Introduction
Infertility is defined as when couples who have an
active sex life without using protective measures for >1 year,
fail to get pregnant, which affects 10–15% (1,2). Of
this, ~25–30% are infertile couples due to problems with the man
(3). Obesity, an acknowledged
major risk factor for male infertility (4–6),
leads to decreased sperm number and reproductive dysfunction
(7,8). Germ cell apoptosis in the testis is a
key pathophysiological process in obesity-induced male
spermatogenesis dysfunction (9),
therefore inhibiting apoptosis in the testis may improve male
spermatogeneses function.
Curcumin, a type of natural active ingredient
derived from rhizoma of Curcuma, has protective effects in a series
of diseases, including cardiovascular disease (10,11),
cancer, Alzheimer's disease (12)
and diabetes (13). Curcumin has
been previously found to serve a significant role in antioxidant,
antimutative, anti-inflammatory and antitumorigenic responses
(14–17), and recent studies indicated that
curcumin can ameliorate high-glucose-induced neural defects by
suppressing cellular stress and apoptosis (18). The present study investigated the
hypothesis that curcumin treatment can improve spermatogenesis
dysfunction induced by a high-fat diet (HFD). Testis/body weight,
histological analysis and serum hormone levels were determined to
reflect spermatogenesis function of adult rats. Since germ cell
apoptosis is an important process during HFD-induced
spermatogenesis dysfunction (9),
apoptosis associated proteins, Fas, B-cell lymphoma (Bcl)-xl,
Bcl-associated X protein (Bax) and cleaved-caspase 3 were also
assessed.
Materials and methods
Animal care and treatment
A total of 30 male Sprague-Dawley rats (permit
number, 42000500002649), weighing 200–250 g were used in the
present study. All animals experiments performed in the present
study were approved by the Institutional Animal Care and Use
Committee of Renmin Hospital of Wuhan University (Wuhan, China).
The animals were allowed free access to food and water at all times
and were maintained on a 12 h light/dark cycle at a controlled
temperature (20–25°C) and humidity (50±5%), which was also
pathogen-free. The composition of the HFD is shown in Table I. Curcumin (Sigma-Aldrich, St.
Louis, MO, USA) was dissolved by olive oil and administered orally
by oral gavage. The rats were randomly divided into three groups:
i) Control; ii) HFD; iii) curcumin treatment groups. All rats were
subjected to a HFD for 8 weeks, with the exception of those in the
control group ad libitum feeding. The rats in curcumin
treatment group were orally administered curcumin treatment (100
mg/kg/day) for 8 weeks, while in the control and HFD groups, an
identical volume vehicle was used. Following treatment, the rats
were anesthetized with sodium pentobarbital (45 mg/kg)
intraperitoneally and blood samples were collected from the
abdominal aorta. The rats were subsequently euthanized with 200
mg/kg sodium pentobarbital intraperitoneally and the testes were
collected to calculate testis/body weight.
 | Table IMacronutrient composition of diets for
rats. |
Table I
Macronutrient composition of diets for
rats.
| Nutrient | Control
| High-fat diet
|
|---|
| g, % | kJ, % | g, % | kJ, % |
|---|
| Protein | 20 | 19 | 20 | 14 |
| Carbohydrate | 76 | 72 | 45 | 31 |
| Saturated fat | 4 | 9 | 35 | 55 |
| kJ/g | 17.5 | | 24.1 | |
Histological analysis
The collected testis tissues were fixed with 4%
paraformaldehyde, dehydrated and embedded in paraffin (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The testis tissue
sections (5 μm) were sectioned with a microtome and stained
with hematoxylin and eosin to examine the morphology. The tissue
sections (5 μm) were observed under light microscopy (Nikon
E100; Nikon, Tokyo, Japan), and the photomicrographs were obtained
by Photo Imaging System (Canon 600D; Canon, Tokyo, Japan). The
diameter of seminiferous tubules was measured by Image-Pro Plus 6.0
(Media Cybernetics, Inc., Rockville, MD, USA). In each group, 120
seminiferous tubules (5 fields/rat, randomized 4 seminiferous
tubules/field) in 6 rats' testes were counted. Additionally, 30
fields (5 fields/rat) in 6 rats/group were randomly selected to
count spermatogenetic cells and interstitial cells.
Hormone levels assay
Blood samples were obtained from the abdominal
aorta. Following centrifugation (1,506 × g) for 10 min at 4°C, the
sera were obtained for further detection. Serum levels of estradiol
(E2), testosterone (T), follicle stimulating hormone (FSH),
luteinizing hormone (LH) and leptin were measured using kits,
according to the manufacturer's protocol (Elabscience Biotechnology
Co., Ltd., Wuhan, China).
Immunohistochemistry
The protein expression levels of Fas, Bcl-xl and
leptin receptors were tested by immunohistochemistry. The sections
were deparaffinized and were subsequently boiled for 15 min in
sodium citrate buffer for antigen retrieval. Following elimination
of internal peroxidase activity, the sections were incubated with
rabbit anti-Fas (1:50; cat no. BA0408), rabbit anti-leptin-receptor
(1:50; cat no. BA1233; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) and rabbit anti-Bcl-xl (1:100; cat. no. 10783-AP;
Proteintech Group, Inc., Chicago, IL, USA) antibodies at 4°C
overnight. The tissue sections were exposed to biotinylated sheep
anti-rabbit immunoglobulin G solution (Proteintech Group, Inc.) at
37°C for 30 min and were subsequently incubated with horseradish
peroxidase-labeled streptavidin (Proteintech Group, Inc.) at 37°C
for 30 min. Finally, the tissue sections were observed under light
microscopy (Nikon E100; Nikon), and the photomicrographs were
obtained by Photo Imaging System (Canon 600D; Canon). In each group
30 fields (5 fields/rat) in 6 rat testis were randomly selected.
Positive expression was assessed using Image-Pro Plus 6.0 and a
mean of the integrated optical density was obtained.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL)
Tissue sections were processed, according to the
manufacturer's protocol for the TUNEL kit (Roche Applied Science,
Indianapolis, IN, USA). Positively labeled nuclei were stained a
brown color, while negatively labeled nuclei were blue. A total of
30 fields were randomly selected in each group (5 fields/rat) and
100 cells were counted in each field under a microscope (Nikon
E100; Nikon), and the number of positive cells was recorded. The
apoptosis index (number of apoptotic cells in each field/100) was
computed for each field.
Western blot analysis
Lysis buffer (720 μl radioimmunoprecipitation
buffer, 100 mmol/l PMSF, 100 μl cocktail, 100 μl
Phos-stop, 20 mmol/l NaF and 100 mmol/l
Na3VO4 in 1 ml) was used to extract the total
proteins from fresh testicular tissues. The protein concentrations
were tested using the Bicinchoninic Acid Protein Assay kit (Thermo
Fisher Scientific, Inc.) and a plate reader (Bio-Tek Instruments,
Inc., Winooski, VT, USA). Equal quantities of protein (30
μg) were separated with denaturing sodium dodecyl sulphate
10% polyacrylamide gels under reducing and denaturing conditions
and were subsequently transferred onto a polyvinylidene difluoride
(PVDF) membrane (EMD Millipore, Billerica, MA, USA). The PVDF
membrane was blocked with 5% (w/v) non-fat milk and 0.1% Tween in
Tris-buffered saline (pH 7.4) at room temperature for 1 h.
Following blocking, the membrane was incubated overnight at 4°C
with the following rabbit primary antibodies: Anti-GAPDH antibody
(1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; cat.
no. sc-25778), anti-Bax antibody (1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA; cat. no. 14796) and
anti-cleaved-caspase 3 antibody (1:1,000; Cell Signaling
Technology, Inc.; cat. no. 9664). Following the incubation with
primary antibody, the membrane was incubated with IRDye
800CW-conjugated secondary antibody (LI-COR Biosciences, Lincoln,
NE, USA; cat. no. 926-32211) for 1 h. Finally, the membrane was
scanned using a two-color infrared imaging system (Odyssey, LI-COR
Biosciences). Densitometric analysis was performed by Odyssey, as
previous described (19), and the
results were expressed as the ratio between targeted proteins and
GAPDH band intensities.
Statistical analysis
All statistical analyses were performed with SPSS
19.0 (IBS SPSS, Chicago, IL, USA). All data are expressed as the
mean ± standard error of the mean. Multiple group comparison was
performed by analysis of variance, followed by the post-hoc least
significant difference test assuming equal variances; otherwise
Tamhane's T2 post-hoc test. All statistical analyses were
two-sided. P<0.05 was considered to indicate a statistically
significant difference.
Results
Histomorphological changes
Following a HFD for 8 weeks, the testis/body weight
was reduced and treatment with curcumin increased the testis/body
weight (Table II; Fig. 1). The testicular structure of the
control group under the light microscope manifested as larger
seminiferous tubules, abundant spermatogenetic and interstitial
cells. As shown in Fig. 1,
atrophic seminiferous tubules and more vacuoles were observed in
the HFD group. Notably, decreased spermatogenetic and interstitial
cells were observed in the HFD group. However, these histological
changes were improved following treatment with curcumin (Table III; Fig. 1).
 | Table IITesticular weight and testicular/body
weight of the three groups. |
Table II
Testicular weight and testicular/body
weight of the three groups.
| Group | n | Body weight
(g) | Testicular weight
(g) | Testicular/body
weight (g/kg) |
|---|
| Control | 10 | 315.80±14.74 | 3.14±0.21 | 9.97±0.93 |
| High-fat diet | 10 |
371.40±12.05a | 2.70±0.16a | 7.27±0.35a |
| Curcumin | 10 | 353.20±5.76 | 2.90±0.12b | 8.21±0.38b |
 | Table IIIComparison of diameters of
seminiferous tubules and the quantity of spermatogenetic cells and
interstitial cells in the three groups. |
Table III
Comparison of diameters of
seminiferous tubules and the quantity of spermatogenetic cells and
interstitial cells in the three groups.
| Group | n | Seminiferous tubule
diameter (μm) | Quantity of
spermatogenetic cells | Quantity of
interstitial cells |
|---|
| Control | 6 | 395.08±19.91 | 25.40±1.14 | 8.16±0.29 |
| High-fat diet | 6 |
273.70±16.55a | 14.80±1.79a | 4.16±0.12a |
| Curcumin | 6 |
319.94±20.55b | 20.60±1.14b | 5.37±0.11b |
Determination of endogenous hormone
Rats fed with an HFD exhibited abnormal serum
hormone levels, manifested as reduced serum levels of T, FSH and
LH, and increased serum levels of leptin and E2. Serum hormone
levels were observed to be restored following treatment with
curcumin (Figs. 2 and 5).
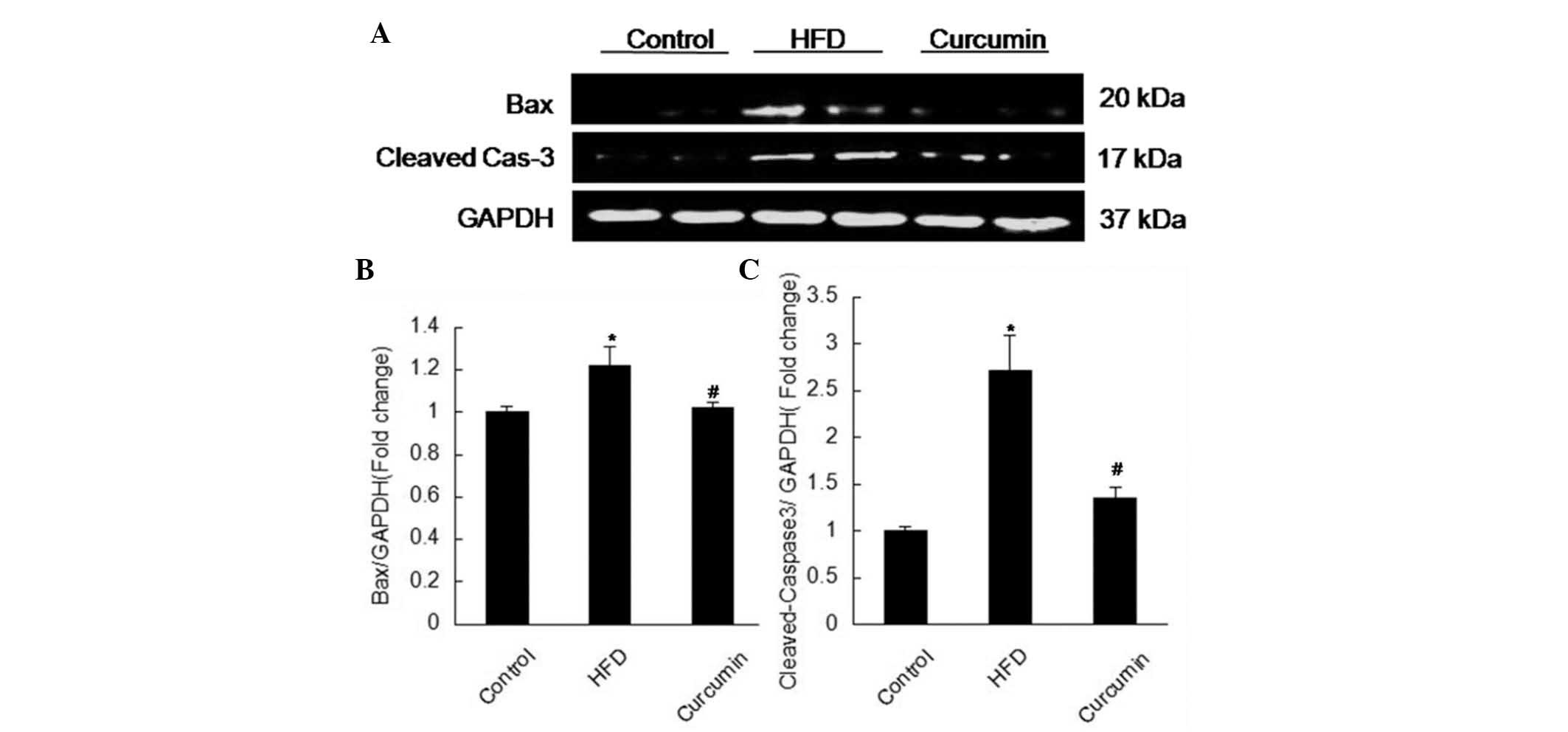
Expression levels of apoptosis associated
proteins and leptin receptors
A HFD resulted in more apoptotic cells in the
testis, characterized by elevated expression levels of Fas, Bax and
cleaved-caspase 3, and reduced expression of Bcl-xl. Following
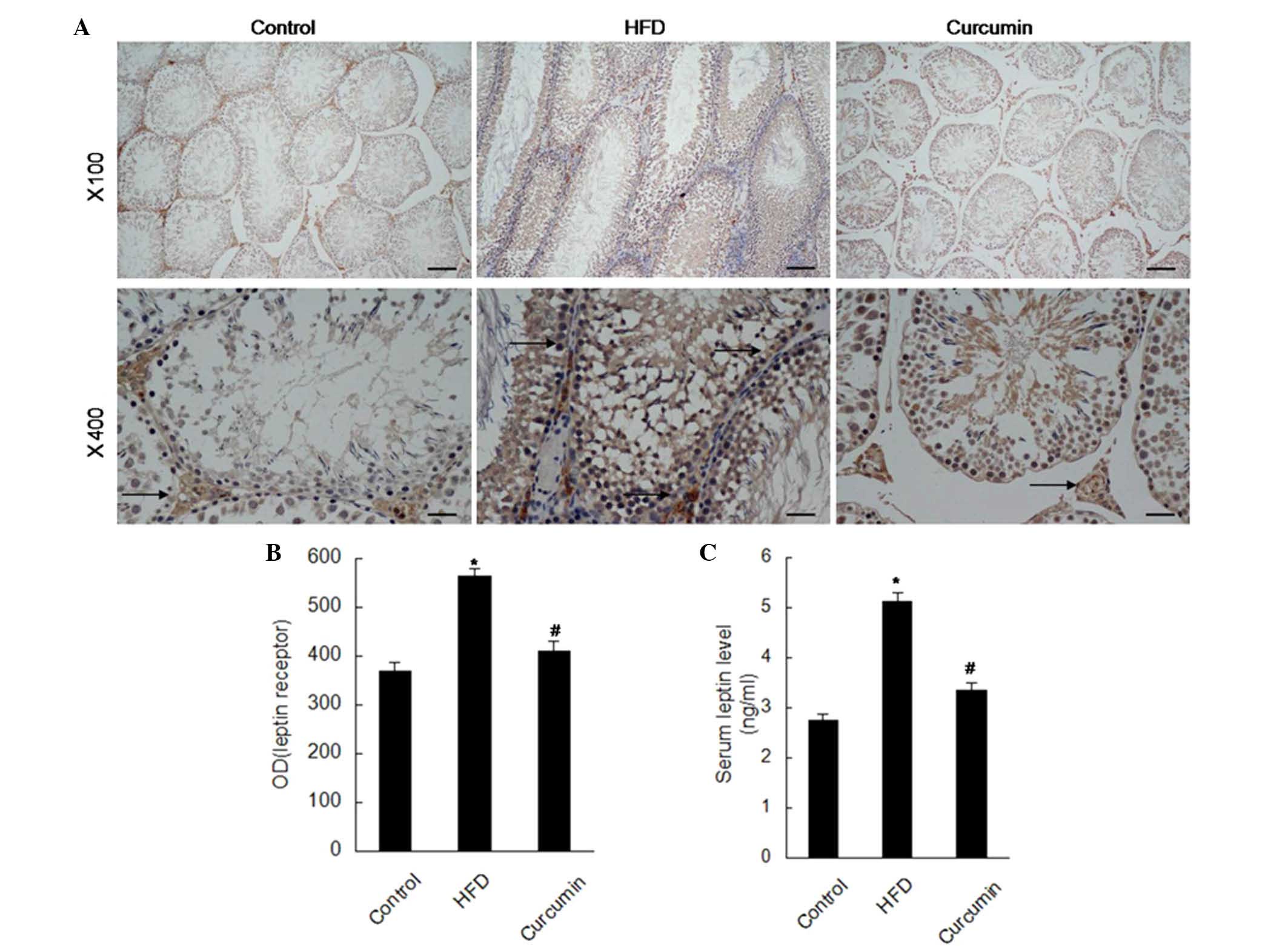
treatment with curcumin, these changes were attenuated (Figs. 3 and 4). The expression of leptin receptors was
found to be increased in the testis of rats fed a HFD by
immunohistochemistry staining, and curcumin treatment reduced these
increased leptin receptors (Fig.
5).
Discussion
In order to investigate the effects of curcumin on
male spermatogenesis function, the present study subjected the rats
to a HFD to induce spermatogenesis dysfunction. The present results
revealed that spermatogenesis function was disturbed in rats fed
with a HFD, characterized as decreased testis/body weight,
atrophied seminiferous tubules, reduced spermatogenetic and
interstitial cells, and abnormal hormone levels, which is
consistent with a previous study (7).
Curcumin has been demonstrated to have a series of
pharmacological actions, including antioxidant, antimutative,
anti-inflammatory and antitumorigenic actions (14–17).
Over the last decade, the therapeutic effects of curcumin on
various cancer types and Alzheimer's disease have been confirmed by
clinical trials (20). The present
study found that curcumin treatment improved atrophied testes
manifested as increased testis/body weight, increased the diameter
of seminiferous tubules, and increased the number of
spermatogenetic and interstitial cells. Additionally, curcumin
treatment improved the abnormal hormone levels. Taken together,
curcumin had protective effects in HFD-induced spermatogenesis
dysfunction.
The balance between cell proliferation and cell
death is important in spermatogenesis function. As a type of cell
death, apoptosis of testicular germ cells was an important
physiological mechanism in regulating the germ cell population
(21–24). Increased germ cell apoptosis was
observed in testicular injury induced by stimuli (25–28),
and additionally, suppressing apoptosis improved the sperm count
(29–33). As shown in the present results,
curcumin treatment decreased the protein expression levels of Fas,
Bax and cleaved-caspase 3 and increased the expression of Bcl-xl.
Notably, curcumin treatment reduced germ cell apoptosis induced by
a HFD.
Leptin, also synthesized by seminiferous tubules
(34,35), was found to have important effects
on reproductive function (36),
which has been confirmed by sterile leptin deficient ob mice
(37). Additionally, leptin
directly acted on the testis and modulated spermatogenesis
(38). In addition, leptin served
an inhibitory role in testicular steroidogenesis (39,40)
and influenced the weight of the testis, the diameter of
seminiferous tubules and the number of germ cells (41). In the present study, the leptin
receptors in the testis and serum leptin level were increased in
rats subjected to a HFD. Treatment with curcumin downregulated the
increased levels of leptin receptors and the serum leptin
level.
In conclusion, the present study revealed that
curcumin reduced HFD-induced spermatogenesis dysfunction and
apoptosis. Curcumin may be a potential novel therapeutic medicine
for male patients suffering from obesity-induced infertility.
Acknowledgments
The present study was partially supported by the Key
Research Project of the Ministry of Public Security (no. 2010
ZDYJHBST007).
References
|
1
|
Botelho F, Figueiredo L, Leite R, Carvalho
A, Tomada N and Vendeira P: Predictive factors of a successful
testicular biopsy and subsequent clinical pregnancy. Andrologia.
44:237–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlin A, Arredi B and Foresta C: Genetic
causes of male infertility. Reprod Toxicol. 22:133–141. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hammoud AO, Gibson M, Peterson CM,
Hamilton BD and Carrell DT: Obesity and male reproductive
potential. J Androl. 27:619–626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pasquali R, Pelusi C, Genghini S, Cacciari
M and Gambineri A: Obesity and reproductive disorders in women. Hum
Reprod Update. 9:359–372. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pantasri T and Norman RJ: The effects of
being overweight and obese on female reproduction: A review.
Gynecol Endocrinol. 30:90–94. 2014. View Article : Google Scholar
|
|
6
|
Jungheim ES, Travieso JL and Hopeman MM:
Weighing the impact of obesity on female reproductive function and
fertility. Nutr Rev. 71(Suppl 1): S3–S8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jensen TK, Andersson AM, Jørgensen N,
Andersen AG, Carlsen E, Petersen JH and Skakkebaek NE: Body mass
index in relation to semen quality and reproductive hormones among
1,558 Danish men. Fertil Steril. 82:863–870. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kort HI, Massey JB, Elsner CW,
Mitchell-Leef D, Shapiro DB, Witt MA and Roudebush WE: Impact of
body mass index values on sperm quantity and quality. J Androl.
27:450–452. 2006. View Article : Google Scholar
|
|
9
|
Palmer NO, Bakos HW, Owens JA, Setchell BP
and Lane M: Diet and exercise in an obese mouse fed a high-fat diet
improve metabolic health and reverse perturbed sperm function. Am J
Physiol Endocrinol Metab. 302:E768–E780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Srivastava G and Mehta JL: Currying the
heart: Curcumin and cardioprotection. J Cardiovasc Pharmacol Ther.
14:22–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wongcharoen W and Phrommintikul A: The
protective role of curcumin in cardiovascular diseases. Int J
Cardiol. 133:145–151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen L and Ji HF: The pharmacology of
curcumin: Is it the degradation products? Trends Mol Med.
18:138–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maheshwari RK, Singh AK, Gaddipati J and
Srimal RC: Multiple biological activities of curcumin: A short
review. Life Sci. 78:2081–2087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li M, Zhang Z, Hill DL, Wang H and Zhang
R: Curcumin, a dietary component, has anticancer,
chemosensitization and radiosensitization effects by
down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2
pathway. Cancer Res. 67:1988–1996. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruck R, Ashkenazi M, Weiss S, Goldiner I,
Shapiro H, Aeed H, Genina O, Helpern Z and Pines M: Prevention of
liver cirrhosis in rats by curcumin. Liver Int. 27:373–383. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shakibaei M, John T, Schulze-Tanzil G,
Lehmann I and Mobasheri A: Suppression of NF-kappaB activation by
curcumin leads to inhibition of expression of cyclo-oxygenase-2 and
matrix metalloproteinase-9 in human articular chondrocytes:
Implications for the treatment of osteoarthritis. Biochem
Pharmacol. 73:1434–1445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen SQ, Zhang Y, Xiang JJ and Xiong CL:
Protective effect of curcumin against liver warm
ischemia/reperfusion injury in rat model is associated with
regulation of heat shock protein and antioxidant enzymes. World J
Gastroenterol. 13:1953–1961. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Y, Wang F, Reece EA and Yang P:
Curcumin ameliorates high glucose-induced neural tube defects by
suppressing cellular stress and apoptosis. Am J Obstet Gynecol.
212:802.e1–e8. 2015. View Article : Google Scholar
|
|
19
|
Liu Y, Jiang XL, Liu Y, Jiang DS, Zhang Y,
Zhang R, Chen Y, Yang Q, Zhang XD, Fan GC and Li H:
Toll-interacting protein (Tollip) negatively regulates pressure
overload-induced ventricular hypertrophy in mice. Cardiovasc Res.
101:87–96. 2014. View Article : Google Scholar :
|
|
20
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: From ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Allan DJ, Harmon BV and Roberts SA:
Spermatogonial apoptosis has three morphologically recognizable
phases and shows no circadian rhythm during normal spermatogenesis
in the rat. Cell Prolif. 25:241–250. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bartke A: Apoptosis of male germ cells, a
generalized or a cell type-specific phenomenon? Endocrinology.
136:3–4. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Billig H, Furuta I, Rivier C, Tapanainen
J, Parvinen M and Hsueh AJ: Apoptosis in testis germ cells:
Developmental changes in gonadotropin dependence and localization
to selective tubule stages. Endocrinology. 136:5–12.
1995.PubMed/NCBI
|
|
24
|
Hikim AP, Wang C, Leung A and Swerdloff
RS: Involvement of apoptosis in the induction of germ cell
degeneration in adult rats after gonadotropin-releasing hormone
antagonist treatment. Endocrinology. 136:2770–2775. 1995.PubMed/NCBI
|
|
25
|
Lee J, Richburg JH, Shipp EB, Meistrich ML
and Boekelheide K: The Fas system, a regulator of testicular germ
cell apoptosis, is differentially up-regulated in Sertoli cell
versus germ cell injury of the testis. Endocrinology. 140:852–858.
1999.PubMed/NCBI
|
|
26
|
Sinha HA, Rajavashisth TB, SinhaHikim I,
Lue Y, Bonavera JJ, Leung A, Wang C and Swerdloff RS: Significance
of apoptosis in the temporal and stage-specific loss of germ cells
in the adult rat after gonadotropin deprivation. Biol Reprod.
57:1193–1201. 1997. View Article : Google Scholar
|
|
27
|
Yin Y, DeWolf WC and Morgentaler A:
Experimental cryptorchidism induces testicular germ cell apoptosis
by p53-dependent and -independent pathways in mice. Biol Reprod.
58:492–496. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hasegawa M, Zhang Y, Niibe H, Terry NH and
Meistrich ML: Resistance of differentiating spermatogonia to
radiation-induced apoptosis and loss in p53-deficient mice. Radiat
Res. 149:263–270. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hockenbery DM, Oltvai ZN, Yin XM, Milliman
CL and Korsmeyer SJ: Bcl-2 functions in an antioxidant pathway to
prevent apoptosis. Cell. 75:241–251. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kane DJ, Sarafian TA, Anton R, Hahn H,
Gralla EB, Valentine JS, Ord T and Bredesen DE: Bcl-2 inhibition of
neural death: Decreased generation of reactive oxygen species.
Science. 262:1274–1277. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wong WY, Merkus HM, Thomas CM, Menkveld R,
Zielhuis GA and Steegers-Theunissen RP: Effects of folic acid and
zinc sulfate on male factor subfertility: A double-blind,
randomized, placebo-controlled trial. Fertil Steril. 77:491–498.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Joshi R, Adhikari S, Patro BS,
Chattopadhyay S and Mukherjee T: Free radical scavenging behavior
of folic acid: Evidence for possible antioxidant activity. Free
Radic Biol Med. 30:1390–1399. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zago MP and Oteiza PI: The antioxidant
properties of zinc: Interactions with iron and antioxidants. Free
Radic Biol Med. 31:266–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Campfield LA, Smith FJ and Burn P: The OB
protein (leptin) pathway-a link between adipose tissue mass and
central neural networks. Horm Metab Res. 28:619–632. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Glander HJ, Lammert A, Paasch U, Glasow A
and Kratzsch J: Leptin exists in tubuli seminiferi and in seminal
plasma. Andrologia. 34:227–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Caprio M, Fabbrini E, Isidori AM, Aversa A
and Fabbri A: Leptin in reproduction. Trends Endocrinol Metab.
12:65–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Proenca R, Maffei M, Barone M,
Leopold L and Friedman JM: Positional cloning of the mouse obese
gene and its human homologue. Nature. 372:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ma Y, Chen B, Wang H, Hu K and Huang Y:
Prediction of sperm retrieval in men with non-obstructive
azoospermia using artificial neural networks: Leptin is a good
assistant diagnostic marker. Hum Reprod. 26:294–298. 2011.
View Article : Google Scholar
|
|
39
|
Fui MN, Dupuis P and Grossmann M: Lowered
testosterone in male obesity: Mechanisms, morbidity and management.
Asian J Androl. 16:223–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Isidori AM, Caprio M, Strollo F, Moretti
C, Frajese G, Isidori A and Fabbri A: Leptin and androgens in male
obesity: Evidence for leptin contribution to reduced androgen
levels. J Clin Endocrinol Metab. 84:3673–3680. 1999.PubMed/NCBI
|
|
41
|
Yuan M, Huang G, Li J, Zhang J, Li F, Li
K, Gao B, Zeng L, Shan W, Lin P and Huang L: Hyperleptinemia
directly affects testicular maturation at different sexual stages
in mice and suppressor of cytokine signaling 3 is involved in this
process. Reprod Biol Endocrinol. 12:152014. View Article : Google Scholar
|