Introduction
Diabetic retinopathy, the leading cause of blindness
worldwide, is characterized by early dysfunction of the retinal
microvasculature. Metabolic alterations resulting from
hyperglycemia are considered to be the cause of diabetic
retinopathy (1). It has been
reported that inflammatory processes are also important in the
pathophysiology of diabetic retinopathy (2). Leukocyte infiltration and the
enhanced expression of chemokines, adhesion molecules, growth
factors and nuclear factors have been observed in diabetic retinal
tissues (3).
The high-mobility group box-1 (HMGB1) protein was
originally described as a nuclear DNA-binding protein (4), which facilitates gene transcription
by stabilizing nucleosome formation (5). HMGB1 can also be released
extracellularly, and acts as a pro-inflammatory cytokine or as an
alarm signal for tissue damage (6). HMGB1 is released from cells through
passive release or by active secretion. Passive release occurs as a
result of cellular necrosis in the majority of eukaryotic cells
(7,8). Active secretion from activated
macrophages and monocytes occurs in response to inflammatory
stimuli, including lipopolysaccharide and tumor necrosis factor
(TNF)-α (9,10), and can trigger a potent
inflammatory response leading to severe tissue injury (11,12).
Extracellular HMGB1 is also involved in the progression of several
inflammatory conditions, including septic shock, rheumatoid
arthritis and atherosclerosis (13–16).
Previously, diabetes has been shown to be associated with an
increase in the expression of HMGB1 in aortic endothelial cells
in vivo, and HMGB1 has been suggested as a causative factor
in diabetic tissue damage (17).
Inflammation has been recognized as being important in the
pathogenesis of diabetic retinopathy, and anti-inflammatory agents
can be beneficial in diabetic retinopathy (18), for example dexamethasone suppressed
upregulation of intercellular adhesion molecule 1, leukostasis, and
prevented retinal vascular leakage in a rat model of diabetic
retinopathy (19). In addition,
Jonas and Söfker (20) reported
that an intravitreal injection of triamcinoloe acetonide
efficiently improved visual acuity in diabetic patients with
macular edema. However, the role of HMGB1 in diabetic retinopathy
remains to be fully elucidated. Therefore, the present study aimed
to investigate the pathogenic function of HMGB1, focusing on its
role in diabetic retinopathy, using primary rat retinal pericytes
and streptozotocin (STZ)-induced diabetic rats. The involvement of
the receptor for advanced glycation end products (RAGE) and the
nuclear factor (NF)-κB signaling pathway were also examined.
Materials and methods
Primary rat retinal pericyte cell
culture
The primary retinal pericytes were isolated from the
retinal microvessels of Sprague-Dawley rats (Orient Bio, Inc.,
Seoul, Korea) using a modified version of a previously published
method (21–24). After one week acclimation period,
eight eyes from four rats were enucleated under deep anesthesia,
following intra peritoneal injection of pentobarbital sodium (30
mg/kg body weight; Hanlim Pharmaceuticals Co., Ltd., Seoul, Korea).
Animals were then sacrificed with an overdose of pentobarbital
sodium (200 mg/kg body weight; Hanlim Pharmaceuticals Co., Ltd.).
The retinas were separated from the eyes, homogenized using a
Teflon-glass homogenizer (Wheaton, Millville, NJ, USA) and filtered
though a 70-μm nylon mesh (BD Biosciences, San Diego, CA,
USA). The remaining retentate was digested in 0.066%
collagenase/dipase (Roche, Mannheim, Germany) in Dulbecco's
phosphate-buffered saline for 1 h at 37°C. The cellular digests
were then filtered through a 40-μm nylon mesh. Purification
of the rat retinal pericytes was achieved using a CELLection Pan
Mouse IgG kit (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) with a mouse anti-desmin monoclonal antibody (cat. no.
MAB3430; EMD Millipore, Billerica, MA, USA), according to the
manufacturer's protocol. The purified cells were maintained in
Dulbecco's modified Eagle's medium containing 10% fetal bovine
serum at 37°C in a humidified atmosphere of a 5% CO2
incubator. The pericytes were identified by their inability to
uptake rhodamine-conjugated, acetylated low-density lipoprotein, as
described by Cacicedo et al (25). The cell cultures in the present
study contained no cells, which reacted with polyclonal rabbit
anti-human antibodies to the endothelial cell marker, von
Willebrand factor (cat. no. A0082; Dako, Carpinteria, CA, USA).
Cells between passages 3 and 5 were used in the present study. The
cells (5×104) were plated onto appropriate culture
dishes and used for experiments upon reaching 80% confluence.
Standard culture medium was replaced with fresh serum-free medium
16 h prior to the experiments at 37°C. To examine the effects of
high glucose, the medium of confluent pericyte cultures was
supplemented with 25 mmol/l D-glucose for 48 h. Galactose or
mannitol were used as a control.
Immunofluorescence staining
The immunofluorescence staining was performed on the
cultured pericytes. The antibody used was monoclonal rabbit
anti-HMGB1 (2639-1; Epitomics, San Fransisco, CA, USA). For the
detection of HMGB1, the cells were incubated with a fluorescein
isothiocyanate (FITC)-conjugated goat anti-rabbit antibody (Santa
Cruz Biotechnology, Santa Cruz, CA, USA). The fluorescence signals
were observed under a fluorescence microscope (Olympus Corporation,
Tokyo, Japan) and analyzed using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Western blot analysis
The nuclear and cytoplasmic extracts were prepared
according to a method described by Schreiber et al (26). Briefly, 1×107 cells were
resuspended in 400 ml of cold buffer A, containing 10 mM HEPES (pH
7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT and 0.5 mM
PMSF. The cells were allowed to swell on ice for 15 min, following
which 25 ml of a 10% solution of was added, and the tube was
vigorously vortexed for 10 sec. The homogenate was centrifuged at
12,000 × g for 30 sec in a microfuge at 4°C, with the supernatant
containing the cytoplasm. The nuclear pellet was resuspended in 50
ml of ice-cold buffer B, containing 20 mM HEPES (pH 7.9), 0.4 M
NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT and 1 mM PMSF, and the tube
was vigorously rocked at 4°C for 15 min on a shaking platform. The
nuclear extract was centrifuged at 12,000 × g for 5 min in a
microfuge at 4°C, and the supernatant was frozen. The protein
content of the fractions were determined using the Bradford assay
method (27). The nuclear and
cytoplasmic extracts (20 μg) were then separated by 12%
SDS-polyacrylamide gel electrophoresis and transferred onto
nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The membranes were probed with rabbit anti-HMGB1 (Epitomics),
polyclonal rabbit anti-RAGE antibody (ab3611; Abcam, Cambridge, MA,
USA), monoclonal mouse anti-β-actin antibody (A5441; Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany), monoclonal mouse
anti-β-tubulin antibody (sc-5274; Santa Cruz Biotechnology, Inc.)
and mouse monoclonal anti-proliferating cellular nuclear antigen
(PCNA) antibody (50-171-599; Upstate Biotechnology, Inc., Lake
Placid, NY, USA), and the immune complexes were then visualized
using an enhanced chemiluminescence detection system (Amersham
Biosciences, Uppsala, Sweden). The protein expression levels were
determined by analyzing the signals captured on the nitrocellulose
membranes using an image analyzer (Las-3000; Fujifilm, Tokyo,
Japan). Anti-β-actin, anti-β-tubulin (28) and anti-PCNA (29) served as loading controls.
Measurement of NF-κB activity
For the electrophoretic mobility shift assay (EMSA),
the nuclear extracts were prepared using a kit, according to the
manufacturer's protocol (NE-PER Nuclear and Cytoplasmic Extraction
kit; Pierce Biotechnology, Inc., Rockville, IL, USA). The EMSA was
performed by incubating 10 μg of nuclear protein extract
with IRDye 700-labeled NF-κB oligonucleotide probe (5′-AGT TGA GGG
GAC TTT CCC AGG C-3′; LI-COR Biosciences, Lincoln, NE, USA) or an
unlabelled probe for cold competition. The EMSA gels were analyzed,
and images were captured and quantified using the LI-COR Odyssey
infrared laser imaging system (LI-COR Biosciecnes).
Chromatin immunoprecipitation (ChIP)
assay
A ChIP assay was performed to analyze the in
vitro interactions of NF-κB with its cognate cis-acting
element in the RAGE promoter. This assay was performed using a ChIP
assay kit (Upstate Biotechnology, Inc.), according to the
manufacturer's protocol. The soluble chromatin was prepared the
from retinal pericytes. Chromatin was mechanically sheared by
sonication to yield fragments with a mean size of 300 bp. Prior to
immunoprecipitation, chromatin (10 μg DNA/assay) was
pretreated with protein A/G agarose beads (Santa-Cruz
Biotechnology, Inc.) for 1 h. The chromatin was immunoprecipitated
with a polyclonal rabbit anti-p65 NF-κB antibody (sc-372; 2
μg each; Santa Cruz Biotechnology, Inc.). Normal rabbit IgG
was used as negative antibody control and DNA from the input (20–40
μg protein-DNA complex) as an internal control.
Antibody-bound chromatin was eluted by heating at 95°C for 30 min.
The DNA was then purified using the QIAquick PCR Purification kit
(Qiagen GmbH, Hilden, Germany). The input DNA and the DNA from Chip
samples were used as a template for polymerase chain reaction
amplification using primer sets for the rat RAGE promoter regions
containing the NF-κB response element. The sequences of the primers
were as follows: Forward 5′-CCCGGCCCTGACTAAGCAGT-3′ and reverse
5′-CCACGGCCTGGAACCCTTA-3′. Quantitative polymerase chain reaction
(qPCR) was performed using SYBR Green PCR Master Mix and Chromo4
Multicolor Real-Time PCR Detection System (Bio-Rad Laboratories,
Inc.,) with with the following cycling conditions: 2 min at 94°C,
followed by 35 cycles of 30 sec at 94°C, 30 sec at 58°C and 1 min
at 72°C, followed by 1 min at 72°C. The copy number for each gene
was determined using iQ5 Optical system software (Bio-Rad
Laboratories, Inc.). The results are reported as the ratio of the
immunoprecipitated DNA to the input DNA.
Animals and experimental design
Six-week-old male Sprague-Dawley rats were purchased
from Orient Bio, Inc. Rats were housed under a 12-h light/12-h dark
cycle at a temperature of 23±1°C and were provided with food and
water ad libitum. After a one week acclimation period,
diabetes was induced by a single injection of STZ (60 mg/kg body
weight; i.p.) in the rats. Age-matched control rats (aged 7 weeks)
were injected with vehicle only. At 1 week post-induction of
diabetes, the blood glucose levels were measured in venous blood
from the tail vein. The glucose assay used was an enzymatic assay
based on glucose oxidase and peroxidase levels (Glucose B-Test;
Wako, Osaka, Japan). Rats with a plasma glucose level >300 mg/dl
were considered to be diabetes-induced rats. The animals were
divided into two groups: Normal rats (n=8) and STZ-induced diabetic
rats (n=8). At 21 weeks of age, blood samples were collected from
the tail vein following a 16-h fast At necropsy, the eye from each
rat was enucleated under deep anesthesia, following intraperitoneal
injection of pentobarbital sodium (30 mg/kg body weight; Hanlim
Pharmaceuticals Co., Ltd.). Animals were then sacrificed with an
overdose of pentobarbital sodium (200 mg/kg body weight; Hanlim
Pharmaceuticals Co., Ltd.). All the procedures involving rats were
approved by the Korea Institute of Oriental Medicine Institutional
Animal Care and Use Committee (Daejeon, Korea). The blood glucose
values were 6.93±0.56 mmol/l for normal rats and 22.74±3.78 mmol/l
for STZ-induced diabetic rats. The body weights were 494.46±8.73 g
(normal rats) and 229.25±10.66 g (diabetic rats).
Trypsin-digested retinal vessel
preparation
The eyes of the animals were enucleated and the
retinas were isolated. The retinal samples were then placed in 10%
formalin for 2 days. Following fixation, the retina was incubated
in trypsin (3% in sodium phosphate buffer) for ~60 min at 37°C. The
vessel structures were isolated from the retinal cells by gentle
rinsing in distilled water. The vascular specimens were then
mounted on a slide. For immunofluorescence staining for HMGB1 and
NF-κB, the vascular specimens were incubated with rabbit anti-HMGB1
(Epitomics) and mouse anti-NF-κB antibody (cat. no. MAB3026;
Chemicon International, Inc., Temecula, CA, USA). For the detection
of HMGB1, the vessels were incubated with FITC-conjugated
polyclonal goat anti-rabbit antibody (sc-2012; Santa Cruz
Biotechnology, Inc.). To detect NF-κB, the vessels were incubated
with polyclonal FITC-conjugated goat anti-mouse IgG (sc-2010; Santa
Cruz Biotechnology, Inc.) and detected using fluorescence
microscopy (Olympus Corporation). For negative controls, the
sections were incubated with non-immune serum instead of the
primary antibodies.
Immunohistochemical staining
The retinal tissues were fixed in 10% formaldehyde
and embedded in paraffin, and 4 μm thick sections were
prepared. The primary antibody used was rabbit anti-RAGE
(Sigma-Aldrich; Merck Millipore). Following incubation in CAS
blocking solution (Zymed Life Technologies, Carlsbad, CA, USA) for
30 min, the sections were incubated in the primary antibody
overnight at 4°C and then washed three times with
phosphate-buffered saline. To detect RAGE in the retinal sections,
the slides were prepared using an Envision kit (Dako), and
immunoreactivity was visualized using a 3,3′-diaminobenzidine
tetrahydrochloride peroxidase substrate kit (Dako).
Statistical analysis
All data are presented as the mean ± standard error.
Comparisons between the two groups were made using Student's
t-test (two-tailed). Statistical analysis was performed
using GraphPad Prism 4.0 software (GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of HMGB1 in retinal
pericytes
To investigate the pathogenic functions of HMGB1 in
retinal pericytes under diabetic conditions, the present study
initially determined whether the protein was expressed in retinal
pericytes using immunofluorescence staining. HMGB1 was detected in
the nuclei and diffusely in the cytoplasm in the high
glucose-treated pericytes, however, in the control, HMGB1 was
expressed in the nuclei only (Fig.
1A; upper panels). Additionally, in diabetic retinal vessels,
HMGB1 was expressed at a high level in the cytoplasm of the retinal
pericytes (Fig. 1A; lower panels).
To confirm that the HMGB1 protein had been translocated into the
cytoplasm of the pericytes, the cells were subcellular
fractionated, and the distribution of HMGB1 was analyzed using
western blot analysis. As shown in Fig. 1B, translocation of HMGB1 from the
nucleus to the cytoplasm was observed at 48 h following high
glucose treatment. In the control pericytes, the majority of the
HMGB1 was found in the nucleus, and the expression of HMGB1 was
lower in the soluble cytoplasm. In the high glucose-treated
pericytes, a lower level of HMGB1 was detected in the nuclear
fraction and a higher level of HMGB1 was detected in the cytoplasm,
compared with the control. These results suggested that HMGB1 was
translocated into the cytoplasm in retinal pericytes in response to
high glucose conditions in vitro and in vivo.
Expression of RAGE
RAGE is a putative receptor for HMGB1 (30). The signaling pathways downstream of
RAGE are key in the cellular responses to stress conditions,
including inflammation (31).
Thus, the present study measured the expression levels of RAGE in
retinal pericytes. The western blot analysis showed that the
protein expression of RAGE was markedly increased in the high
glucose-treated pericytes, compared with the control cells
(Fig. 2A). Similarly,
immunohistochemical staining for RAGE showed that the expression
level of RAGE was markedly higher in the diabetic rat retinas,
compared with the normal rat retinas (Fig. 2B).
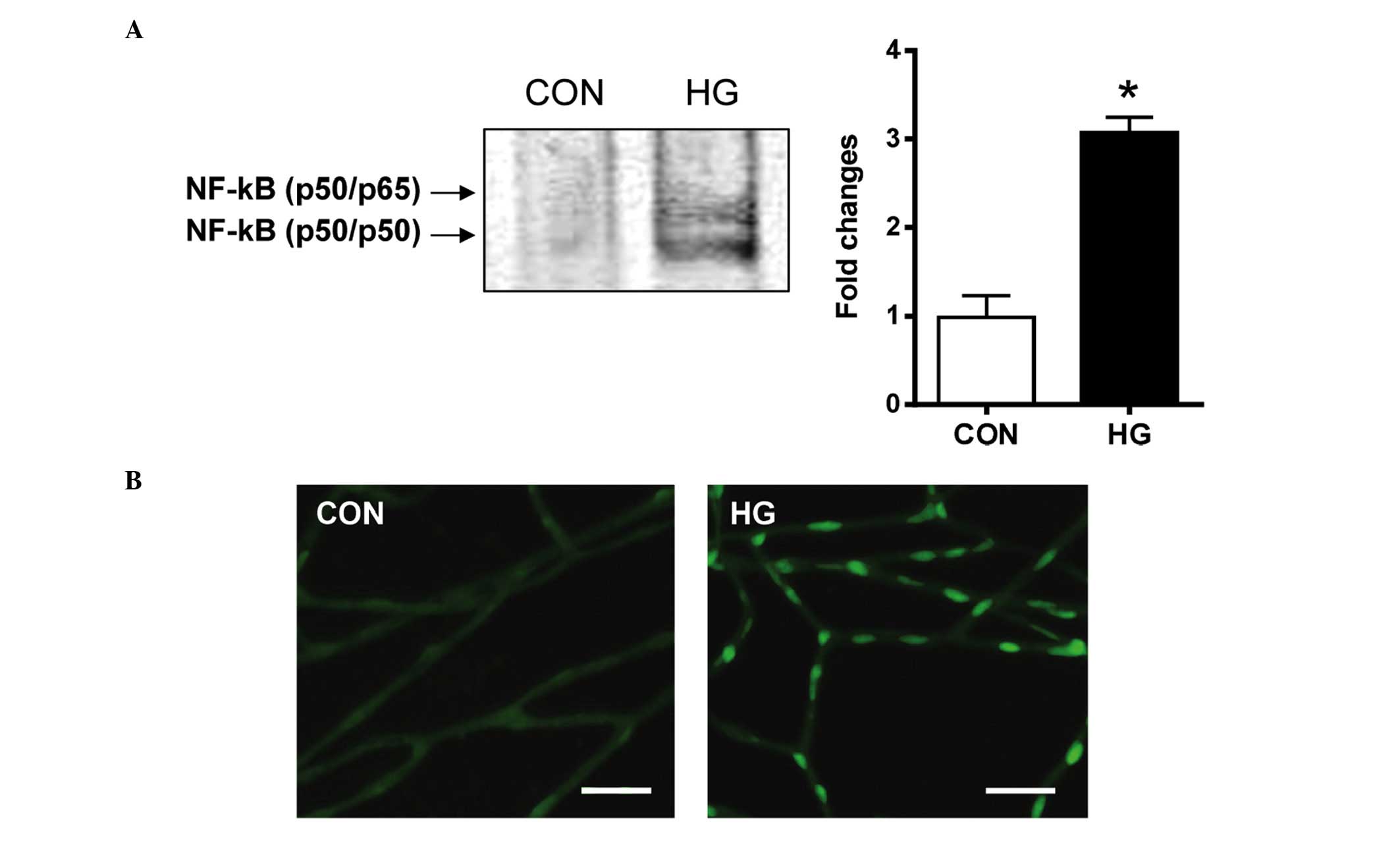
Activation of NF-κB
The present study investigated whether the
downstream effect of RAGE activation was associated mechanistically
with the NF-κB pathway. The nuclear extracts from the retinal
pericytes were analyzed for NF-κB DNA-binding activity using an
EMSA. The results of the EMSA showed that the NF-κB DNA-binding
activity was significantly increased in the high glucose-treated
pericytes, compared with the control cells (Fig. 3A). In the diabetic rats, activated
NF-κB was found in the nucleus of the retinal pericytes. However,
in the control rats, minimal positive signals of activated NF-κB
were detected (Fig. 3B). These
observations indicated that diabetic conditions markedly induced
NF-κB DNA-binding activity in the retinal pericytes.
Role of NF-κB in the mRNA expression of
RAGE
To evaluate the role of NF-κB in the high
glucose-induction of the mRNA expression of RAGE in the retinal
pericytes, the binding of NF-κB p65 to the RAGE promoter was
determined using a ChIP assay. As shown in Fig. 4, high glucose conditions increased
the binding of this transcription factor to the RAGE promoter. This
result suggested that the expression of RAGE expression was
regulated by NF-κB.
Discussion
The present study showed the first evidence, to the
best of our knowledge, of HMGB1 translocatton into the cytoplasm of
retinal pericytes in response to high glucose in vitro and
in diabetic conditions in vivo. Through immunofluorescence
staining and western blot analyses for HMGB1, the present study
determined that HMGB1 translocated from the nucleus to the
cytoplasm in response to high glucose. These observations indicated
that high glucose was an important regulator of the subcelluar
distribution of HMGB1 in the retinal pericytes.
Retinal pericytes wrap around the microvascular
endothelium to provide vascular stability and regulate capillary
blood flow. The retina has the highest number of pericytes in the
body (32), and their loss is
considered to be a hallmark of early diabetic retinopathy (33). Pericytes are important in the
barrier function of microvessels and assist in regulating
inflammatory processes, including the leakage of plasma proteins
(34). Diabetes has been shown to
be associated with the upregulation of various pro-inflammatory
mediators in the retina, including intercellular adhesion molecule
1, vascular endothelial growth factor, NF-κB, inducible nitric
oxide synthase and transforming growth factor-β, and localized
inflammatory processes are considered to be involved in the
development of diabetic retinopathy (35,36).
The data presented in the present study showed that high glucose
significantly induced the cytoplasmic expression of the
pro-inflammatory mediator, HMGB1, in retinal pericytes, suggesting
that increased inflammation is an important contributing factor to
the progressive injury of pericytes during the development of
diabetic retinopathy.
The present study also showed that the expression of
RAGE was markedly enhanced in high glucose-treated retinal
pericytes. HMGB1 is a specific ligand for RAGE (30). RAGE is expressed at low levels in a
variety of cell types, however, its expression is increased by the
cellular activation, which occurs during inflammation (37,38).
RAGE was originally identified by its ability to bind advanced
glycation end products (AGEs). Increased glucose levels lead to the
formation of AGEs. However, the importance of AGEs as ligands of
RAGE in vivo remains controversial, as proteins modified by
AGEs to the extent necessary to bind to RAGE are unlikely to exist
in physiological systems in vivo (39–41).
By contrast, the HMGB1 protein is present at sites of inflammation
in vivo at concentrations, which activate RAGE (42). RAGE is also found on retinal
pericytes (43). This suggests
that HMGB1 is a functional mediator, which is involved in the
induction of diabetic retinopathy by signaling through these
receptors.
The present study also found that high
glucose-induced HMGB1 increased the activity of NF-κB. It has been
reported that the NF-κB system is a major intracellular signaling
pathway downstream of RAGE (44).
The NF-κB transcription factor is a key regulator of inflammation,
immune response, cell survival and cell proliferation (45). In the present study, it was found
that the transcriptional activity of NF-κB was significantly
enhanced in retinal pericytes by high glucose in vitro and
under diabetic conditions in vivo. This is consistent with
reports that the constitutive activation of NF-κB is essential for
retinal pericyte damage (46–48).
The pro-inflammatory role of NF-κB has been implicated in pericyte
injury through the transcriptional activation of NF-κB-dependent
inflammatory mediators. Pro-inflammatory cytokines, including tumor
necrosis factor-α and monocyte chemoattractant protein-1, are also
regulated by NF-κB in retinal pericytes (48,49).
In addition, ChIP analysis showed that high glucose also increased
the binding of NF-κB to the RAGE promoter. These results
demonstrated that the increased expression of RAGE was a
consequence of the HMGB1-induced activation of NF-κB.
In conclusion, the results of the present study
indicated that hyperglycemia-induced HMGB1 release may induce
retinal pericyte injury under diabetic conditions. It was also
demonstrated that the pathogenic role of HMGB1 may be dependent on
the NF-κB-dependent regulation of RAGE. Whether the markedly
elevated protein levels of HMGB1 in patients with diabetic
retinopathy is responsible for the retinal pericyte injury remains
to be elucidated. If this is the case, such knowledge may be
considered in the design of potential therapeutic strategies for
the treatment of diabetic retinopathy.
Acknowledgments
This study was supported by a grant (grant no.
K15270 and K16817) from the Korea Institute of Oriental
Medicine.
References
|
1
|
The DCCT Research Group: The effect of
intensive treatment of diabetes on the development and progression
of long-term complications in insulin-dependent diabetes mellitus.
N Engl J Med. 329:977–986. 1993. View Article : Google Scholar
|
|
2
|
Joussen AM, Poulaki V, Le ML, Koizumi K,
Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B,
et al: A central role for inflammation in the pathogenesis of
diabetic retinopathy. FASEB J. 18:1450–1452. 2004.PubMed/NCBI
|
|
3
|
King GL: The role of inflammatory
cytokines in diabetes and its complications. J Periodontol.
79:1527–1534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muller S, Ronfani L and Bianchi ME:
Regulated expression and subcellular localization of HMGB1, a
chromatin protein with a cytokine function. J Intern Med.
255:332–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bianchi ME: Significant (re)location: How
to use chromatin and/or abundant proteins as messages of life and
death. Trends Cell Biol. 14:287–293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ulloa L and Messmer D: High-mobility group
box 1 (HMGB1) protein: Friend and foe. Cytokine Growth Factor Rev.
17:189–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bianchi ME and Manfredi A: Chromatin and
cell death. Biochim Biophys Acta. 1677:181–186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang W, Li J, Gallowitsch-Puerta M,
Tracey KJ and Pisetsky DS: The effects of CpG DNA on HMGB1 release
by murine macrophage cell lines. J Leukoc Biol. 78:930–936. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riedemann NC, Guo RF and Ward PA: The
enigma of sepsis. J Clin Invest. 112:460–467. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fiuza C, Bustin M, Talwar S, Tropea M,
Gerstenberger E, Shelhamer JH and Suffredini AF:
Inflammation-promoting activity of HMGB1 on human microvascular
endothelial cells. Blood. 101:2652–2660. 2003. View Article : Google Scholar
|
|
13
|
Wang H, Bloom O, Zhang M, Vishnubhakat JM,
Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et
al: HMG-1 as a late mediator of endotoxin lethality in mice.
Science. 285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taniguchi N, Kawahara K, Yone K,
Hashiguchi T, Yamakuchi M, Goto M, Inoue K, Yamada S, Ijiri K,
Matsunaga S, et al: High mobility group box chromosomal protein 1
plays a role in the pathogenesis of rheumatoid arthritis as a novel
cytokine. Arthritis Rheum. 48:971–981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inoue K, Kawahara K, Biswas KK, Ando K,
Mitsudo K, Nobuyoshi M and Maruyama I: HMGB1 expression by
activated vascular smooth muscle cells in advanced human
atherosclerosis plaques. Cardiovasc Pathol. 16:136–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalinina N, Agrotis A, Antropova Y,
DiVitto G, Kanellakis P, Kostolias G, Ilyinskaya O, Tararak E and
Bobik A: Increased expression of the DNA-binding cytokine HMGB1 in
human atherosclerotic lesions: Role of activated macrophages and
cytokines. Arterioscler Thromb Vasc Biol. 24:2320–2325. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao D and Brownlee M:
Hyperglycemia-induced reactive oxygen species increase expression
of the receptor for advanced glycation end products (RAGE) and RAGE
ligands. Diabetes. 59:249–255. 2010. View Article : Google Scholar
|
|
18
|
Lupo G, Motta C, Giurdanella G, Anfuso CD,
Alberghina M, Drago F, Salomone S and Bucolo C: Role of
phospholipases A2 in diabetic retinopathy: In vitro and in vivo
studies. Biochem Pharmacol. 86:1603–1613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamura H, Miyamoto K, Kiryu J, Miyahara S,
Katsuta H, Hirose F, Musashi K and Yoshimura N: Intravitreal
injection of corticosteroid attenuates leukostasis and vascular
leakage in experimental diabetic retina. Invest Ophthalmol Vis Sci.
46:1440–1444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jonas JB and Söfker A: Intraocular
injection of crystalline cortisone as adjunctive treatment of
diabetic macular edema. Am J Ophthalmol. 132:425–427. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai J, Kehoe O, Smith GM, Hykin P and
Boulton ME: The angiopoietin/Tie-2 system regulates pericyte
survival and recruitment in diabetic retinopathy. Invest Ophthalmol
Vis Sci. 49:2163–2171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Capetandes A and Gerritsen ME: Simplified
methods for consistent and selective culture of bovine retinal
endothelial cells and pericytes. Invest Ophthalmol Vis Sci.
31:1738–1744. 1990.PubMed/NCBI
|
|
23
|
Kondo T, Hosoya K, Hori S, Tomi M, Ohtsuki
S, Takanaga H, Nakashima E, Iizasa H, Asashima T, Ueda M, et al:
Establishment of conditionally immortalized rat retinal pericyte
cell lines (TR-rPCT) and their application in a co-culture system
using retinal capillary endothelial cell line (TR-iBRB2). Cell
Struct Funct. 28:145–153. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang YL, Hui YN, Guo B and Ma JX:
Strengthening tight junctions of retinal microvascular endothelial
cells by pericytes under normoxia and hypoxia involving
angiopoietin-1 signal way. Eye (Lond). 21:1501–1510. 2007.
View Article : Google Scholar
|
|
25
|
Cacicedo JM, Benjachareowong S, Chou E,
Ruderman NB and Ido Y: Palmitate-induced apoptosis in cultured
bovine retinal pericytes: Roles of NAD (P)H oxidase, oxidant
stress, and ceramide. Diabetes. 54:1838–1845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schreiber E, Matthias P, Müller MM and
Schaffner W: Rapid detection of octamer binding proteins with
'mini-extracts', prepared from a small number of cells. Nucleic
Acids Res. 17:64191989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
von Knethen A, Callsen D and Brüne B:
NF-kappaB and AP-1 activation by nitric oxide attenuated apoptotic
cell death in RAW 264.7 macrophages. Mol Biol Cell. 10:361–372.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vos MD, Ellis CA, Elam C, Ulku AS, Taylor
BJ and Clark GJ: RASSF2 is a novel K-Ras-specific effector and
potential tumor suppressor. J Biol Chem. 278:28045–28051. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hori O, Brett J, Slattery T, Cao R, Zhang
J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, et al: The
receptor for advanced glycation end products (RAGE) is a cellular
binding site for amphoterin. Mediation of neurite outgrowth and
co-expression of rage and amphoterin in the developing nervous
system. J Biol Chem. 270:25752–25761. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Clynes R, Moser B, Yan SF, Ramasamy R,
Herold K and Schmidt AM: Receptor for AGE (RAGE): Weaving tangled
webs within the inflammatory response. Curr Mol Med. 7:743–751.
2007. View Article : Google Scholar
|
|
32
|
Motiejunaite R and Kazlauskas A: Pericytes
and ocular diseases. Exp Eye Res. 86:171–177. 2008. View Article : Google Scholar
|
|
33
|
Kuwabara T and Cogan DG: Retinal vascular
patterns. VII. Acellular change. Invest Ophthalmol. 4:1049–1064.
1965.PubMed/NCBI
|
|
34
|
Sims DE: Diversity within pericytes. Clin
Exp Pharmacol Physiol. 27:842–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Joussen AM, Murata T, Tsujikawa A,
Kirchhof B, Bursell SE and Adamis AP: Leukocyte-mediated
endothelial cell i njury and death in the diabetic retina. Am J
Pathol. 158:147–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kern TS: Contributions of inflammatory
processes to the development of the early stages of diabetic
retinopathy. Exp Diabetes Res. 2007:951032007. View Article : Google Scholar
|
|
37
|
Hofmann MA, Drury S, Fu C, Qu W, Taguchi
A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al: RAGE
mediates a novel proinflammatory axis: A central cell surface
receptor for S100/calgranulin polypeptides. Cell. 97:889–901. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bierhaus A, Schiekofer S, Schwaninger M,
Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Klöting
I, et al: Diabetes-associated sustained activation of the
transcription factor nuclear factor-kappaB. Diabetes. 50:2792–2808.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thornalley PJ: Dietary AGEs and ALEs and
risk to human health by their interaction with the receptor for
advanced glycation endproducts (RAGE)-an introduction. Mol Nutr
Food Res. 51:1107–1110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ramasamy R, Yan SF and Schmidt AM: Arguing
for the motion: Yes, RAGE is a receptor for advanced glycation
endproducts. Mol Nutr Food Res. 51:1111–1115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Heizmann CW: The mechanism by which
dietary AGEs are a risk to human health is via their interaction
with RAGE: Arguing against the motion. Mol Nutr Food Res.
51:1116–1119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Foell D, Wittkowski H, Vogl T and Roth J:
S100 proteins expressed in phagocytes: A novel group of
damage-associated molecular pattern molecules. J Leukoc Biol.
81:28–37. 2007. View Article : Google Scholar
|
|
43
|
Yamagishi S, Takeuchi M, Matsui T,
Nakamura K, Imaizumi T and Inoue H: Angiotensin II augments
advanced glycation end product-induced pericyte apoptosis through
RAGE overexpression. FEBS Lett. 579:4265–4270. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yan SD, Schmidt AM, Anderson GM, Zhang J,
Brett J, Zou YS, Pinsky D and Stern D: Enhanced cellular oxidant
stress by the interaction of advanced glycation end products with
their receptors/binding proteins. J Biol Chem. 269:9889–9897.
1994.PubMed/NCBI
|
|
45
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: The control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar
|
|
46
|
Kim J, Kim OS, Kim CS, Kim NH and Kim JS:
Cytotoxic role of methylglyoxal in rat retinal pericytes:
Involvement of a nuclear factor-kappaB and inducible nitric oxide
synthase pathway. Chem Biol Interact. 188:86–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim J, Son JW, Lee JA, Oh YS and Shinn SH:
Methylglyoxal induces apoptosis mediated by reactive oxygen species
in bovine retinal pericytes. J Korean Med Sci. 19:95–100. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Romeo G, Liu WH, Asnaghi V, Kern TS and
Lorenzi M: Activation of nuclear factor-kappaB induced by diabetes
and high glucose regulates a proapoptotic program in retinal
pericytes. Diabetes. 51:2241–2248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang SX, Wang JJ, Dashti A, Wilson K, Zou
MH, Szweda L, Ma JX and Lyons TJ: Pigment epithelium-derived factor
mitigates inflammation and oxidative stress in retinal pericytes
exposed to oxidized low-density lipoprotein. J Mol Endocrinol.
41:135–143. 2008. View Article : Google Scholar : PubMed/NCBI
|