC proliferation
when used at 3 (P=0.0013) and 5 µM (P=0.0005; Fig. 1A). The positive effects of SPC were
still efficacious at day 3 (Fig.
1B). Conversely, PTX exhibited a direct dose-dependent
cytotoxic effect on EPCs (Fig.
1C), whereas VPC23019 had no effect on proliferation (Fig. 1D). To confirm the positive effects
of SPC on EPC growth, cells were co-treated with SPC and VPC23019.
EPC growth was promoted by treatment with SPC, which was suppressed
following the addition of VPC23019 (Fig. 1E).
Effects of SPC on EPC angiogenesis
In vitro tube formation was increased
following treatment of EPCs with SPC in a dose-dependent manner
(Fig. 2A). RT-qPCR was conducted
on total RNA extracted from EPCs treated with various
concentrations of SPC, which detected increased mRNA expression
levels of HGF, IL-6, MMP-2, MMP-9, TNF-α and VEGF, as compared with
the control group. HGF (P=0.021), IL-6 (P=0.010), MMP-2 (P=0.047)
and VEGF (P=0.026) expression was significantly increased following
treatment with 5 µM SPC. MMP-9 (P=0.025/P=0.006) and TNF-α
(P=0.016/P=0.036) expression significantly increased at 3 and 5
µM SPC (Fig. 2B).
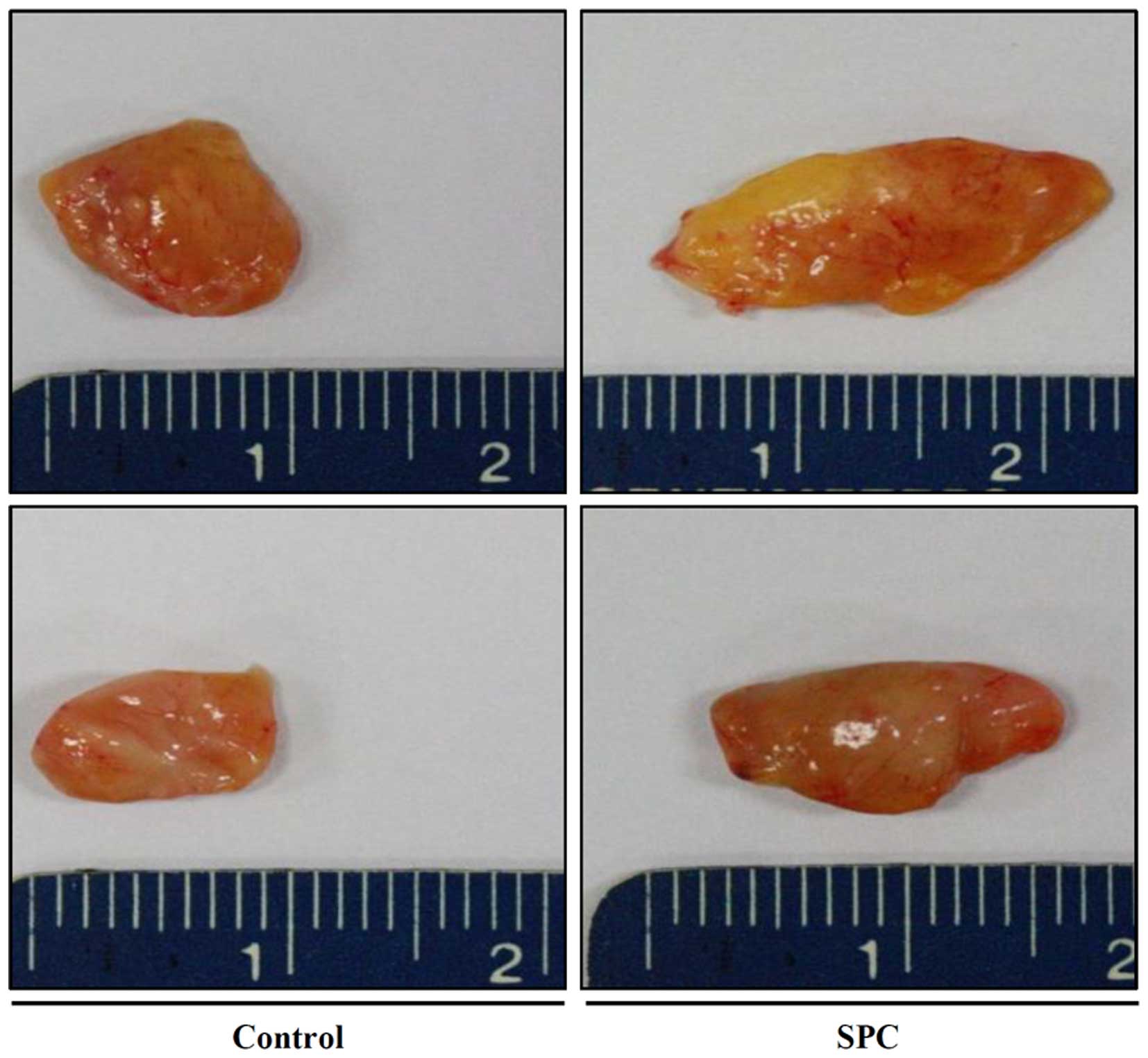
Effects of SPC on cryopreserved fat
tissue survival
Grafted fat was retrieved from the mice and the
gross findings exhibited a significant bulk increase in the 3
µM SPC-treated group (Fig.
3). Weight and volume measurements indicated that grafted fat
survival was increased when treated with various concentrations of
SPC. Statistical analysis was performed and the 3 µM
SPC-treated group exhibited a statistically significant increase
(P=0.04) in both weight and volume (Table I).
 | Table IEffect of SPC on fat graft weight and
volume. |
Table I
Effect of SPC on fat graft weight and
volume.
| Group | Weight (Median,
25th–75th) | P-value (vs.
Control) | Volume (Median,
25th–75th) | P-value (vs.
Control) |
|---|
| Control (n=8) | 0.15 (0.14–0.17) | | 0.17 (0.15–0.19) | |
| SPC 1 µM
(n=8) | 0.16 (0.13–0.19) | 0.72 | 0.18 (0.15–0.20) | 0.72 |
| SPC 3 µM
(n=8) | 0.19 (0.17–0.21) | 0.04a | 0.21 (0.19–0.23) | 0.04a |
| SPC 5 µM
(n=8) | 0.17 (0.15–0.19) | 0.20 | 0.18 (0.17–0.20) | 0.33 |
| SPC 10 µM
(n=8) | 0.15 (0.13–0.19) | 0.96 | 0.16 (0.15–0.20) | 0.96 |
| SPC 15 µM
(n=8) | 0.14 (0.11–0.18) | 0.57 | 0.17 (0.13–0.19) | 0.72 |
Effects of SPC on cryopreserved fat
tissue angiogenesis
Increased angiogenesis of the SPC-treated fat graft
tissue of the grafted fat tissue was detected by
immunohistochemistry, as evidenced by increased CD31 expression
(Fig. 4A). RT-qPCR analysis of
total RNA extracted from SPC-treated fat tissue revealed increased
mRNA expression levels of MMP9 (P=0.0001) and TNF-α (P=0.0024)
compared with the control group (Fig.
4B).
Discussion
SPC is reportedly associated with various cellular
functions of the cardiovascular system, skin, neurons, and immune
cells (3,4,9,11);
however, its effects on the survival of grafted fat tissues have
yet to be reported.
The present study demonstrated that SPC exerted
strong mitogenic effects on endothelial cells, and an in
vitro study was conducted to elucidate the direct mitogenic
effect of SPC on EPCs. EPC proliferation was increased following
SPC treatment, and proliferation peaked when the cells were treated
with 3 or 5 µM SPC; this effect lasted for 3 days.
Co-treatment with VPC23019, an inhibitor of the downstream SPC
mediator sphingosine-1-phosphate, confirmed that significant EPC
proliferation resulted from SPC treatment. In addition, tube
formation assay and RT-qPCR demonstrated that SPC was able to
increase the angiogenic potential of EPCs.
An in vivo experiment using cryopreserved
human fat tissue to conduct a fat graft in mice demonstrated
statistically significant differences in weight and volume between
the control and 3 µM SPC-treated groups.
With the favorable histological outcome,
immunohistochemical result, and RT-qPCR findings of the present
study, SPC may increase the survival rate of cryopreserved fat
tissue by improving its angiogenic potential.
Previous studies have reported that SPC can
stimulate the proliferation of human adipose tissue-derived
mesenchymal stem cells (hADSC) (12,13).
Therefore, the increased survival of cryopreserved fat detected in
the present study may also be caused by SPC-induced hADSC
proliferation. These results suggested that with an SPC-induced
intensified EPC role in transplanted fat tissue, the cryopreserved
fat survival rate could be further increased. In addition, these
results indicated that specific concentrations of SPC have a
favorable role in grafted cryopreserved human fat tissue, and this
positive effect may be promoted by increased
angiogenesis-associated mRNA expression.
Acknowledgments
The present study was supported by the Biomedical
Research Institute, Pusan National University Hospital (grant no.
2012-12).
References
|
1
|
Gamboa GM and Ross WA: Autogenous fat
transfer in aesthetic facial recontouring. Ann Plast Surg.
70:513–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baran CN, Celebioğlu S, Sensöz O, Ulusoy
G, Civelek B and Ortak T: The behavior of fat grafts in recipient
areas with enhanced vascularity. Plast Reconstr Surg.
109:1646–1651. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyer zu Heringdorf D, Himmel HM and
Jakobs KH: Sphingosylphosphorylcholine-biological functions and
mechanisms of action. Biochim Biophys Acta. 1582:178–189. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun L, Xu L, Henry FA, Spiegel S and
Nielsen TB: A new wound healing agent -
sphingosylphosphorylcholine. J Invest Dermatol. 106:232–237. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yi C, Pan Y, Zhen Y, Zhang L, Zhang X, Shu
M, Han Y and Guo S: Enhancement of viability of fat grafts in nude
mice by endothelial progenitor cells. Dermatol Surg. 32:1437–1443.
2006.
|
|
7
|
Bae YH, Park HJ, Kim SR, Kim JY, Kang Y,
Kim JA, Wee HJ, Kageyama R, Jung JS, Bae MK and Bae SK: Notch1
mediates visfatin-induced FGF-2 up-regulation and endothelial
angiogenesis. Cardiovasc Res. 89:436–445. 2011. View Article : Google Scholar
|
|
8
|
Ferguson RE, Cui X, Fink BF, Vasconez HC
and Pu LL: The viability of autologous fat grafts harvested with
the LipiVage system: A comparative study. Ann Plast Surg.
60:594–597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pietrzak WS and Eppley BL: Platelet rich
plasma: Biology and new technology. J Craniofac Surg. 16:1043–1054.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ayhan M, Senen D, Adanali G, Görgü M,
Erdoğan B and Albayrak B: Use of beta blockers for increasing
survival of free fat grafts. Aesthetic Plast Surg. 25:338–342.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nixon GF, Mathieson FA and Hunter I: The
multi-functional role of sphingosylphosphorylcholine. Prog Lipid
Res. 47:62–75. 2008. View Article : Google Scholar
|
|
12
|
Jeon ES, Song HY, Kim MR, Moon HJ, Bae YC,
Jung JS and Kim JH: Sphingosylphosphorylcholine induces
proliferation of human adipose tissue-derived mesenchymal stem
cells via activation of JNK. J Lipid Res. 47:653–664. 2006.
View Article : Google Scholar
|
|
13
|
Moon HJ, Jeon ES, Kim YM, Lee MJ, Oh CK
and Kim JH: Sphingosylphosphorylcholine stimulates expression of
fibronectin through TGF-beta1-Smad-dependent mechanism in human
mesenchymal stem cells. Int J Biochem Cell Biol. 39:1224–1234.
2007. View Article : Google Scholar : PubMed/NCBI
|