Introduction
Intercellular junctions include gap junctions,
adhering junctions and tight junctions. Gap junctions chemically
and electrically link adjacent cells. Tight junctions build the
intercellular barrier. Together, these junctions modulate
intracellular and intercellular signaling and transport (1). These junctions perform a number of
roles, including acting as barriers against the extracellular
environment, regulating cellular permeability and polarity, and
serving as intermediates/transducers in cell signaling cascades
(2).
Tight junction proteins are commonly classified into
three types: Transmembrane, cytoskeletal and cytoplasmic plaque
proteins (3). Claudin (CLDN),
occludin (OCLN) and junction adhesion molecule A (JAM-A) are
transmembrane proteins, whereas zona occludens 1 (ZO-1) is a
cytoplasmic plaque protein.
CLDNs are integral transmembrane proteins (4), containing four transmembrane domains
and two extracellular loops. The first extracellular loop is long
and influences paracellular charge selectivity. The second
extracellular loop is shorter and facilitates CLDN-CLDN
interactions (5,6). CLDNs contribute to the polarity of
epithelial cells and regulate the paracellular selective
permeability of ions (7). The
expression pattern and function of each CLDN varies depending on
the organ (8). OCLN is a primary
transmembrane protein consisting of four transmembrane domains, two
extracellular loops and three cytoplasmic domains (9). The unique charged structures of the
extracellular loops may enable cells to attach to each other
(10). OCLN acts as a paracellular
barrier, maintains cell surface polarity through interaction with
ZO-1 and regulates the permeability of endothelial cells (11,12).
Expression of this protein has been reported in numerous organs and
tissues, including the brain, testis, epididymis, kidney, liver,
lung, stomach, duodenum, ileum, colon, skin and blood vessels
(13,14). JAM-A is an integral transmembrane
protein with numerous functions, including intercellular junction
assembly, paracellular barrier formation, promotion of leukocyte
migration, platelet activation and angiogenesis (15). ZO-1 interacts directly with
transmembrane factors including CLDNs, OCLN and JAM-A, and is known
to organize and regulate the structure of tight junctions (16). In addition, ZO-1 shuttles between
the nucleus and plasma membrane (17), modulates paracellular permeability
and is involved in the control of gene expression (18). ZO-1 is expressed in numerous organ
types (19).
Tight junction proteins are physiologically
important and have been associated with numerous diseases.
Therefore, a number of investigations have been conducted to
examine the expression and regulation of tight junction proteins in
animals and humans. However, few studies have been performed using
dogs as the experimental model. In particular, comparisons of mRNA
and protein expression levels in canine organs remain to be
performed. Therefore, the aim of the present study was to measure
the expression and localization of CLDNs (CLDN1, 2, 4 and 5), OCLN,
JAM-A and ZO-1 in dogs. The organ-specific mRNA and protein
expression levels of these factors in canine duodenum, lung, liver
and kidney were measured using reverse transcription-polymerase
chain reaction (RT-PCR), RT-quantitative PCR (RT-qPCR) and western
blot analysis. Localization of proteins in the canine organs was
determined by immunohistochemistry.
Materials and methods
Experimental animals
A total of 3 female beagle dogs (age, 3 years; mean
weight, 9.5 kg; Central Lab Animal Inc., Seoul, Korea) were
sacrificed for the present study. The dogs were fed a commercial
diet (Natural Balance Korea, Inc., Suwon, Korea) and tap water and
were housed in stainless steel cages in a controlled environment
maintained with 12-h light/dark cycles (temperature, 23±2°C;
relative humidity, 50±10%; ventilation, 17±1 times/min). The dogs
were euthanized by administering 20–30 ml KCl (100 g KCl dissolved
in 1 l water) intravenously. A midline incision was made to collect
samples from the duodenum, lung, liver and kidney. The
Institutional Animal Care and Use Committee of Chungbuk National
University (Cheongju, Korea) approved all experimental
procedures.
Total RNA extraction, RT-PCR and
RT-qPCR
Organs were placed in TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
homogenized with ULTRA-TURRAX® (IKA-Works, Breisgau,
Germany), according to the manufacturer's instructions. RNA (1
μg) was reverse transcribed using the first strand Maloney
murine leukemia virus reverse transcriptase (Intron Biotechnology,
Inc., Seongnam, Korea) and random 9-mer primer (Takara Bio, Inc.,
Otsu, Japan) to synthesize complementary DNA (cDNA).
β-actin, a housekeeping control gene, served as a
loading control and an endogenous reference for normalization of
target gene expression levels for RT-PCR and RT-qPCR. The following
dog-specific primers were used: CLDN1, sense, 5′-AAG ACG ATG AGG
TGC AGA AG-3′ and antisense, 5′-GTG AAG AGA GCC TGA CCA AA-3′;
CLDN2, sense, 5′-TGA GAT GCA CTG TCT TCT GC-3′ and antisense,
5′-AGC TTC TCC GAT CTC GAA CT-3′; CLDN4, sense, 5′-TCA TGG TCG TCA
GCA TCA-3′ and antisense, 5′-AGT CCC GGA TGA TAT TGT TG-3′; CLDN5
sense, 5′-GGC CAT TGT GCA GAA GAA-3′ and antisense, 5′-TGA CTC AAC
AGT CTG TCC TCC T-3′; OCLN sense, 5′-AAG AAC TCT CTC GCC TGG AT-3′
and antisense, 5′-GAT GTG GGA CAA TTT GCT CT-3′; JAM-a, sense,
5′-CTT CGA TCC TGT GTC AGC TT-3′ and antisense, 5′-TCT ATA GGC GAA
CCA GAT GC-3′; ZO-1 sense, 5′-GGA GAG GTG TTT CGT GTT GT-3′ and
antisense, 5′-ACT GCT CAG CCC TGT TCT TA-3′; β-actin, sense, 5′-AAG
TCC AGC TTC TGT TTC CTC-3′ and antisense, 5′-GCA GTG ATC TCC TTC
TGC AT-3′.
For RT-PCR, genes were amplified in a 20 μl
PCR reaction containing 1 unit of i-StarTaq™ DNA polymerase (Intron
Biotechnology, Inc.), 1.5 mM MgCl2, 2 mM dNTP and 20
pmol primers. The cycling conditions were as follows: 30 cycles of
denaturation at 95°C for 30 sec, annealing at 58°C for 30 sec and
extension at 72°C for 30 sec. PCR products (10 μl) were
separated on a 2.3% agarose gel and stained with ethidium bromide.
Gel images were captured under UV illumination using a Gel Doc EQ
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
RT-qPCR was performed on 1 μl cDNA using
SYBR® (Takara Bio, Inc.) or TaqMan® (Applied
Biosystems; Thermo Fisher Scientific, Inc.) probe methods,
according to the manufacturer's instructions. The cycling
conditions were as follows: A total of 40 cycles of denaturation at
95°C for 15 sec, annealing at 58°C for 15 sec and extension at 72°C
for 30 sec. Data for each sample were analyzed by comparing
quantification cycle (Cq) values at constant
fluorescence intensity. The quantity of transcript was inversely
associated with the observed Cq, and for every two-fold
dilution of the transcript, the Cq was expected to
increase by one increment. Relative expression (R) was calculated
using the equation: R=2−ΔΔCq (20).
Western blotting
Organs were placed in 50 or 1,000 μl
Pro-prep™ Protein Extraction solution (Intron Biotechnology, Inc.)
depending on organ volume, and were then homogenized. Total protein
(60 μg) was loaded onto 5–12.5% sodium dodecyl
sulfate-polyacrylamide gels, electrophoresed, and transferred onto
polyvinylidene difluoride membranes (PerkinElmer, Inc., Waltham,
MA, USA). After blocking in 5% skim milk for 1 h, membranes were
subsequently incubated overnight at 4°C with the following primary
antibodies: Mouse anti-CLDN1 (dilution, 1:1,000; cat. no. 37-4900;
Invitrogen; Thermo Fisher Scientific, Inc.), mouse anti-CLDN2
(dilution, 1:1,000; cat. no. 32-5600; Invitrogen; Thermo Fisher
Scientific, Inc.), mouse anti-CLDN5 (dilution, 1:1,000; cat. no.
35-2500; Invitrogen; Thermo Fisher Scientific, Inc.), rabbit
anti-CLDN4 (dilution, 1:500; cat. no. 32-9400; Invitrogen; Thermo
Fisher Scientific, Inc.), rabbit anti-OCLN (dilution, 1:1,000; cat.
no. 71-1500; Invitrogen; Thermo Fisher Scientific, Inc.), rabbit
anti-ZO-1 (dilution, 1:1,000; cat. no. 40-2200; Invitrogen; Thermo
Fisher Scientific, Inc.), rabbit anti-JAM-A (dilution, 1:1,000;
cat. no. sc-25629; Santa Cruz Biotechnology Inc., Dallas, TX, USA)
and rabbit anti-β-actin (dilution, 1:1,000; cat. no. sc-47778;
Santa Cruz Biotechnology, Inc.), diluted in 5% bovine serum albumin
(BSA; cat. no. 0903; Amresco, LLC, Solon, OH, USA) solution
dissolved in phosphate-buffered saline (PBS). The horseradish
peroxidase-conjugated secondary antibodies, poly-clonal anti-mouse
(dilution, 1:2,000; cat. no. bs-0330R-HRP; Bioss, Woburn, MA, USA)
and polyclonal anti-rabbit (dilution, 1:2,000; cat. no. sc-2004;
Santa Cruz Biotechnology, Inc.), were diluted to 1:3,000 in 2.5%
skim milk dissolved in Tris-buffered saline containing Tween 20 and
were incubated for 2 h at room temperature. Protein bands were
visualized with an Enhanced Chemiluminescence reagent (Santa Cruz
Biotechnology, Inc.) and exposed to Biomax™ Light film (Kodak,
Rochester, NY, USA) for 1–5 min. Signal specificity was confirmed
by blotting without the primary antibody, and bands were normalized
to β-actin. Signal intensity for each band was measured using
ImageJ software (version 1.50e; National Institutes of Health,
Bethesda, MD, USA).
Immunohistochemistry
Organ-specific localization of tight junction
proteins was investigated by immunohistochemistry. After 1 week of
10% formalin fixation, tissue processing was performed using the
Tissue-Tek VIP 5 (Sakura Finetek USA, Inc., Torrance, CA, USA) to
embed tissues in paraffin. Embedded blocks were sectioned at 3.5
μm. Each slide was boiled in buffered citrate solution for
20 min to effect antigen retrieval and washed with TBS-T for 5 min.
To block endogenous peroxidase activity, slides were placed in 3%
hydrogen peroxide for 30 min at room temperature. To prevent
non-specific antibody binding, sections were incubated with 10%
goat serum (Vector Laboratories, Inc., Burlingame, CA, USA) in
phosphate-buffered saline for 1 h at room temperature. The slides
were incubated with the primary antibodies listed in the western
blotting section diluted 1:200 in 5% BSA dissolved in PBS,
overnight at room temperature in a moist chamber. Biotinylated
anti-mouse or anti-rabbit secondary antibodies (dilution, 1:400;
cat. nos. BA-9200 and BA-1000, respectively; Vector Laboratories,
Inc.) were added and incubated at 37°C for 2 h. Slides were washed
as described previously. Elite ABC kit (Vector Laboratories, Inc.)
was added to slides and incubated at 37°C for 2 h. Diaminobenzidine
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) was used as a
chromogen. Sections were counterstained with Harris hematoxylin
(Sigma-Aldrich; Merck Millipore).
Statistical analysis
The results of all experiments are presented as the
mean ± standard deviation, which was calculated using GraphPad
Prism software program, (version 4.0; GraphPad Software, Inc., La
Jolla, CA, USA).
Results
Organ-specific mRNA expression levels of
CLDN family members
Organ-specific mRNA expression levels of CLDN1, 2, 4
and 5 in duodenum, lung, liver and kidney tissues were analyzed by
RT-PCR and RT-qPCR. β-actin served as an internal control. The
expression patterns of CLDNs varied among the different organs.
CLDN1 was expressed in all organs examined with the greatest
expression levels in lung. Expression levels were relatively high
in the kidney and low in the liver and duodenum (Fig. 1A). CLDN2 expression levels were
high in the kidney, low in liver and undetectable in duodenum and
lung (Fig. 1B). CLDN4 was
expressed in all the examined organs except for the duodenum; the
greatest expression levels were observed in the kidney (Fig. 1C). CLDN5 mRNA was barely detected;
however, its expression levels were greatest in the kidney
(Fig. 1D).
 | Figure 1Organ-specific mRNA and protein
expression levels of CLDN1, 2, 4 and 5 in the canine duodenum,
lung, liver and kidney. mRNA expression levels of (A) CLDN1 were
greatest in the lung and kidney, whereas (B) CLDN2 and (C) CLDN4
mRNA was primarily expressed in kidney. (D) CLDN5 mRNA expression
levels were low. (E) CLDN1 protein expression levels were high in
the kidney, low in the lung and undetectable in duodenum and liver.
(F) CLDN2, (G) CLDN4 and (H) CLDN5 protein expression was detected
only in the kidney. mRNA and protein expression were normalized to
β-actin. Data are presented as the mean ± standard deviation. CLDN,
claudin; ACTB, β-actin; D, duodenum; Lu, lung; Li, liver; K,
kidney. |
Organ-specific protein expression levels
and localization of CLDN family members
Organ-specific protein expression levels of CLDN1,
2, 4 and 5 were determined in the duodenum, lung, liver and kidney
samples obtained from all three dogs by western blotting (Fig. 1E–H). β-actin served as an internal
control. Greater protein expression levels of CLDN1 were detected
in the kidney compared with the lung; however, CLDN1 protein was
not detected in the duodenum or liver. High protein expression
levels of CLDN2, 4 and 5 were detected in the kidney; however,
these proteins were not detected in other organs, suggesting that
CLDN family members may have tissue-specific roles in the
kidney.
Localization of CLDN1, 2, 4 and 5 in the duodenum,
lung, liver and kidney was evaluated by immunohistochemical
analysis. CLDN1, 2, 4 and 5 was detected in the kidney tissues, but
not within the duodenum, lung or liver tissues (Fig. 2). Within the kidney the
localization sites of all CLDNs were identical, with expression
detected in the renal distal tubules.
Organ-specific mRNA expression levels of
OCLN, JAM-A and ZO-1
RT-PCR and qPCR results demonstrated that OCLN mRNA
expression levels were greatest in the duodenum compared with the
other organs. OCLN expression levels were moderate in the lung and
kidney and low in the liver (Fig.
3A). JAM-A mRNA expression levels were greatest in the duodenum
followed (in descending order) by the lung, kidney and liver
(Fig. 3B). ZO-1 mRNA expression
levels were greater in the duodenum compared with the other organs.
As for OCLN and JAM-A, ZO-1 expression levels were greatest in the
duodenum followed (in descending order) by the lung, kidney and
liver (Fig. 3C).
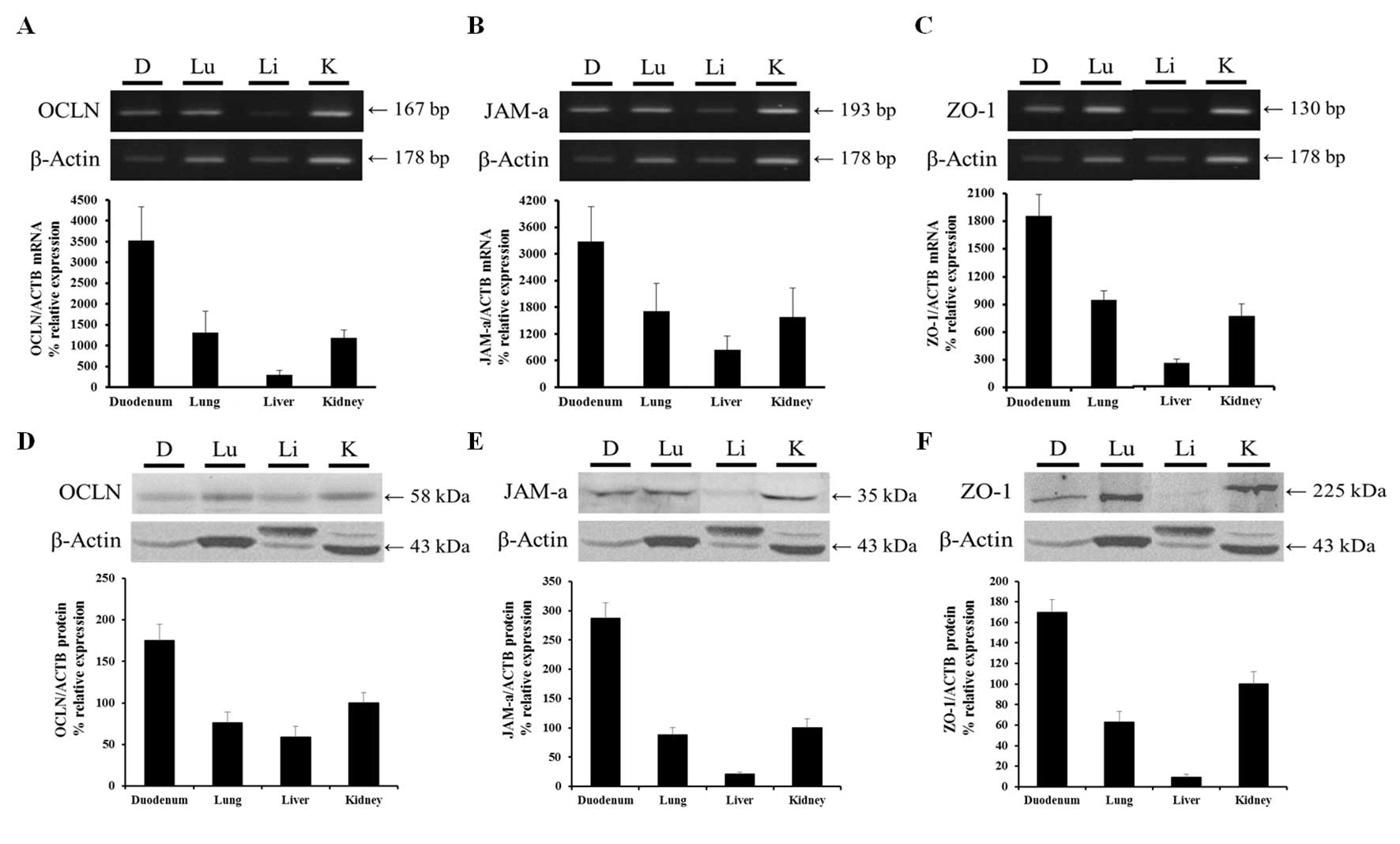 | Figure 3Organ-specific mRNA and protein
expression levels of OCLN, JAM-A and ZO-1 in canine duodenum, lung,
liver and kidney. mRNA expression levels of (A) OCLN, (B) JAM-A and
(C) ZO-1 were greatest in the duodenum, moderate in the lung and
kidney, and low in the liver. Protein expression levels of (D)
OCLN, (E) JAM-A and (F) ZO-1 followed a similar pattern to mRNA
expression levels. mRNA and protein expression were normalized to
β-actin. Data are presented as the mean ± standard deviation. OCLN,
occludin; JAM-A, junction adhesion molecule A; ZO-1, zona occludens
1; ACTB, β-actin; D, duodenum; Lu, lung; Li, liver; K, kidney. |
Organ-specific protein expression levels
of OCLN, JAM-A and ZO-1
Western blotting identified that OCLN was expressed
in all the examined organs, with a pattern of expression similar to
that of mRNA. Protein expression levels were greatest in the
duodenum followed (in descending order) by the kidney, lung and
liver (Fig. 3D). JAM-A protein was
expressed in all the examined organs with the greatest levels
observed in the duodenum. The expression levels in the kidney and
lung were similar and relatively high compared with the liver,
which were in agreement with the JAM-A mRNA expression levels
(Fig. 3E). ZO-1 protein was
expressed in all the examined organs, and the pattern of protein
expression was similar to that of mRNA. The ZO-1 protein expression
levels were greatest in the duodenum, moderate in the kidney and
lung and low in the liver (Fig.
3F).
Organ-specific localization of OCLN,
JAM-A and ZO-1
OCLN-specific immunohistochemical staining was
observed in all the examined organs. This protein was observed in
intestinal villi of the duodenum, bronchiolar epithelium of the
lung, connective tissue stroma of the hepatocytes and renal distal
tubules (Fig. 4A). JAM-A
immunohistochemical staining was observed in all the examined
organs, in intestinal villi of the duodenum, alveolar walls of the
lung, hepatocytes and in renal distal tubules (Fig. 4B). Immunohistochemistry indicated
that ZO-1 was localized in muscularis mucosae and submucosal glands
of the duodenum, alveolar walls of the lung, hepatocytes, and
distal tubules and glomeruli of the kidney (Fig. 4C).
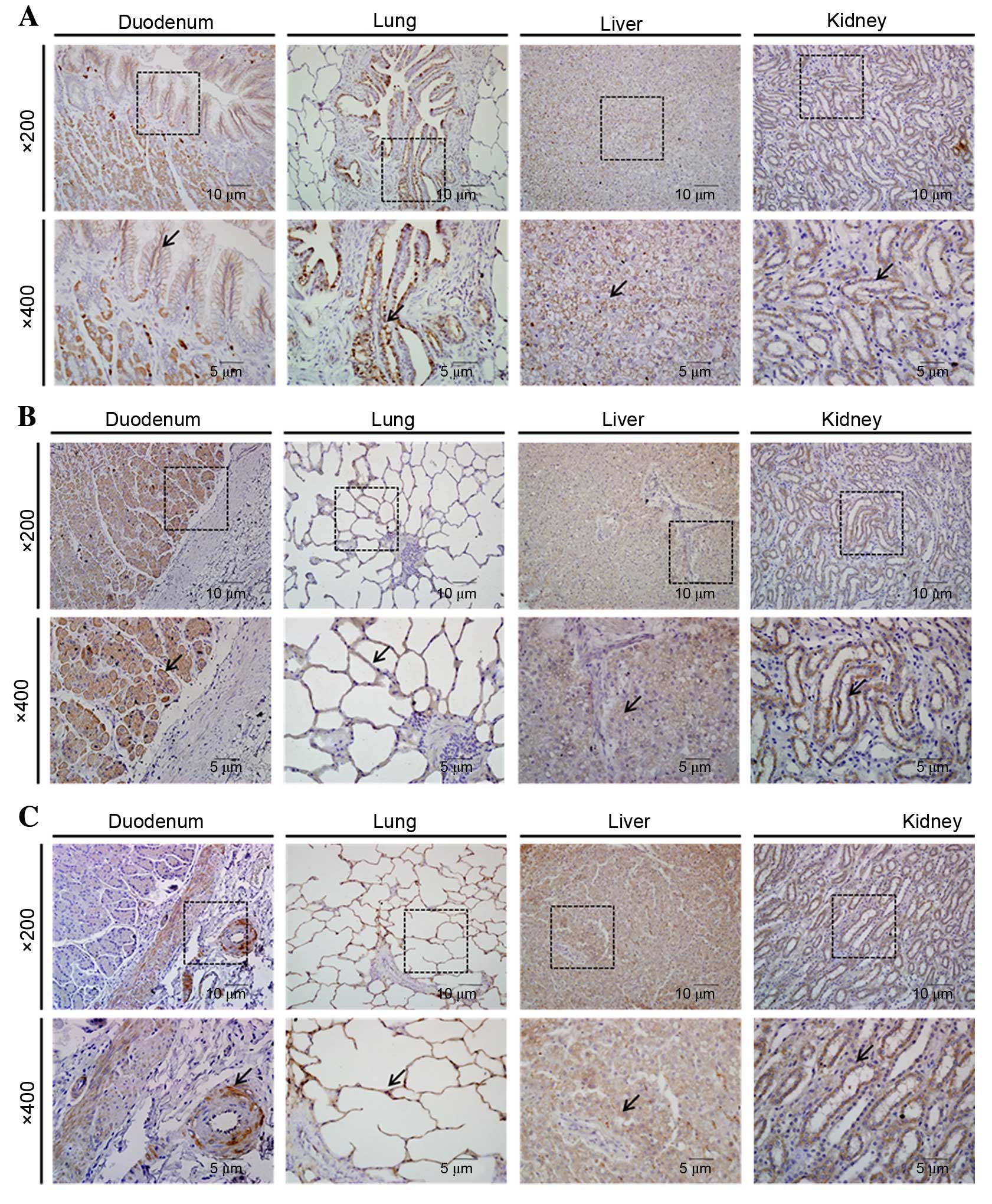 | Figure 4Organ-specific localization of OCLN,
JAM-A and ZO-1 in canine duodenum, lung, liver, and kidney, as
detected by immunohistochemistry. (A) OCLN, (B) JAM-A and (C) ZO-1
immunostaining was detected in all organs examined. Boxes in the
upper panels (magnification, ×200) are areas magnified in the lower
panels (magnification, ×400). Arrows indicate positive staining.
OCLN, occludin; JAM-A, junction adhesion molecule A; ZO-1, zona
occludens 1. |
Discussion
The present study examined the organ-specific
expression and localization of transmembrane proteins (CLDNs, OCLN
and JAM-A) and a cytoplasmic plaque protein (ZO-1) in canine
organs. Organ-specific variations in transelectrical resistance and
paracellular ionic selectivity among epithelia have been
demonstrated to be determined by the differential expression and
distribution of CLDNs (21). The
results of the present study demonstrated that CLDN mRNA and
protein were highly expressed in the kidney and commonly localized
to the renal distal tubules. This expression and distribution
pattern in canine organs differs from those in other animals
(22) and humans (23). Compared with humans, low expression
levels of CLDN 1, 2, 4 and 5 have been observed in the duodenum,
lung and liver of mice (22). The
gatekeeper function of CLDNs determines the renal epithelial tight
junction paracellular permeability; therefore, CLDNs are associated
with renal physiology and pathology. Abnormal expression of CLDNs
induces mineral imbalances, impedes recovery from ischemic acute
renal injury and disrupts acid/base homeostasis in humans and rats
(24). Based on data from the
literature and the results of the present study, CLDNs may be
critical for maintaining renal physiology in dogs.
In contrast to CLDNs, mRNA and protein expression of
OCLN, JAM-A and ZO-1 were detected in all the examined organs.
Expression patterns of three factors were similar. As for CLDNs,
the expression and distribution patterns of OCLN, JAM-A and ZO-1
were different compared with other species (25). For instance, high expression levels
of ZO-1 and JAM-A have been observed in the livers of mice
(25), whereas, in the present
study, low expression levels of these factors were observed in
canine livers when compared with other organs. Expression of OCLN,
JAM-A and ZO-1 was greater in canine duodenum compared with other
organs, and was localized to the intestinal villi and submucosal
gland. Numerous groups have demonstrated that tight junction
molecules act as epithelial barriers and regulators of inflammatory
responses in the intestine. Tight junctions regulate mucosal
permeability and enteric pathogens disrupt intestinal physiological
functions in various intestinal inflammatory diseases, including
inflammatory bowel disease and Crohn's disease (26). As these diseases are observed in
dogs, tight junctions may be associated with canine intestinal
physiology and pathology. In support of this, a previous study
demonstrated that CLDN2 expression in the superficial colonic
epithelium is upregulated in dogs with idiopathic colitis (27). OCLN, JAM-A and ZO-1 mRNA and
protein expression was observed in the lung, where tight junctions
form the alveolar membrane barrier. In addition, tight junctions of
the alveolar epithelial and capillary endothelial cells maintain
alveolar wall permeability (28).
In a previous study, reduction of OCLN expression induced by acute
lung injury led to reduced alveolar membrane integrity and elevated
alveolar membrane permeability (25). Furthermore, tight junctions have
been revealed to help prevent microbial invasion and interact with
dendritic cells in pulmonary epithelia (29). Renal expression of OCLN, JAM-A and
ZO-1 was localized to the distal tubules and glomeruli. In the
kidney, tight junctions of the renal epithelia are widely
distributed in renal tubules, and have a transepithelial potential
difference and various morphological and functional
characteristics. In general, tight junctions regulate the transfer
of various solutes and water, and inhibit the interaction between
apical and basolateral plasmolemmal domains. It has been determined
that ZO-1 acts as a coxsackievirus and adenovirus receptor in
glomerular podocytes, and serves as a barrier that controls the
movement of macromolecules and ions (30). Thus, tight junctions are important
in viral infection of the kidney and renal disease development. The
mRNA and protein expression levels of OCLN, JAM-A and ZO-1 in the
liver were observed to be relatively low compared with the other
examined organs. Hepatocytes closely interact with each other
through intercellular junctions to form hepatocytic plates and
maintain liver function. Tight junctions of hepatocytes tightly
encircle the bile canaliculi, and the cells form a blood-biliary
barrier and maintain epithelial polarity (24). In addition, tight junction
molecules bind to hepatitis C virus to mediate viral infection and
fatty liver-associated disease. In addition, dysfunction and
downregulation of tight junctions is associated with primary and
metastatic tumor development (31).
In conclusion, the results of the present study
demonstrated that the canine tight junction proteins, CLDNs, OCLN,
JAM-A and ZO-1 were expressed and regulated in a tissue-specific
manner. The data suggest that these proteins may perform unique
physiological roles in tissues where they are highly expressed.
Therefore, these observations may serve as a basis for further
studies of tight junction proteins and their roles in canine
physiological or pathological conditions.
Acknowledgments
The present study was supported by the National
Research Foundation of Korea (grant no. NRF-2013R1A2A2A05004582)
and the Korea Foundation for the Advancement of Science &
Creativity, funded by the Korean government.
References
|
1
|
Green KJ, Getsios S, Troyanovsky S and
Godsel LM: Intercellular junction assembly, dynamics, and
homeostasis. Cold Spring Harb Perspect Biol. 2:a0001252010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin TA and Jiang WG: Tight junctions
and their role in cancer metastasis. Histol Histopathol.
16:1183–1195. 2001.PubMed/NCBI
|
|
3
|
Gumbiner BM: Cell adhesion: The molecular
basis of tissue architecture and morphogenesis. Cell. 84:345–357.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamura A, Yamazaki Y, Hayashi D, Suzuki K,
Sentani K, Yasui W and Tsukita S: Claudin-based paracellular proton
barrier in the stomach. Ann N Y Acad Sci. 1258:108–114. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
González-Mariscal L, Betanzos A, Nava P
and Jaramillo BE: Tight junction proteins. Prog Biophys Mol Biol.
81:1–44. 2003. View Article : Google Scholar
|
|
6
|
Findley MK and Koval M: Regulation and
roles for claudin-family tight junction proteins. IUBMB Life.
61:431–437. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Angelow S, Ahlstrom R and Yu AS: Biology
of claudins. Am J Physiol Renal Physiol. 295:F867–F876. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsukita S and Furuse M: The structure and
function of claudins, cell adhesion molecules at tight junctions.
Ann NY Acad Sci. 915:129–135. 2000. View Article : Google Scholar
|
|
9
|
Tsukita S and Furuse M: Occludin and
claudins in tight-junction strands: Leading or supporting players?
Trends Cell Biol. 9:268–273. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ando-Akatsuka Y, Saitou M, Hirase T, Kishi
M, Sakakibara A, Itoh M, Yonemura S, Furuse M and Tsukita S:
Interspecies diversity of the occludin sequence: cDNA cloning of
human, mouse, dog, and rat-kangaroo homologues. J Cell Biol.
133:43–47. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balda MS, Whitney JA, Flores C, González
S, Cereijido M and Matter K: Functional dissociation of
paracellular permeability and transepithelial electrical resistance
and disruption of the apical-basolateral intramembrane diffusion
barrier by expression of a mutant tight junction membrane protein.
J Cell Biol. 134:1031–1049. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McCarthy KM, Skare IB, Stankewich MC,
Furuse M, Tsukita S, Rogers RA, Lynch RD and Schneeberger EE:
Occludin is a functional component of the tight junction. J Cell
Sci. 109:2287–2298. 1996.PubMed/NCBI
|
|
13
|
Gye MC: Expression of occludin in canine
testis and epididymis. Reprod Domest Anim. 39:43–47. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kimura Y, Shiozaki H, Hirao M, Maeno Y,
Doki Y, Inoue M, Monden T, Ando-Akatsuka Y, Furuse M, Tsukita S and
Monden M: Expression of occludin, tight-junction-associated
protein, in human digestive tract. Am J Pathol. 151:45–54.
1997.PubMed/NCBI
|
|
15
|
Mandell KJ and Parkos CA: The JAM family
of proteins. Adv Drug Deliv Rev. 57:857–867. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niessen CM: Tight junctions/adherens
junctions: Basic structure and function. J Invest Dermatol.
127:2525–2532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Islas S, Vega J, Ponce L and
Gonzalez-Mariscal L: Nuclear localization of the tight junction
protein ZO-2 in epithelial cells. Exp Cell Res. 274:138–148. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Balda MS and Matter K: The tight junction
protein ZO-1 and an interacting transcription factor regulate
ErbB-2 expression. EMBO J. 19:2024–2033. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stevenson BR, Siliciano JD, Mooseker MS
and Goodenough DA: Identification of ZO-1: A high molecular weight
polypeptide associated with the tight junction (zonula occludens)
in a variety of epithelia. J Cell Biol. 103:755–766. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Ohta H, Yamaguchi T, Rajapakshage BK,
Murakami M, Sasaki N, Nakamura K, Hwang SJ, Yamasaki M and
Takiguchi M: Expression and subcellular localization of apical
junction proteins in canine duodenal and colonic mucosa. Am J Vet
Res. 72:1046–1051. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hwang I, Yang H, Kang HS, Ahn CH, Lee GS,
Hong EJ, An BS and Jeung EB: Spatial expression of claudin family
members in various organs of mice. Mol Med Rep. 9:1806–1812.
2014.PubMed/NCBI
|
|
23
|
Hewitt KJ, Agarwal R and Morin PJ: The
claudin gene family: Expression in normal and neoplastic tissues.
BMC Cancer. 6:1862006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balkovetz DF: Tight junction claudins and
the kidney in sickness and in health. Biochim Biophys Acta.
1788:858–863. 2009. View Article : Google Scholar
|
|
25
|
Hwang I, An BS, Yang H, Kang HS, Jung EM
and Jeung EB: Tissue-specific expression of occludin, zona
occludens-1, and junction adhesion molecule A in the duodenum,
ileum, colon, kidney, liver, lung, brain, and skeletal muscle of
C57BL mice. J Physiol Pharmacol. 64:11–18. 2013.PubMed/NCBI
|
|
26
|
Laukoetter MG, Nava P, Lee WY, Severson
EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E,
Campbell JA, et al: JAM-A regulates permeability and inflammation
in the intestine in vivo. J Exp Med. 204:3067–3076. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ridyard AE, Brown JK, Rhind SM, Else RW,
Simpson JW and Miller HR: Apical junction complex protein
expression in the canine colon: Differential expression of
claudin-2 in the colonic mucosa in dogs with idiopathic colitis. J
Histochem Cytochem. 55:1049–1058. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hartsock A and Nelson WJ: Adherens and
tight junctions: Structure, function and connections to the actin
cytoskeleton. Biochim Biophys Acta. 1778:660–669. 2008. View Article : Google Scholar
|
|
29
|
Lambrecht BN and Hammad H: Biology of lung
dendritic cells at the origin of asthma. Immunity. 31:412–424.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagai M, Yaoita E, Yoshida Y, Kuwano R,
Nameta M, Ohshiro K, Isome M, Fujinaka H, Suzuki S, Suzuki J, et
al: Coxsackievirus and adenovirus receptor, a tight junction
membrane protein, is expressed in glomerular podocytes in the
kidney. Lab Invest. 83:901–911. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee NP and Luk JM: Hepatic tight
junctions: From viral entry to cancer metastasis. World J
Gastroenterol. 16:289–295. 2010. View Article : Google Scholar : PubMed/NCBI
|


















