Introduction
Rheumatoid arthritis (RA), a chronic multisystem
inflammatory autoimmune disorder, is characterized by destructive
synovitis, systemic inflammation and acceleration of
atherosclerosis (1,2). The imbalance of inflammatory
cytokines, including tumor necrosis factor (TNF) and interleukin
(IL)-6, is responsible for this disease (3). It has been previously estimated that
~0.5%–1.0% of the general population are affected by RA (4). The prevalence of RA in China is
0.28%, with a 6:1 female:male ratio (5). RA leads to various functional
disabilities, pain and low health-associated quality of life,
imposing a large economic and mental burden on society and
individuals with the disease (6,7). The
predominant selective and effective therapeutic options for RA
include conventional-synthesized disease-modifying antirheumatic
drugs (DMARDs) and biological DMARDs, for example, anti-TNF-α
monoclonal antibodies and tocilizumab. Although the management of
RA has achieved considerable success, various approaches are
expensive and no DMARD achieves long-term drug-free remission
(8,9). Thus, it is necessary to develop novel
and more effective therapies for RA.
Previous studies have investigated mesenchymal stem
cells (MSCs) due to their immunosuppressive ability via regulation
of T and B cell proliferation and differentiation (10). MSCs are considered as attractive
therapeutic targets for treatment of immune disorders, including RA
(11–13). The major common sources of MSCs are
bone marrow, peripheral blood and adipose tissue. However,
obtaining human MSCs is invasive, except when extracted from
umbilical cord (UC), as it is a postnatal organ discarded following
birth. Additionally, UC-MSCs have a higher proliferative potential
than bone marrow-derived MSCs (BM-MSCs) (14,15).
Furthermore, it has previously been well demonstrated that UC-MSCs
also possess immunoregulatory capabilities (15). Therefore, UC-MSCs are now regarded
as an alternative source of stem cells and require further
investigation (16). In a previous
report, Liu et al (17)
suggested that there is therapeutic potential in using human
UC-MSCs for the treatment of RA. However, little information is
available regarding the co-culture of fibroblast-like synoviocytes
(FLS) with UC-MSCs.
The present study investigated the effect induced by
co-culture of FLS with UC-MSCs and the potential mechanism by which
this may affect RA. The levels of certain pro-inflammatory
cytokines and chemokines, aggrecan, collagen type II and
apoptosis-associated proteins, and the percentage of apoptotic
cells were analyzed.
Materials and methods
Isolation and phenotypic identification
of human UC-MSCs
The protocol of the present study was approved by
the ethics committee of The Second Affiliated Hospital of Hunan
University of Chinese Medicine (Changsha, China) and informed
consent was obtained from all the participants prior to the
research. The methods for isolation and culture of human UC-MSCs
were described a previous study (18). Fresh human umbilical cords (n=5;
gestational age, 39–40 weeks) were obtained from Department of
Obstetrics and Gynecology of The Second Affiliated Hospital of
Hunan University of Chinese Medicine following normal birth. The
tissues were collected and transferred into a sterile container in
α-modified Minimum Essential Medium (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 100 U/ml penicillin and
100 µg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA).
Following washing with phosphate-buffered saline (PBS), the tissues
were divided into 1–2 mm3 pieces, incubated with 0.075%
type II collagenase (Sigma-Aldrich) and 0.125% trypsin (Thermo
Fisher Scientific, Inc.) then passed through a 100-µm cell
strainer (BD Biosciences, San Jose, CA, USA). The cells
(1×106 cells/cm2) were then transferred to a
flask containing the growth medium (GM) and cultured in a
humidified atmosphere with 5% CO2 at 37°C for 3 to 4
days. The GM included Dulbecco's modified Eagle medium (DMEM;
Sigma-Aldrich) and 10% fetal bovine serum (FBS) (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10 ng/ml vascular
endothelial growth factor (VEGF; Gibco; Thermo Fisher Scientific,
Inc.), 10 ng/ml epidermal growth factor (EGF; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin, 100 µg/ml
streptomycin, and 2 mM L-glutamine (Sigma-Aldrich). Following
culture, the medium was changed and non-adherent cells were
discarded. The medium was replaced twice weekly. The adherent cells
(1×104 cells/cm2) were maintained in GM for
expansion when the confluence reached 60–80%. For phenotypic
identification, the cells were detached and washed with PBS
supplemented with 0.5% bovine serum albumin (BSA; Sigma-Aldrich),
and maintained in primary antibodies for 30 min at 4°C. To examine
intracellular antigens, the cells were fixed with 4%
paraformaldehyde for 15 min at 4°C, blocked with normal goat serum,
and then permeabilized with 0.1% saponin (Sigma-Aldrich) for 1 h at
room temperature. Primary antibodies were used as follows: cluster
of differentiation (CD)105, CD29, CD44, CD166, CD14, CD34, and CD45
(BD Biosciences). An antibody of the same isotope from the same
species without a target served as the negative control. The cells
were then washed with PBS containing 0.5% BSA and incubated with
fluorescein isothiocyanate (FITC) and phycoerythrin (PE)-conjugated
secondary antibodies for 30 min at 4°C. Following washing three
times, the cells were resuspended in PBS and detected by flow
cytometry by using FACSCalibur flow cytometer (BD Biosciences) and
the CellQuest Pro 3.0 software (BD Biosciences) (19).
Isolation of FLS and co-culture with
UC-MSCs
The FLS were prepared from synovial tissues of 6
patients with RA who had undergone total joint replacement surgery.
The diagnosis of RA was based on the revised criteria from the
American College of Rheumatology (20). The FLS were isolated according to a
previously described method (21).
Briefly, the tissues were washed with PBS and homogenized, then
treated with type I collagenase (1 mg/ml; Sigma-Aldrich) and 2
mg/ml testicular hyaluronidase (Sigma-Aldrich) in DMEM for 2 h at
37°C. Then, the culture was washed with PBS and maintained
overnight in DMEM supplemented with 10% FBS, 100 U/ml penicillin,
100 µg/ml streptomycin and 2 mM L-glutamine in a 5%
CO2 incubator. FLS at passages 4–6 were used for each
experiment.
For the co-culture, FLS (1.5×104) were
seeded in 24-well plates and cultured overnight in DMEM containing
10% FBS. FLS were then washed with serum-free DMEM and UC-MSC
suspensions were added directly onto the FLS at a cell number ratio
of 1:1 for 24 h.
Enzyme-linked immunosorbent assay
(ELISA)
Following co-culture, the supernatants from each
group (FLS alone or FLS + UC-MSCs) were collected and centrifuged
to remove cellular debris. The cell-free culture supernatants were
assayed for IL-1β, IL-6 and chemokine (C-C motif) ligand (CCL)-2
using commercial ELISA kits (R&D Systems, Inc., Minneapolis,
MN, USA). All samples and standards were assessed in triplicate.
The absorbance of each well was determined at 450 nm with a
spectrophotometer (SpectraMax 250, Molecular Devices, LLC,
Sunnyvale, CA, USA). The reference wavelength was 540 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA in the FLS alone or FLS + UC-MSCs groups
was isolated using RNeasy Mini kit (Qiagen, Inc., Valencia, CA,
USA) according to the manufacturer's instructions. Total RNA was
quantified and complementary DNA (cDNA) was synthesized using
SuperScript Reverse Transcriptase (Promega Corporation, Madison,
WI, USA) following the manufacturer's recommendations. The cDNA was
subsequently used for qPCR. The IL-1β, IL-6, and chemokine CCL-2
mRNA levels were determined using PrimeScript RT Reagent kit
(Takara Bio, Inc., Otsu, Japan) and SYBR Premix Ex Taq (Takara Bio,
Inc.). The qPCR cycling conditions were as follows: 95°C for 10
min; 40 cycles at 95°C for 15 sec, 60°C for 2 min, 70°C for 2 min,
and 77°C for 30 sec, 99.9°C for 10 min; the reaction was stopped at
4°C. The following primers were used: Forward,
5′-GTCATGACTTGCCCTCAGCA-3′ and reverse, 5′-GCAGGTGACCCAGTTTTTGG-3′
for IL-1β; forward, 5′-AAATTCGGTACATCCTCGACGGCA-3 and reverse,
5′-AGTGCCTCTTTGCTGCTTTCACAC-3′ for IL-6; forward,
5′-CGCTCAGCCAGATGCAATCAAT-3′ and reverse,
5′-GCTGGTGATTCTTCTATAGCTCGCG-3′ for CCL-2; and forward,
5′-CCAAGGTCATCCATGACAAC-3′ and reverse, 5′-TGTCATACCAGGAAATGAGC-3′
for GAPDH. The relative expression levels of the genes were
calculated by using the 2−ΔΔCq method (22). GAPDH was used as a reference gene.
The reactions were performed in triplicate.
Flow cytometry assay
Cell apoptosis rate was determined by an Annexin
V-FITC apoptosis detection kit (Sigma-Aldrich) according to the
manufacturer's protocol. Briefly, cells were harvested and washed
with cold PBS 3 times. The cells were then resuspended in 0.5 ml
binding buffer, and incubated with 10 µl Annexin V-FITC and
10 µl propidium iodide at room temperature in the dark for
15 min. The cells were subsequently analyzed using a FACSCalibur
flow cytometer. The percentage of total apoptotic cells, including
the early and late stage, were calculated. The data were analyzed
using CellQuest Pro 3.0 software.
Chondrogenesis assay
For chondrogenic differentiation, FLS with or
without UC-MSCs (1×106) were seeded in 24-well plates
with chondrogenic basal medium and cultured for 28 days at 37°C
with 5% CO2 and 95% air. The medium was replaced every
3–4 days. Control cells were cultured in medium without growth
factors. Cells were harvested and the mRNA expression of aggrecan
and collagen type II was measured following co-culture at 7, 14, 21
and 28 days using NucleoSpin RNAII kit (Machery-Nagel GmbH, Düren,
Germany). cDNA was prepared by reverse transcription of total RNA.
The primer sequences for aggrecan, collagen type II and the
housekeeping gene were listed as follows: Forward,
5′-AGGAGACAGAGGGACACGTC-3′ and reverse, 5′-TCCACTGGTAGTCTTGGGCAT-3′
for aggrecan, and forward, 5′-TTCAGCTATGGAGATGACAATC-3′ and
reverse, 5′-AGAGTCCTAGAGTGACTGAG-3′ for collagen type II. GAPDH
served as the housekeeping gene. cDNA was then quantified by qPCR
using SYBR Green dye. The details of cycling conditions and the
quantification method were as described above.
Western blot analysis
Western blotting was performed to determine the
activation of Bcl-2 apoptosis regulator, Bcl-2-associated X
apoptosis regulator (Bax), p53, phosphorylation of AKT serine
threonine kinase and growth differentiation factor-5 (GDF-5).
Protein was exacted using radioimmunoprecipitation assay buffer
(Sigma-Aldrich), and the concentration was measured with the
Bio-Rad DC Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Protein samples (20 µl) were separated on a 10–12%
sodium dodecyl sulfate-polyacrylamide gel, transferred onto
polyvinylidene difluoride membranes (Merck Millipore, Darmstadt,
Germany), blocked in 5% non-fat milk powder in PBS for 2 h at room
temperature and probed with rabbit monoclonal anti-Bcl-2 (cat. no.
4223), rabbit monoclonal anti-Bax (cat. no. 5023), anti-p53 (cat.
no. 2527), anti-phosphorylated (p)-Akt (cat. no. 4060) and
anti-GDF-5 (Abcam, Cambridge, MA, USA; cat. no. ab93855) primary
antibodies (diluted 1:1,000) overnight at 4°C, followed by
incubation with the appropriate goat anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (1:5,000; cat. no. 7071)
for 1 h at 4°C. All antibodies, unless otherwise stated, were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Rabbit monoclonal GAPDH (Cell Signaling Technology, Inc.; cat. no.
5174) was used as a control. Finally, the samples were detected by
enhanced chemiluminescence (Pierce ECL Western Blotting Substrate;
Thermo Fisher Scientific, Inc.) and densitometry analysis was
performed at least three times using ImageLab™ software version
2.0.1 (Bio-Rad Laboratories, Inc.).
Statistical analysis
The experimental data are presented as the mean ±
standard deviation. Statistical analyses were performed using SPSS
statistical software (version 19.0; IBM SPSS, Armonk, NY, USA).
Student's t-test was performed to comparison between 2 groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Phenotypic identification of UC-MSCs
The UC-MSCs were successfully isolated and expanded
and flow cytometry was performed to determine the cell phenotype.
The percentages of the positive cells were calculated They were
negative for CD14 (1.31±0.82%), CD34 (0.70±0.30%) and CD45
(0.28±0.21%), but positive for CD105 (99.01±0.04%), CD29
(99.23±0.31%), CD44 (98.06±0.71%) and CD166 (87.63±0.07%),
indicating that UC-MSCs exhibited a fibroblast-like phenotype.
Effect of co-culture on expression of
pro-inflammatory cytokines and chemokines
To investigate the effect of co-culture on
expression levels of inflammatory cytokines and chemokines, ELISA
and RT-qPCR were performed to determine the protein and mRNA levels
of IL-1β, IL-6 and CCL-2. The ELISA results (Fig. 1A) demonstrated that co-culture of
FLS with UC-MSCs significantly downregulated the protein expression
of IL-1β (P=0.000), IL-6 (P=0.004), and chemokine CCL-2 (P=0.023)
compared with FLS cultured alone. RT-qPCR analysis demonstrated
similar results. IL-1β (P=0.006), IL-6 (P=0.036) and CCL-2
(P=0.005) mRNA levels were significantly reduced in the co-culture
group compared with the FLS only (Fig.
1B). These results indicate that co-culture of FLS with UC-MSCs
inhibits the expression of pro-inflammatory cytokines and
chemokines.
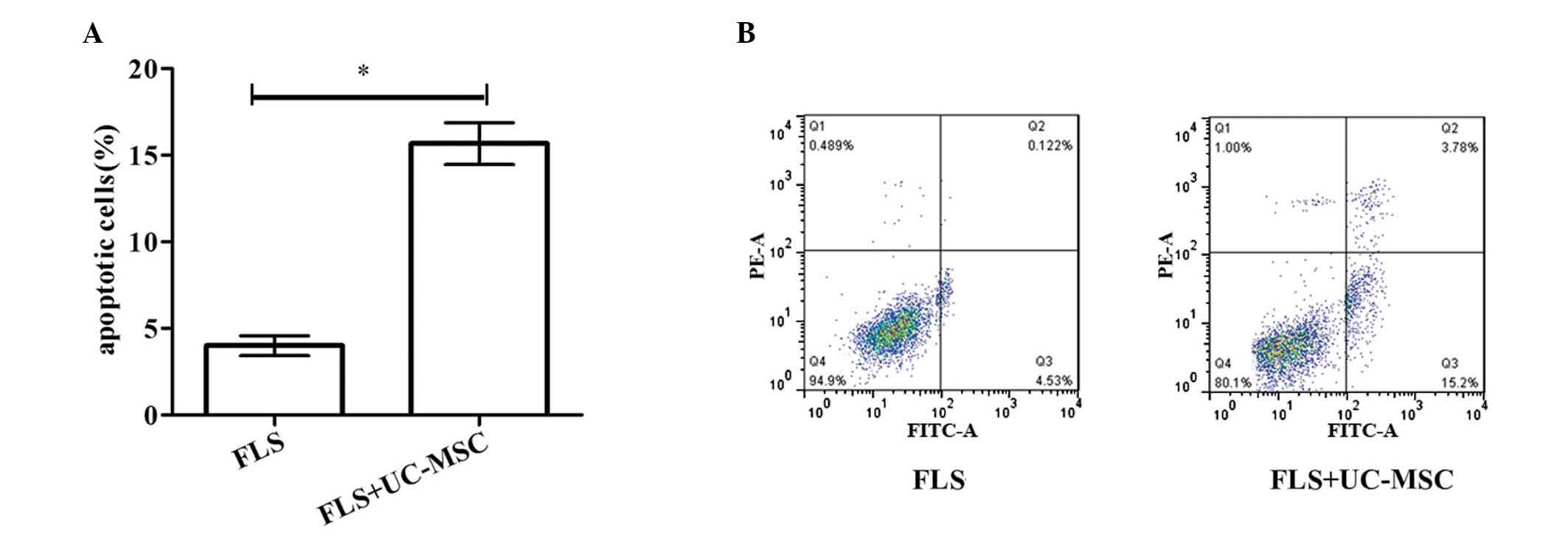
Effect of co-culture on FLS apoptosis and
chondrogenesis
To investigate the effect of co-culture with UC-MCSs
on FLS apoptosis and chondrogenesis, flow cytometry and
chondrogenesis assay were performed, respectively. As demonstrated
in Fig. 2A and B, the percentage
of apoptotic cells was significantly higher in the co-culture group
compared with the FLS group (P=0.039). Additionally, the relative
RNA expression levels of aggrecan and collagen type II at 7, 14, 21
and 28 days were significantly higher in the co-culture group
compared with the FLS group (Fig.
3). These results demonstrated that co-culture of FLS with
UC-MSCs induces FLS apoptosis and promotes chondrogenic
differentiation.
Effect of co-culture on the protein
expression levels of apoptosis-associated proteins and GDF-5
To further confirm the mechanisms of apoptosis and
chondrogenesis induced by the co-culture, the expression levels of
apoptosis-associated proteins (Bcl-2, Bax, p53, and p-AKT) and
GDF-5 were determined by western blot analysis. As demonstrated in
Fig. 4, compared with the levels
in the FLS group, the expression levels of anti-apoptotic Bcl-2
(P=0.026) and p-AKT (P=0.019) were significantly decreased and the
levels of pro-apoptotic p53 (P= 0.001) and Bax (P= 0.006) were
significantly increased by co-culture of FLS with UC-MSCs.
Additionally, the expression level of GDF-5 was significantly
increased by co-culture of FLS with UC-MSCs compared with FLS alone
(P= 0.005). These results indicate that co-culture of FLS with
UC-MSCs induces apoptosis of FLS by regulating the expression of
pro/anti-apoptotic proteins.
Discussion
The present study provided evidence indicating that
co-culture of FLS with UC-MSCs exerts a profound inhibitory effect
on the expression of pro-inflammatory cytokines and chemokines
(IL-1β, IL-6 and CCL-2), induces FLS apoptosis and promotes
chondrogenic differentiation. Furthermore, the current study
demonstrated that the apoptosis and chondrogenesis induced by
co-culture of FLS with UC-MSCs may be regulated via changes to the
expression of pro/anti-apoptotic proteins and GDF-5.
RA is a heterogeneous autoimmune disease that not
only causes progressive joint deterioration, but also causes damage
to multiple organs and tissues (2). Although the pathogenic mechanisms
that mediate RA remain to be elucidated, a hallmark of RA pathology
is intra-articular inflammation. It has previously been well
demonstrated that various active cytokines and chemokines,
including TNF-α, IL-1β, IL-6 and C-X-C motif chemokine ligand
8/IL-8, are abundant in the arthritic synovium and serum of
patients with RA (23), which may
impact disease progression leading to articular deterioration and
the co-morbidities of RA (24,25).
Furthermore, FLS, the resident cells of synovial joints, are
associated with the formation of pannus and stimulate inflammatory
responses (26). The interaction
between inflammatory/immune cell infiltration and FLS is
responsible for the progression of joint damage progression and
immune activation. Thus, in addition to inhibiting inflammatory
responses, targeting FLS may be another important treatment
strategy for RA (27).
Targeted therapies have been previously developed
and achieved substantial success. For example, TNF-α competitive
inhibitor, TNF-α monoclonal antibodies and B-cell-depleting
therapies have been used to treat patients with RA (28,29).
However, these approaches are usually expensive and have potential
side-effects (30). Additionally,
traditional medications cannot repair joint damage (31), and most patients develop clinical
relapse and disease progression. Thus, it is critical to develop
novel and effective therapeutic approaches to improve the clinical
outcomes. MSCs have previously been demonstrated to be effective
for the treatment of various diseases, including autoimmune
arthritis (32), due to their
self-regenerating, differentiation and immunosuppressive
capability. MSCs are also present in the synovium where they are
thought to maintain tissues and contribute to repair processes
(33). However, MSCs from patients
with RA exhibit lower clonogenic potential and proliferative
capability compared with normal MSCs (34), making allogenic MSCs accessible for
clinical trials. Furthermore, although bone marrow has been
considered as the main source of MSCs, BM-MSCs are not always a
viable option for clinical use. Compared with BM-MSCs, UC-MSCs have
higher proliferative effectiveness, more robust differentiation
ability, and lower risks of contamination and immunogenicity
(18). Thus, UC-MSCs demonstrate
greater therapeutic potential for the treatment of a number of
diseases, compared with BM-MSCs. However, the effect of co-culture
of FLS with UC-MSCs on RA has not been widely investigated.
The present study isolated and cultured FLS and
UC-MSCs. While the stroma of the synovium includes FLS and MSCs,
their association is poorly understood. Therefore, the phenotype of
UC-MSCs was subsequently identified. The data of the current study
demonstrated that UC-MSCs had been successfully isolated and
expanded. The cells were negative for CD14, CD34 and CD45, but
positive for CD105, CD29, CD44 and CD166, demonstrating that
UC-MSCs exhibited a fibroblast-like morphology. FLS and MSCs may be
from the same lineage but at different functional stages. Following
identification of the UC-MSC phenotype, FLS and UC-MSCs were
co-cultured to observe the effect on the expression of
pro-inflammatory cytokines and chemokines, and on apoptosis and
chondrogenesis. The results of the present study demonstrated that
co-culture of FLS with UC-MSCs significantly reduces the expression
of IL-1β, IL-6 and CCL-2. Among the pro-inflammatory cytokines,
IL-1β has previously been reported strongly inhibit tissue repair
during RA (35). IL-6 and CCL-2
are also thought to be important in the pathology of RA.
Additionally, the current study demonstrated that co-culture of FLS
with UC-MSCs significantly increased FLS apoptosis and promoted
chondrogenesis. Furthermore, the mechanisms that mediated the
increases apoptosis and chondrogenesis were explored. The results
demonstrated that the levels of anti-apoptotic protein level (Bcl-2
and p-Akt) were significantly decreased, whereas, pro-apoptotic
proteins (p53 and Bax) were significantly increased by co-culture
of FLS with UC-MSCs, suggesting that the increased apoptosis may be
induced via regulation of the expression of pro/anti-apoptotic
proteins. The present study also demonstrated that the protein
expression level of GDF-5 was significantly increased by co-culture
of FLS with UC-MSCs. GDF-5, a member of bone morphogenetic protein
and transforming growth factor-β families, is expressed by
fibroblasts, articular cartilage chondrocytes and odontoblasts
(36–38). It was previously reported that
GDF-5 aggregates the mesenchyme, increases glycosaminoglycan
synthesis, promotes chondrogenic differentiation in vitro,
and induces cartilage and bone formation in vivo (37,39).
A previous report suggested that GDF-5 is present in the synovium
membrane and cartilage of patients with RA, and is actively
involved in the regulation of cartilage maintenance and repair
(40). The present study
demonstrated that GDF-5 may be involved in chondrogenesis induced
by co-culture of FLS with UC-MSCs.
In conclusion, co-culture of FLS with UC-MSCs exerts
an inhibitory effect on the expression of pro-inflammatory
cytokines and chemokines, induces FLS apoptosis and promotes
chondrogenesis. The results of the current study suggest that
co-culture of FLS with UC-MSCs may be clinically useful and
potentially important for the treatment of RA.
References
|
1
|
Scott DL, Wolfe F and Huizinga TW:
Rheumatoid arthritis. Lancet. 376:1094–1108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McInnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. New Engl J Med. 365:2205–2219. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smolen JS, Emery P, Fleischmann R, van
Vollenhoven RF, Pavelka K, Durez P, Guérette B, Kupper H, Redden L,
Arora V and Kavanaugh A: Adjustment of therapy in rheumatoid
arthritis on the basis of achievement of stable low disease
activity with adalimumab plus methotrexate or methotrexate alone:
The randomised controlled OPTIMA trial. Lancet. 383:321–332. 2014.
View Article : Google Scholar
|
|
4
|
Sangha O: Epidemiology of rheumatic
diseases. Rheumatology (Oxford). 39(Suppl 2): 3–12. 2000.
View Article : Google Scholar
|
|
5
|
Li R, Sun J, Ren LM, Wang HY, Liu WH,
Zhang XW, Chen S, Mu R, He J, Zhao Y, et al: Epidemiology of eight
common rheumatic diseases in China: A large-scale cross-sectional
survey in Beijing. Rheumatology (Oxford). 51:721–729. 2012.
View Article : Google Scholar
|
|
6
|
Cross M, Smith E, Hoy D, Carmona L, Wolfe
F, Vos T, Williams B, Gabriel S, Lassere M, Johns N, et al: The
global burden of rheumatoid arthritis: Estimates from the global
burden of disease 2010 study. Ann Rheum Dis. 73:1316–1322. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uhlig T, Moe RH and Kvien TK: The burden
of disease in rheumatoid arthritis. Pharmacoeconomics. 32:841–851.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hyrich KL, Symmons DP, Watson KD and
Silman AJ; British Society for Rheumatology Biologics Register:
Comparison of the response to infliximab or etanercept monotherapy
with the response to cotherapy with methotrexate or another
disease-modifying anti-rheumatic drug in patients with rheumatoid
arthritis: Results from the British Society for Rheumatology
Biologics Register. Arthritis Rheum. 54:1786–1794. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Listing J, Strangfeld A, Rau R, Kekow J,
Gromnica-Ihle E, Klopsch T, Demary W, Burmester GR and Zink A:
Clinical and functional remission: Even though biologics are
superior to conventional DMARDs overall success rates remain
low–results from RABBIT, the German biologics register. Arthritis
Res Ther. 8:R662006. View
Article : Google Scholar
|
|
10
|
Gonzalez-Rey E, Gonzalez MA, Varela N,
O'Valle F, Hernandez-Cortes P, Rico L, Büscher D and Delgado M:
Human adipose-derived mesenchymal stem cells reduce inflammatory
and T cell responses and induce regulatory T cells in vitro in
rheumatoid arthritis. Ann Rheum Dis. 69:241–248. 2010. View Article : Google Scholar
|
|
11
|
Sun L, Akiyama K, Zhang H, Yamaza T, Hou
Y, Zhao S, Xu T, Le A and Shi S: Mesenchymal stem cell
transplantation reverses multiorgan dysfunction in systemic lupus
erythematosus mice and humans. Stem Cells. 27:1421–1432. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Bari C: Are mesenchymal stem cells in
rheumatoid arthritis the good or bad guys? Arthritis Res Ther.
17:1132015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papadopoulou A, Yiangou M, Athanasiou E,
Zogas N, Kaloyannidis P, Batsis I, Fassas A, Anagnostopoulos A and
Yannaki E: Mesenchymal stem cells are conditionally therapeutic in
preclinical models of rheumatoid arthritis. Ann Rheum Dis.
71:1733–1740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stolzing A, Jones E, McGonagle D and Scutt
A: Age-related changes in human bone marrow-derived mesenchymal
stem cells: Consequences for cell therapies. Mech Ageing Dev.
129:163–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baksh D, Yao R and Tuan RS: Comparison of
proliferative and multilineage differentiation potential of human
mesenchymal stem cells derived from umbilical cord and bone marrow.
Stem Cells. 25:1384–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weiss ML, Anderson C, Medicetty S,
Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D and McIntosh KR:
Immune properties of human umbilical cord Wharton's jelly-derived
cells. Stem Cells. 26:2865–2874. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Mu R, Wang S, Long L, Liu X, Li R,
Sun J, Guo J, Zhang X, Guo J, et al: Therapeutic potential of human
umbilical cord mesenchymal stem cells in the treatment of
rheumatoid arthritis. Arthritis Res Ther. 12:R2102010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X,
Gong W, Han ZB, Xu ZS, Lu YX, Liu D, et al: Isolation and
characterization of human umbilical cord mesenchymal stem cells
with hematopoiesis-supportive function and other potentials.
Haematologica. 91:1017–1026. 2006.PubMed/NCBI
|
|
19
|
Cao Y, Sun Z, Liao L, Meng Y, Han Q and
Zhao RC: Human adipose tissue-derived stem cells differentiate into
endothelial cells in vitro and improve postnatal neovascularization
in vivo. Biochem Biophys Res Commun. 332:370–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arnett FC, Edworthy SM, Bloch DA, McShane
DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS,
et al: The American Rheumatism Association 1987 revised criteria
for the classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho CS, Cho ML, Min SY, Kim WU, Min DJ,
Lee SS, Park SH, Choe J and Kim HY: CD40 engagement on synovial
fibroblast up-regulates production of vascular endothelial growth
factor. J Immunol. 164:5055–5061. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Moelants EA, Mortier A, Van Damme J and
Proost P: Regulation of TNF-α with a focus on rheumatoid arthritis.
Immunol Cell Biol. 91:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brennan FM and McInnes IB: Evidence that
cytokines play a role in rheumatoid arthritis. J Clin Invest.
118:3537–3545. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kokkonen H, Söderström I, Rocklöv J,
Hallmans G, Lejon K and Rantapää Dahlqvist S: Up-regulation of
cytokines and chemokines predates the onset of rheumatoid
arthritis. Arthritis Rheum. 62:383–391. 2010.PubMed/NCBI
|
|
26
|
Kasperkovitz PV, Timmer TC, Smeets TJ,
Verbeet NL, Tak PP, van Baarsen LG, Baltus B, Huizinga TW,
Pieterman E, Fero M, et al: Fibroblast-like synoviocytes derived
from patients with rheumatoid arthritis show the imprint of
synovial tissue heterogeneity: Evidence of a link between an
increased myofibroblast-like phenotype and high-inflammation
synovitis. Arthritis Rheum. 52:430–441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noss EH and Brenner MB: The role and
therapeutic implications of fibroblast-like synoviocytes in
inflammation and cartilage erosion in rheumatoid arthritis. Immunol
Rev. 223:252–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dass S, Vital EM and Emery P: Rituximab:
Novel B-cell depletion therapy for the treatment of rheumatoid
arthritis. Opin Pharmacother. 7:2559–2570. 2006. View Article : Google Scholar
|
|
29
|
Colmegna I, Ohata BR and Menard HA:
Current understanding of rheumatoid arthritis therapy. Clin
Pharmacol Ther. 91:607–620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giles JT, Bartlett SJ, Gelber AC, Nanda S,
Fontaine K, Ruffing V and Bathon JM: Tumor necrosis factor
inhibitor therapy and risk of serious postoperative orthopedic
infection in rheumatoid arthritis. Arthritis Rheum. 55:333–337.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moreland LW, Schiff MH, Baumgartner SW,
Tindall EA, Fleischmann RM, Bulpitt KJ, Weaver AL, Keystone EC,
Furst DE, Mease PJ, et al: Etanercept therapy in rheumatoid
arthritis. A randomized, controlled trial. Ann Intern Med.
130:478–486. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Augello A, Tasso R, Negrini SM, Cancedda R
and Pennesi G: Cell therapy using allogeneic bone marrow
mesenchymal stem cells prevents tissue damage in collagen-induced
arthritis. Arthritis Rheum. 56:1175–1186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Bari C, Dell'Accio F, Karystinou A,
Guillot PV, Fisk NM, Jones EA, McGonagle D, Khan IM, Archer CW,
Mitsiadis TA, et al: A biomarker-based mathematical model to
predict bone-forming potency of human synovial and periosteal
mesenchymal stem cells. Arthritis Rheum. 58:240–250. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kastrinaki MC, Sidiropoulos P, Roche S,
Ringe J, Lehmann S, Kritikos H, Vlahava VM, Delorme B, Eliopoulos
GD, Jorgensen C, et al: Functional, molecular and proteomic
characterisation of bone marrow mesenchymal stem cells in
rheumatoid arthritis. Ann Rheum Dis. 67:741–749. 2008. View Article : Google Scholar
|
|
35
|
Catterall JB, Carrère S, Koshy PJ, Degnan
BA, Shingleton WD, Brinckerhoff CE, Rutter J, Cawston TE and Rowan
AD: Synergistic induction of matrix metalloproteinase 1 by
interleukin-1alpha and oncostatin M in human chondrocytes involves
signal transducer and activator of transcription and activator
protein 1 transcription factors via a novel mechanism. Arthritis
Rheum. 44:2296–2310. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hötten G, Neidhardt H, Jacobowsky B and
Pohl J: Cloning and expression of recombinant human
growth/differentiation factor 5. Biochem Biophys Res Commun.
204:646–652. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bobacz K, Gruber R, Soleiman A, Graninger
WB, Luyten FP and Erlacher L: Cartilage-derived morphogenetic
protein-1 and-2 are endogenously expressed in healthy and
osteoarthritic human articular chondrocytes and stimulate matrix
synthesis. Osteoarthritis Cartilage. 10:394–401. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morotome Y, Goseki-Sone M, Ishikawa I and
Oida S: Gene expression of growth and differentiation factors-5,
-6, and -7 in developing bovine tooth at the root forming stage.
Biochem Biophys Res Commun. 244:85–90. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hötten GC, Matsumoto T, Kimura M, Bechtold
RF, Kron R, Ohara T, Tanaka H, Satoh Y, Okazaki M, Shirai T, et al:
Recombinant human growth/differentiation factor 5 stimulates
mesenchyme aggregation and chondrogenesis responsible for the
skeletal development of limbs. Growth Factors. 13:65–74. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu FL, Lin LH, Sytwu HK and Chang DM:
GDF-5 is suppressed by IL-1beta and enhances TGF-beta3-mediated
chondrogenic differentiation in human rheumatoid fibroblast-like
synoviocytes. Exp Mol Pathol. 88:163–170. 2010. View Article : Google Scholar
|