Introduction
Liraglutide is a synthetic analogue of glucagon-like
peptide-1 (GLP-1) that is commonly used in the management of type 2
diabetes (T2D) and obesity. Liraglutide shares 97% sequence
identity with native human GLP-1 and induces similar biological
effects, including enhanced glucose-dependent insulin secretion,
accelerated insulin β-cell proliferation and inhibition of β-cell
apoptosis (1). A recent study
demonstrated that liraglutide aids the prevention of T2D
complications, such as diabetic nephropathy, caused by exposure to
high glucose concentrations (2).
The plasma half-life of liraglutide is ~13 h, compared with the
1.5–2.1 min half-life of native GLP-1, increasing its
bioavailability compared with native GLP-1 (3). Liraglutide is also effective in
assisting with weight loss, as it delays gastric emptying, slows
carbohydrate absorption and increases satiety (3). Liraglutide was previously considered
to have minor side effects, and received marketing authorization
from medical agencies in Europe, Canada, Japan, and the USA,
however, the drug has now been associated with the development of
hypoglycemia, acute pancreatitis and thyroid carcinoma (4). Thus, the future clinical application
of liraglutide requires comprehensive consideration.
Bone formation is largely dependent on the
transformation (differentiation) of osteoblasts into mature
osteocytes, which make up >90% of all bone cells in the adult
skeleton (5). Osteoblastic
differentiation is a carefully orchestrated process, and is tightly
regulated by signaling pathways, including adenosine
monophosphate-activated protein kinase (AMPK)-mediated signaling.
AMPK stimulates the proliferation, mineralization and
differentiation of osteoblastic MC3T3-E1 cells, when activated by
the AMP analog 5-aminoimidazole-4-carboxamide ribonucleotide, the
adiponectin adipokine and the lipid-lowering agent, Bezafibrate
(6,7). However, AMPK activated by the
adipokine C1q/tumor necrosis factor-related protein 3, is reported
to repress the differentiation of osteoblastic MC3T3-E1 cells
(8). Additionally, Wei et
al (9) observed that glucose
uptake favors osteoblast differentiation by inhibiting the
functions of AMPK in vivo and in vitro. The reason
for the discrepancy of AMPK function on osteoblastic
differentiation in these studies remains unclear; however,
variations in downstream effectors activated by AMPK under
different conditions may be a contributing factor.
Liraglutide has recently been demonstrated to
prevent the osteoblastic differentiation of human vascular smooth
muscle cells, which is reported to slow the process of arterial
calcification (10,11). As arterial calcification is
considered to be similar to bone formation, this observation
indicated that liraglutide may be implicated in the osteoblastic
differentiation of osteoblasts. Furthermore, AMPK/mammalian target
of rapamycin (mTOR) signaling cascades are involved in the
mechanisms that mediate the antidiabetic effects of liraglutide
(12). Thus, it is hypothesized
that liraglutide is involved in osteoblastic differentiation by
modulating AMPK/mTOR signaling.
Alkaline phosphatase (Alp) is responsible for
hydrolyzing pyrophosphate in osteoblasts to generate inorganic
phosphate, an essential component of the mineralized matrix of
osteoblasts (13), while mature
osteoblasts are typically characterized by high osteocalcin (OC)
expression (5). OC, a structural
protein of bone, has a high affinity for hydroxyapatite and
regulates the generation of osteoblast mineralized matrix (14,15).
Due to the reciprocal association between Alp/OC expression and
osteoblastic differentiation (16), Alp and OC are commonly used as
osteoblastic differentiation markers. Mineralized matrix formation
in osteoblasts is an important process during osteoblastic
differentiation and is associated with the deposition of calcium
phosphate salts (14,17). Therefore, examination of the
calcium phosphate salt content in osteoblasts is another marker of
the extent of osteoblastic differentiation. Matrix mineralization
can be assessed via Alizarin red S staining, which detects both
microcrystalline and noncrystalline calcium phosphate salts in
osteoblasts (18).
The present study aimed to investigate the effects
of liraglutide treatment on osteoblastic differentiation of
MC3T3-E1 cells, a clonal osteogenic cell line that maintains
characteristics of osteoblasts, and is frequently used to
investigate osteoblastic differentiation and function in
vitro (19), using Alp, OC and
mineralized matrix formation as markers of osteoblastic
differentiation.
Materials and methods
Reagents and cell treatments
Compound C (AMPK inhibitor) and MHY1485 (mTOR
activator) were purchased from Calbiochem (EMD Millipore,
Billerica, MA, USA) and Selleck Chemicals (Shanghai, China),
respectively. Rabbit anti-Alp antibody (1:10,000; catalog no.
ab190931; Abcam, Cambridge, UK), mouse anti-OC antibody (1:400;
catalog no. sc-74495; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), rabbit anti-AMPK antibody (1:1,000; catalog no. ab32047;
Abcam), rabbit anti-phosphorylated (p)-AMPK antibody (Thr172;
1:1,000; catalog no. ab133448; Abcam), rabbit anti-mTOR antibody
(1:1,000; catalog no. 2972; CST Biological Reagents Co., Ltd.,
Shanghai, China), rabbit anti-p-mTOR antibody (Ser2448; 1:1,000;
catalog no. 2971; CST Biological Reagents Co., Ltd.), rabbit
anti-transforming growth factor-β (TGF-β) antibody (1:500; catalog
no. bs-0103R; BIOSS, Beijing, China) and mouse
anti-glyceraldehyde-3 phosphate dehydrogenase (GAPDH) antibody
(1:500; catalog no. sc-365062; Santa Cruz Biotechnology) were
purchased for western blotting and immunocytochemistry.
MC3T3-E1 cells, obtained from the American Type
Culture Collection (Manassas, VA, USA), were maintained in
Dulbecco's modified Eagle's medium (DMEM, Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine
serum (FBS, Hyclone; GE Healthcare Life Sciences), 100 U/ml
penicillin and 100 mg/ml streptomycin at 37°C in a humidified
atmosphere of 5% CO2. Having reached 70% confluence, the
culture medium was switched to commercial osteogenic
differentiation medium (catalog no. MUCMX-90021; Cyagen
Biotechnology Co., Ltd., Taicang, China). MC3T3-E1 cells were
cultured in the osteogenic differentiation medium for 14 days,
following by culture in DMEM supplemented with varying
concentrations of liraglutide (catalog no. HY-P0014; MedChem
Express, Shanghai, China) for a further 14 days. MC3T3-E1 cells
treated with 4 nM liraglutide were cultured in the presence or
absence of Compound C or MHY1485. MC3T3-E1 cells maintained in DMEM
for 28 days in the absence of any treatment were used as the
negative control (NC); cells cultured in commercial osteogenic
differentiation medium for 14 days and in DMEM without liraglutide
for an additional 14 days were used as the positive control
(PC).
Western blotting
Cells were lysed with NP-40 buffer (1% NP-40, 0.15 M
NaCl, 50 mM Tris, pH 8.0) containing phosphatase inhibitors
(okadaic acid; catalog no. ab120375; Abcam). Protein concentration
was measured using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology, Shanghai, China). Equal amounts of
protein (20 µg) were separated by sodium dodecyl
sulfate-polyacryl-amide gel electrophoresis on 12% gels and
transferred onto nitrocellulose membranes. After blocking with 5%
non-fat milk in Tris-buffered saline containing Tween (TBST) for 1
h, membranes were incubated with primary antibody in TBST overnight
at 4°C and subsequently incubated with anti-mouse (catalog no.
A9309; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) or
anti-rabbit (catalog no. ab97051; Abcam) horse radish
peroxidase-conjugated secondary antibodies at a 1:2,000 dilution
for 2 h at room temperature. Reactive proteins were detected using
Pierce Enhanced Chemiluminscent and SuperSignal™ Chemiluminescent
substrates (Thermo Fisher Scientific, Inc., Waltham, MA, USA). To
control for loading efficiency, the blots were stripped with Mild
Stripping Buffer (Abcam) at room temperature for 5–10 min and
reprobed with GAPDH antibody (1:600).
Immunocytochemistry (ICC)
The cells were fixed by 4% paraformaldehyde at room
temperature for 10 min and methanol at −20°C for 20 min. Normal
goat serum (10%; Hyclone; GE Healthcare Life Sciences) was added to
cells for 30 min to block nonspecific binding sites. The fixed
cells were immunostained with primary antibodies targeting Alp
(1:300) and OC (1:200) overnight at 4°C and the Alexa Fluor
488-conjugated goat anti-rabbit secondary antibody (1:500; catalog
no. SP-9000; ZSGB-BIO, Beijing, China) for 1 h at 37°C. Images were
acquired with a high-resolution CoolSNAP™ CCD camera (Photometrics
Inc., Tucson, AZ, USA) under the control of a computer using Leica
FW4000 software version 1.2 (Leica Microsystems, Ltd., Milton
Keynes, UK).
Mineralization assay
The extent of matrix mineralization of MC3T3-E1
cells were determined by Alizarin Red S staining. Cells were fixed
with 2% formaldehyde for 10 min at room temperature, followed by
exposure to 2% Alizarin Red S for 40 min. Cells were then washed
with phosphate-buffered saline to remove excess dye, and cells were
examined under a BA410 Optical Microscope plus Absorbance Analyzer
(Motic Medical Diagnostic Systems Co., Ltd., Xiamen, China).
Absorbance was measured at 550 nm. The mineralization values were
normalized to the relative value of the NC.
Statistical analysis
Data were analyzed using SPSS 12.0 software (SPSS,
Inc., Chicago, IL, USA). One-way analysis of variance followed by
Scheffe's post-hoc test was used for multiple comparisons
between each group. P<0.05 was considered to indicate a
statistically significant difference.
Results
Osteoblastic differentiation of MC3T3-E1
cells is induced by commercial osteogenic differentiation
medium
Commercial osteogenic differentiation medium was
used to induce the differentiation of osteoblastic MC3T3-E1 cells
towards mature osteocytes. The protein expression levels of two
crucial osteoblastic differentiation markers, Alp and OC, were
evaluated on days 0, 3, 5, 7, and 14 of incubation in osteogenic
differentiation medium by western blotting. Protein expression
levels of Alp and OC gradually increased during the time course,
with ~3- and 4-fold higher expression on day 14 compared with day
0, respectively (P=0.024 and P=0.027; Fig. 1), indicating a trend of
osteoblastic MC3T3-E1 cell differentiation to mature osteocytes
over this time period. In parallel with the osteoblastic
differentiation of MC3T3-E1 cells, p-AMPK expression levels
progressively decreased over the same period, with the lowest
expression levels at 14 days (P=0.036 vs. day 0). However, protein
expression levels of p-mTOR and TGF-β gradually increased over the
time course. Expression levels were significantly increased on day
14 compared with day 0, (P=0.039 and P=0.017, respectively). No
significant differences were observed in AMPK and mTOR expression
levels (Fig. 1). These data
suggested a close association between osteoblastic differentiation
of MC3T3-E1 cells and p-AMPK/p-mTOR/TGF-β expression levels.
 | Figure 1Relative protein expression of Alp,
OC, p-AMPK, p-mTOR and TGF-β during osteogenic differentiation.
Commercial osteogenic differentiation medium was used to induce
osteoblastic differentiation of MC3T3-E1 cells, and the relative
protein expression of indicated proteins was analyzed by western
blotting on days 0, 3, 5, 7 and 14. Quantification was relative to
GAPDH. Data are presented as the mean ± standard error (n=5).
*P<0.05 vs. day 0. Alp, alkaline phosphatase; OC,
osteocalcin; p-AMPK, phosphorylated AMPK; p-mTOR, phosphorylated
mTOR; TGF-β, transforming growth factor-β; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
Increased expression of Alp and OC on day 14
compared with day 0 was also observed by ICC (Fig. 2A). Alizarin red S staining was used
to evaluate mineral matrix formation in osteoblasts. Increased
intensity of Alizarin red S staining, as determined by
spectrophotometric readings, were observed over the time-course of
14 days (Fig. 2B). Notably,
optical intensity was significantly increased on day 14 compared
with day 0 (P=0.008). These data indicated an increased mineral
matrix formation over this time period. Osteoblastic
differentiation of MC3T3-E1 cells was, therefore, induced by the
commercial osteogenic differentiation medium.
Liraglutide dose-dependently attenuates
the osteoblastic differentiation of osteoblasts
MC3T3-E1 cells cultured in commercial osteogenic
differentiation medium for 14 days, were transferred into basal
culture medium and maintained for an additional 14 days in various
concentrations of liraglutide (0, 0.1, 0.5, 1, 2 and 4 nM). Protein
expression levels of Alp and OC were analyzed by western blotting,
revealing that liraglutide dose-dependently decreased protein
expression levels of Alp and OC compared with untreated PC cells;
treatment with 4 nm liraglutide resulted in ~1/3 of the protein
expression levels of both Alp and OC compared with untreated PC
cells (P=0.035 and P=0.038; Fig.
3A). In addition, matrix mineralization of osteoblasts was
attenuated by liraglutide in a dose-dependent manner, as determined
by Alizarin red S staining (Fig.
3B). Liraglutide at a concentration of 4 nM decreased the
absorbance to the lowest level of all treatment groups (P=0.009 vs.
PC group). These results indicated that liraglutide may inhibit
and/or reverse osteoblastic differentiation.
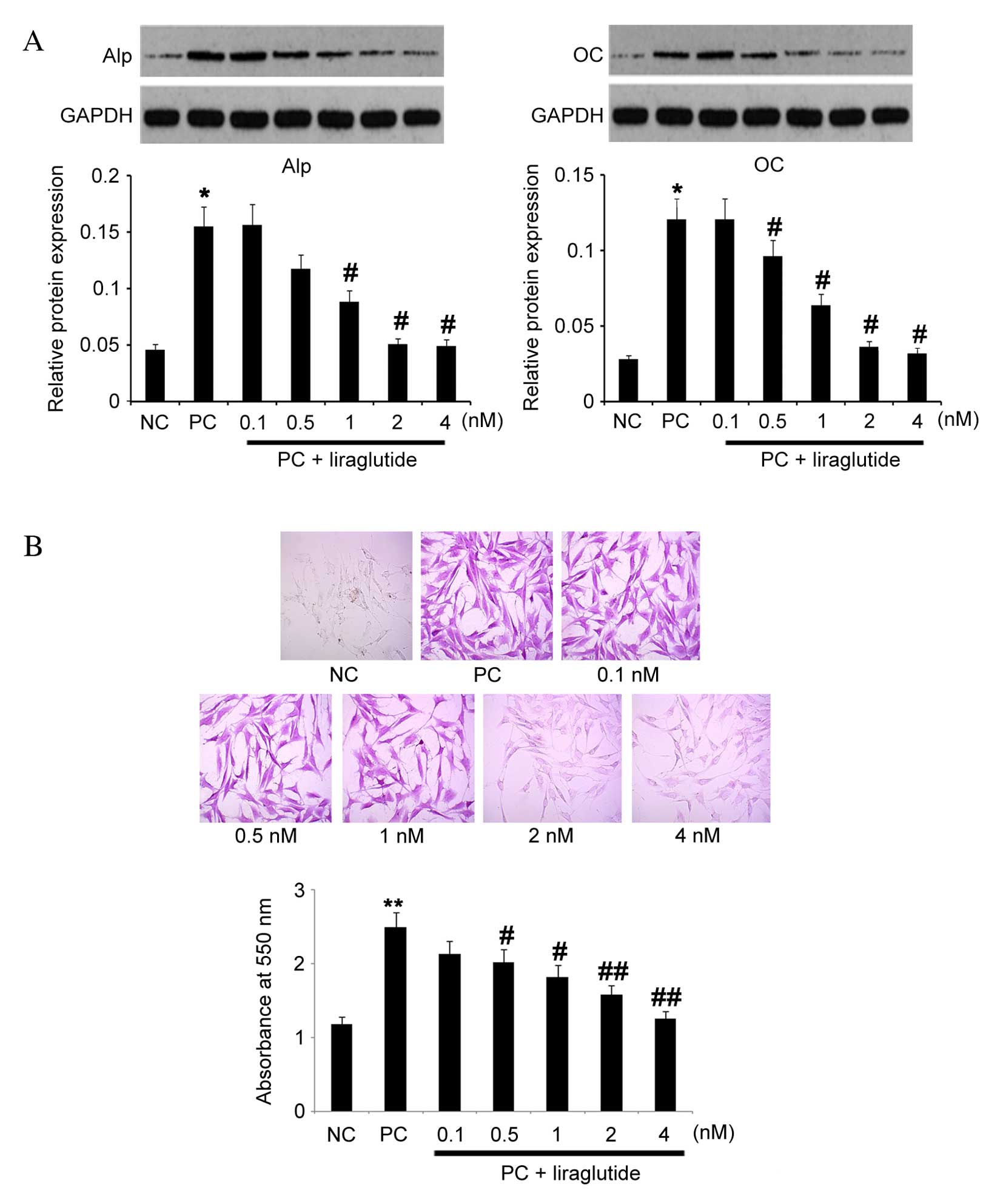 | Figure 3Effects of liraglutide treatment on
Alp and OC protein expression levels, and matrix mineralization.
Following culture in commercial osteogenic differentiation medium
for 14 days, MC3T3-E1 cells were cultured for an additional 14 days
in basal culture medium with various concentrations of liraglutide
(0, 0.1, 0.5, 1, 2 and 4 nM). MC3T3-E1 cells maintained in DMEM for
28 days with no treatment were used as the NC; cells cultured in
the commercial osteogenic differentiation medium for 14 days and in
DMEM medium without liraglutide for a further 14 days were used as
the PC. (A) Relative protein expression of Alp and OC was analyzed
by western blot and quantified relative to GAPDH (n=5). (B) Matrix
mineralization was determined by Alizarin Red S staining (n=10).
Magnification, ×200. Data are presented as the mean ± standard
error. *P<0.05, **P<0.05 vs. NC;
#P<0.05, ##P<0.01 vs. PC. Alp, alkaline
phosphatase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NC,
negative control; PC, positive control; OC, osteocalcin. |
AMPK/mTOR signaling is involved in the
inhibitory effect of liraglutide on osteoblastic
differentiation
MC3T3-E1 cells cultured in commercial osteogenic
differentiation medium for 14 days, followed by a further 14 days
culturing in basal culture medium with 4 nM liraglutide exhibited
significantly lower formation of mineralized matrix compared with
untreated PC cells (P=0.009; Fig.
4A) and significantly reduced protein expression levels of Alp
(P=0.035; Fig. 4B) and OC
(P=0.038; Fig. 4B). Liraglutide
treatment significantly increased p-AMPK protein expression levels
compared with untreated PC cells (P=0.041) whereas the levels of
p-mTOR and TGF-β were significantly reduced compared with PC
(P=0.041 and P=0.035, respectively). However, co-treatment of 4 nM
liraglutide and 10 µM Compound C, an inhibitor of AMPK,
prevented the liraglutide-induced increase in p-AMPK levels
compared with liraglutide treated cells, and restored the levels of
Alp, OC, p-mTOR and TGF-β (Fig.
4B), and matrix mineralization (Fig. 4A) to levels comparable with
positive control cells. Similarly, co-treatment with 4 nM
liraglutide and 1 µM MHY1485, an mTOR activator, resulted in
protein expression levels of Alp, OC, p-mTOR and TGF-β (Fig. 4B) and matrix mineralization
(Fig. 4A) comparable to those of
positive control cells cells. However, MHY1485 did not similarly
attenuate the liraglutide-induced increase in p-AMPK levels,
resulting in significantly increased protein expression levels of
p-AMPK compared with positive control cells (P=0.04; Fig. 4B). Protein expression levels of
AMPK and mTOR were unaffected by liraglutide and the AMPK and mTOR
inhibitors. These data suggested that liraglutide affects the
activities of AMPK and mTOR but not their expression, and that the
AMPK/mTOR axis is potentially involved in the effect of liraglutide
on osteoblastic differentiation.
 | Figure 4Effects of liraglutide treatments and
AMPK inhibitor/mTOR activator on matrix mineralization and relative
protein levels of Alp, OC, p-AMPK, p-mTOR and TGF-β. Following
culture in commercial osteogenic differentiation medium for 14
days, MC3T3-E1 cells were cultured for a further 14 days in basal
culture medium with 4 nM liraglutide, and 4 nM liraglutide plus
AMPK inhibitor, 10 µM compound C, or 1 µM mTOR
activator, MHY1485. MC3T3-E1 cells maintained in DMEM for 28 days
with no treatment were used as the NC; cells cultured in the
commercial osteogenic differentiation medium for 14 days and in
DMEM without liraglutide for a further 14 days were used as the PC.
(A) Matrix mineralization was determined by Alizarin Red S staining
(n=10). Magnification, ×200. (B) Relative protein expression was
analyzed by western blot and quantified relative to GAPDH (n=5).
Data are presented as the mean ± standard error. Bars with letters
means they significantly differ with positive control (P<0.05).
*P<0.05, **P<0.05 vs. NC;
#P<0.05, ##P<0.01 vs. PC. NC, negative
control; PC, positive control; Alp, alkaline phosphatase; OC,
osteocalcin; p-, phosphorylated; AMPK, adenosine
monophosphate-activated protein kinase; mTOR, mechanistic target of
rapamycin; TGF-β, transforming growth factor-β; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
The present study demonstrated that liraglutide
attenuated osteoblastic differentiation, based on the decreased
protein levels of Alp and OC resulting from liraglutide treatment,
and prevention of mineralized matrix formation in osteoblast
MC3T3-E1 cells following exposure to liraglutide. Protein
expression levels of Alp and OC in osteoblasts were decreased by
liraglutide in a dose-dependent manner, indicating that liraglutide
inhibits differentiation of osteoblast MC3T3-E1 cells. Liraglutide
also dose-dependently decreased Alizarin red S staining of MC3T3-E1
cells, indicating that liraglutide inhibits calcium phosphate salt
deposition, resulting in diminished osteoblastic differentiation of
MC3T3-E1 cells.
Since previous studies have suggested that AMPK
signaling may be involved in both osteoblastic differentiation and
other biological functions of liraglutide (6–9,12),
the present study investigated AMPK signaling to provide insights
into the adverse effects of liraglutide on osteoblastic
differentiation. AMPK activity, assessed through detection of
phosphorylated AMPK, was decreased during osteoblastic
differentiation of MC3T3-E1 cells induced by commercial osteogenic
differentiation medium. However, when such differentiation was
inhibited using liraglutide, AMPK was activated at similar levels
as in undifferentiated cells. Furthermore, inhibition of AMPK
signaling through treatment with compound C abolished the
inhibitory effect of liraglutide on osteoblast differentiation.
These data, therefore, support the hypothesis that AMPK signaling
serves a critical role in the liraglutide-associated attenuation of
osteoblastic differentiation.
AMPK signaling has been previously demonstrated to
attenuate or enhance osteoblastic differentiation, according to the
manner in which AMPK is activated and which downstream targets of
AMPK are regulated (6–9). mTOR, an important down-stream target
of AMPK, stimulates osteoblastic differentiation (10,20).
The present study demonstrated that mTOR phosphorylation is
negatively associated with that of AMPK, but differentiation of
MC3T3-E1 osteoblasts is functionally associated with increased mTOR
activity. Importantly, both the AMPK inhibitor and mTOR activator
restored mTOR activity (mTOR phosphorylation) and abolished the
inhibitory effect of liraglutide on osteoblast differentiation.
However, the mTOR activator had no effect on the levels of p-AMPK,
indicating that liraglutide attenuates osteoblastic differentiation
of MC3T3-E1 cells via AMPK-mediated mTOR suppression.
TGF-β is the most abundant cytokine in bone, with a
key role in osteoblastic differentiation (21,22).
To initiate cellular responses TGF-β receptors are required to
transduce the TGF-β signal and activate intracellular transcription
factors (23). Osteoblasts have
been demonstrated to secrete TGF-β, and to express a large variety
of high affinity TGF-β receptors, suggesting that TGF-β acts as an
autocrine and paracrine cytokine, regulating multiple physiological
processes in osteoblasts (15,21).
Although the effect of TGF-β on the regulation of osteoblastic
differentiation remains unclear, previous studies have demonstrated
that TGF-β promotes early differentiation of osteoblasts (24,25).
The present study revealed that protein expression levels of TGF-β
followed similar trends to p-mTOR, and that both the AMPK inhibitor
and mTOR activator abolished the inhibitory effect of liraglutide
on osteoblast differentiation. Previous studies have demonstrated
that ribosomal protein S6 kinase B1, a well-characterized
downstream target of mTOR, regulates the functions and synthesis of
multiple cytokines to modulate osteogenesis, including
platelet-derived growth factor, fibroblast growth factor and
vascular endothelial growth factor (26). TGF-β is, therefore, speculated to
mediate the effects of mTOR on osteoblastic differentiation.
The clinical implications of liraglutide attenuation
of osteoblastic differentiation remain unknown. Attenuated
osteoblastic differentiation inhibits bone formation, which may,
therefore, increase the risk of osteoporosis and bone fractures
(27). However, the
liraglutide-induced reduction in endogenous TGF-β expression
demonstrated in the present study may inhibit the generation of
osteophytes, as demonstrated in previous in vivo studies
(28,29). Additionally, as osteoblastic
differentiation is associated with calcium and phosphorus
metabolism (26), it is possible
that negative regulation of osteoblastic differentiation by
liraglutide may also impact calcium and phosphorus metabolism.
In summary, the present study demonstrated that
liraglutide attenuated osteoblastic differentiation and inhibited
the expression of osteoblastic differentiation markers, Alp and OC,
and the formation of mineralized matrix in osteoblasts. The protein
expression level of p-AMPK was significantly increased when
osteoblastic differentiation of MC3T3-E1 cells was attenuated by
liraglutide, whereas levels of p-mTOR and TGF-β were decreased
under the same conditions. An AMPK inhibitor and mTOR activator
both attenuated the inhibitory effect of liraglutide on
osteoblastic differentiation and restored p-mTOR protein levels to
those of untreated cells. However, the mTOR activator did not
restore p-AMPK protein expression levels to that of untreated
cells. Thus, the present study demonstrated that liraglutide
attenuated osteoblastic differentiation of MC3T3-E1 cells via
modulation of AMPK/mTOR signaling. The present study revealed a
novel function of liraglutide, which contributes to the
understanding of its pharmacological and physiological effects in
clinical settings.
Acknowledgments
This study was partially funded by the National
Natural Science Foundation of China (grant no. 81271940) and the
Project of Furong Scholar of Hunan Province.
References
|
1
|
Jendle J, Nauck MA, Matthews DR, Frid A,
Hermansen K, Düring M, Zdravkovic M, Strauss BJ and Garber AJ;
LEAD-2 and LEAD-3 Study Groups: Weight loss with liraglutide, a
once-daily human glucagon-like peptide-1 analogue for type 2
diabetes treatment as monotherapy or added to metformin, is
primarily as a result of a reduction in fat tissue. Diabetes Obes
Metab. 11:1163–1172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao X, Liu G, Shen H, Gao B, Li X, Fu J,
Zhou J and Ji Q: Liraglutide inhibits autophagy and apoptosis
induced by high glucose through GLP-1R in renal tubular epithelial
cells. Int J Mol Med. 35:684–692. 2015.PubMed/NCBI
|
|
3
|
Shyangdan D, Cummins E, Royle P and Waugh
N: Liraglutide for the treatment of type 2 diabetes. Health Technol
Assess. 15(Suppl 1): S77–S86. 2011. View Article : Google Scholar
|
|
4
|
Gough SC: Liraglutide: From clinical
trials to clinical practice. Diabetes Obes Metab. 14(Suppl 2):
S33–S40. 2012. View Article : Google Scholar
|
|
5
|
Lind T, Sundqvist A, Hu L, Pejler G,
Andersson G, Jacobson A and Melhus H: Vitamin a is a negative
regulator of osteoblast mineralization. PLoS One. 8:e823882013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanazawa I, Yamaguchi T, Yano S, Yamauchi
M, Yamamoto M and Sugimoto T: Adiponectin and AMP kinase activator
stimulate proliferation, differentiation, and mineralization of
osteoblastic MC3T3-E1 cells. BMC Cell Biol. 8:512007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong X, Xiu LL, Wei GH, Liu YY, Su L, Cao
XP, Li YB and Xiao HP: Bezafibrate enhances proliferation and
differentiation of osteoblastic MC3T3-E1 cells via AMPK and eNOS
activation. Acta Pharmacol Sin. 32:591–600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JY, Min JY, Baek JM, Ahn SJ, Jun HY,
Yoon KH, Choi MK, Lee MS and Oh J: CTRP3 acts as a negative
regulator of osteoclastogenesis through AMPK-c-Fos-NFATc1 signaling
in vitro and RANKL-induced calvarial bone destruction in vivo.
Bone. 79:242–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei J, Shimazu J, Makinistoglu MP, Maurizi
A, Kajimura D, Zong H, Takarada T, Iezaki T, Pessin JE, Hinoi E and
Karsenty G: Glucose uptake and Runx2 synergize to orchestrate
osteoblast differentiation and bone formation. Cell. 161:1576–1591.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhan JK, Wang YJ, Wang Y, Tang ZY, Tan P,
Huang W and Liu YS: Adiponectin attenuates the osteoblastic
differentiation of vascular smooth muscle cells through the
AMPK/mTOR pathway. Exp Cell Res. 323:352–358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhan JK, Wang YJ, Wang Y, Tang ZY, Tan P,
Huang W and Liu YS: The protective effect of GLP-1 analogue in
arterial calcification through attenuating osteoblastic
differentiation of human VSMCs. Int J Cardiol. 189:188–193. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miao XY, Gu ZY, Liu P, Hu Y, Li L, Gong
YP, Shu H, Liu Y and Li CL: The human glucagon-like peptide-1
analogue liraglutide regulates pancreatic beta-cell proliferation
and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides.
39:71–79. 2013. View Article : Google Scholar
|
|
13
|
de la Croix Ndong J, Makowski AJ,
Uppuganti S, Vignaux G, Ono K, Perrien DS, Joubert S, Baglio SR,
Granchi D, Stevenson DA, et al: Corrigendum: Asfotase-α improves
bone growth, mineralization and strength in mouse models of
neurofibromatosis type-1. Nat Med. 21:4142015. View Article : Google Scholar
|
|
14
|
D'Alonzo RC, Kowalski AJ, Denhardt DT,
Nickols GA and Partridge NC: Regulation of collagenase-3 and
osteocalcin gene expression by collagen and osteopontin in
differentiating MC3T3-E1 cells. J Biol Chem. 277:24788–24798. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hughes-Fulford M and Li CF: The role of
FGF-2 and BMP-2 in regulation of gene induction, cell proliferation
and mineralization. J Orthop Surg Res. 6:82011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Owen TA, Aronow M, Shalhoub V, Barone LM,
Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB and Stein
GS: Progressive development of the rat osteoblast phenotype in
vitro: Reciprocal relationships in expression of genes associated
with osteoblast proliferation and differentiation during formation
of the bone extracellular matrix. J Cell Physiol. 143:420–430.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang L, Cheng P, Chen C, He HB, Xie GQ,
Zhou HD, Xie H, Wu XP and Luo XH: miR-93/Sp7 function loop mediates
osteoblast mineralization. J Bone Miner Res. 27:1598–1606. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paul H, Reginato AJ and Schumacher HR:
Alizarin red S staining as a screening test to detect calcium
compounds in synovial fluid. Arthritis Rheum. 26:191–200. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singha UK, Jiang Y, Yu S, Luo M, Lu Y,
Zhang J and Xiao G: Rapamycin inhibits osteoblast proliferation and
differentiation in MC3T3-E1 cells and primary mouse bone marrow
stromal cells. J Cell Biochem. 103:434–446. 2008. View Article : Google Scholar
|
|
21
|
Ehnert S, Baur J, Schmitt A, Neumaier M,
Lucke M, Dooley S, Vester H, Wildemann B, Stöckle U and Nussler AK:
TGF-β1 as possible link between loss of bone mineral density and
chronic inflammation. PLoS One. 5:e140732010. View Article : Google Scholar
|
|
22
|
Janssens K, ten Dijke P, Janssens S and
Van Hul W: Transforming growth factor-beta1 to the bone. Endocr
Rev. 26:743–774. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Latella G, Vetuschi A, Sferra R, Speca S
and Gaudio E: Localization of ανβ6 integrin-TGF-β1/Smad3, mTOR and
PPARγ in experimental colorectal fibrosis. Eur J Histochem.
57:e402013. View Article : Google Scholar
|
|
24
|
Manzano-Moreno FJ, Medina-Huertas R,
Ramos-Torrecillas J, García-Martínez O and Ruiz C: The effect of
low-level diode laser therapy on early differentiation of
osteoblast via BMP-2/TGF-β1 and its receptors. J Craniomaxillofac
Surg. 43:1926–1932. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki E, Ochiai-Shino H, Aoki H, Onodera
S, Saito A, Saito A and Azuma T: Akt activation is required for
TGF-β1-induced osteoblast differentiation of MC3T3-E1
pre-osteoblasts. PLoS One. 9:e1125662014. View Article : Google Scholar
|
|
26
|
Xiang X, Zhao J, Xu G, Li Y and Zhang W:
mTOR and the differentiation of mesenchymal stem cells. Acta
Biochim Biophys Sin (Shanghai). 43:501–510. 2011. View Article : Google Scholar
|
|
27
|
Wanachewin O, Boonmaleerat K, Pothacharoen
P, Reutrakul V and Kongtawelert P: Sesamin stimulates osteoblast
differentiation through p38 and ERK1/2 MAPK signaling pathways. BMC
Complement Altern Med. 12:712012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scharstuhl A, Glansbeek HL, van Beuningen
HM, Vitters EL, van der Kraan PM and van den Berg WB: Inhibition of
endogenous TGF-beta during experimental osteoarthritis prevents
osteophyte formation and impairs cartilage repair. J Immunol.
169:507–514. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scharstuhl A, Vitters EL, van der Kraan PM
and van den Berg WB: Reduction of osteophyte formation and synovial
thickening by adenoviral overexpression of transforming growth
factor beta/bone morphogenetic protein inhibitors during
experimental osteoarthritis. Arthritis Rheum. 48:3442–3451. 2003.
View Article : Google Scholar : PubMed/NCBI
|


















