Introduction
Myocardial infarction (MI) inhibits blood flow to
the heart and damages cardiac muscle, which may lead to heart
failure, an irregular heartbeat or cardiac arrest (1). MI is one of the predominant global
diseases due to its high mortality and morbidity rate, it is also a
burden on the healthcare system in various countries (2).
The communication between the brain and the heart is
considered to be bidirectional (3–5).
During cardiac disease, the brain signals to the heart; cardiac
dysfunction initially occurs and then the brain provides feedback
to the heart via sympathetic drive or fluid regulation to sustain
the cardiac disease state (2,6).
Previous studies have examined the central
structures or brain neurons associated with MI and heart failure
(7–11). Monitoring of proto-oncogene c-Fos
(c-Fos) expression levels has been established as a reliable
technique to identify neural populations of metabolically activated
brain regions (12,13). The selective expression of c-Fos
has previously been observed in the rat brain following heart
failure (11). It is generally
accepted that neuronal activation or changes in the brain occur
following MI, however, the mechanisms involved remain poorly
understood. The paraventricular nucleus of the hypothalamus (PVNH)
and the paraventricular nucleus of the thalamus (PVNT), which
exhibit c-Fos expression following stresses, including
capsaicin-induced nociceptive pain in rats (14). However, to the best of our
knowledge, this effect has not been reported during MI. Therefore,
the present study examined the changes in c-Fos expression levels
in the PVNH and PVNT of rats to understand the pathophysiology and
improve the management of MI (15,16).
Materials and methods
Induction of MI
Male Sprague-Dawley rats (12 weeks of age; body
weight, 300–320 g) were obtained from the Experimental Animal
Center at Kangwon National University (Chuncheon, South Korea). A
total of 98 male rats were housed in individual cages (temperature,
23°C; humidity, 60%) under a 12 h light/dark cycle, and provided
with commercial chow and water ad libitum and throughout the
experimental period. The procedures for animal handling and care
were in compliance with the Guide for the Care and Use of
Laboratory Animals, and were approved by the Institutional Animal
Care and Use Committee at Kangwon National University (Chuncheon,
South Korea).
MI was induced as described in our previous study
(17). Briefly, the animals were
intubated and ventilated with a small animal ventilator (model
SAR-830/P; CWE, Inc., Ardmore, PA, USA). The left coronary artery
was permanently ligated below the left atrial appendage.
Sham-operated animals were subjected to the same surgical
procedures without ligation of the left coronary artery.
Tissue processing for histology
The rats were anesthetized with intraperitoneal
injection of pentobarbital sodium (30 mg/kg; JW Pharmaceutical,
Seoul, Korea) at 1, 3, 7, 14, 28 and 56 days (n=7 at each time
point) after MI induction. Subsequently, they were perfused via the
abdominal aorta with 0.1 M phosphate-buffered saline (PBS; pH 7.4)
followed by 4% paraformaldehyde in 0.1 M phosphate-buffer (pH 7.4).
The hearts were excised and embedded in paraffin blocks and
sectioned into 6 μm sections at 600 μm intervals. The
brains were removed and embedded in tissue-freezing medium and
serially sectioned into 30 μm coronal sections using a
cryostat (Leica Microsystems GmbH, Wetzlar, Germany).
Masson's trichrome staining
The heart sections were stained to examine the
histology of the heart using Masson's trichrome staining as
previously described by Ahmet et al (15) the sections were placed in the
Biebrich scarlet-acid fuchsin solution (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) and aniline blue (Sigma-Aldrich;
Merck Millipore) to detect the area of infarction in the heart
tissue of MI-induced rats. Images were obtained using a light
microscope (BX53, Olympus, Hamburg, Germany) equipped with a
digital camera (DP72, Olympus) connected to a PC monitor. Whole
images of the heart were merged by image analyzing system Optimas
version 6.5 (CyberMetrics, Phoenix, AZ, USA).
Crystal violet (CV) and Fluoro-Jade B
(F-J B) histofluorescence staining
To examine neuronal damage in the diencephalon
following MI, CV staining and F-J B histofluorescence staining were
performed as previously described (18). In brief, the sections were stained
with 1.0% (w/v) CV acetate (Sigma-Aldrich; Merck Millipore) and
dehydrated. For F-J B histofluorescence, the sections were immersed
in 0.0004% F-J B (Histo-Chem, Inc., Jefferson, AR, USA) staining
solution. The sections were washed with 0.1 M PBS and examined
using an epifluorescent microscope (Zeiss GmbH, Göttingen, Germany)
with blue (450–490 nm) excitation light and a barrier filter. Five
randomly selected microscope fields (×400 magnification) were
photographed to represent each rat.
Immunohistochemistry for c-Fos
c-Fos immunohistochemistry in the diencephalon was
performed according to our previous study (18). Briefly, the sections were incubated
with primary rabbit anti-c-Fos (cat. no.. sc-52; 1:200; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), followed with secondary
antibody incubation at room temperature for 2 h (cat. no. BA1000;
1:200; Vector Laboratories, Inc., Burlingame, CA, USA) and
developed using a Vectastain ABC kit (Vector Laboratories, Inc.).
They were visualized with 3,3′-diaminobenzidine in 0.1 M Tris-HCl
buffer. Five randomly selected digital images of the PVNH and PVNT
groups per rat were captured using Olympus light microscope
equipped with a digital camera (DP72, Olympus) connected to a PC
monitor
Western blot analysis
To obtain the accurate data for changes in level of
c-Fos protein in the PVNH and PVNT following MI, the animals (n=7
at each time point) were sacrificed at 1, 3, 7, 14, 28 and 56 day
following the induction of MI and the PVNH and PVNT tissue used for
western blot analysis. As previously described (19), the brain was transversely cut into
400-μm thick sections on a vibratome (Leica Microsystems
GmbH). The PVNH and the PVNT were dissected with a surgical blade
under stereoscopic microscope. The tissues were homogenized in 50
mM PBS (pH 7.4) containing 0.1 mM ethylene glycol bis (2-aminoethyl
ether)-N,N,N′,N′ tetraacetic acid (pH 8.0), 0.2% Nonidet P-40, 10
mM ethylendiamine tetraacetic acid (pH 8.0), 15 mM sodium
pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2
mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride and 1
mM dithiothreitol (DTT). Following centrifugation at in 16,000 × g
for 20 min at 4°C, the protein level in the supernatant was
determined using a Micro BCA protein assay kit with bovine serum
albumin as the standard (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Aliquots containing 20 μg total protein
were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM
DTT, 6% sodium dodecyl sulfate, 0.3% bromophenol blue and 30%
glycerol. Subsequently, each aliquot was loaded onto a 12.5%
polyacrylamide gel for electrophoresis. The gels were then
transferred to nitrocellulose transfer membranes (Pall Corporation,
East Hills, NY, USA). To reduce background staining, the membranes
were incubated with 5% non-fat dry milk in PBS containing 0.1%
Tween 20 for 45 min and then with rabbit anti-c-Fos (cat. no..
sc-52; 1:1,500; Santa Cruz Biotechnology, Inc.) for 24 h at
4°C, followed by incubation at room temperature for 1 h with
peroxidase-conjugated goat anti-rabbit IgG (cat. no. A0545; 1:200;
Sigma-Aldrich; Merck Millipore) and an enhanced chemiluminescence
kit (Pierce; Thermo Fisher Scientific, Inc.) was used for
detection. Loading controls were performed using β-actin incubated
at 4°C overnight (cat. no. ab8227; 1:5,000; Abcam,
Cambridge, MA, USA). Densitometric analysis for the quantification
of the bands was performed using ImageJ version 1.46 software
(National Institutes of Health, Bethesda, MD, USA), which was used
to determine the relative optical density (ROD). The levels of
c-Fos were normalized to the level of β-actin. A ratio of the ROD
was calibrated as the percentage, with the PM 3 group designated as
100%
Data analysis
The size of the MI induced was calculated using the
method previously described by Ahmet et al (15) as an average percentage of the left
ventricular endocardial and epicardial circumferences that were
identified as the infarcted area in the Masson's trichrome-stained
tissues. The mean number of c-Fos-immunoreactive (+)
cells was counted in a 250×250 μm square in five randomly
selected images of PVNH and PVNT in each rat. Cell counts were
obtained by averaging the total number of cells from each animal
per group.
Statistical analysis
Data are presented as the mean ± standard error.
One-way analysis of variance and Tukey's post-hoc test were used in
order to compare the difference of c-Fos immunoreactivity and
protein levels between sham- and MI-operated rat groups. SAS
software version 9.2 (SAS Institute Inc., Cary, NC, USA) was used
to perform the statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
MI induction
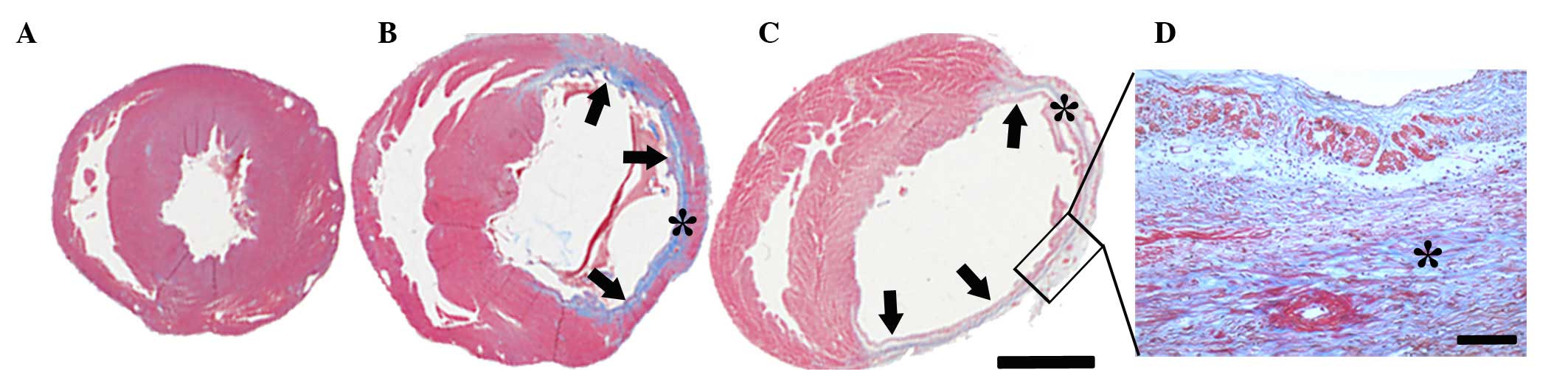
Masson's trichrome staining determined that the
infarcted region encompassed between 41 and 46% of the ventricular
circumference of the left ventricle following MI (Fig. 1). No difference in the infarcted
area was observed between the different time-point groups. Viable
myocardium was stained red and fibrosis caused by infarction damage
was stained blue (Fig. 1). The
sham-operated group was used as a control group and no blue
staining was observed. By contrast, the infarcted zone in the
MI-operated group had pronounced blue scar tissue. The infarct wall
of the left ventricle was thinner (indicated with arrows in
Fig. 1) following MI, whereas the
non-infarct wall exhibited hypertrophy (Fig. 1B and C). Collagen and
myofibroblasts accumulated in the infarct wall over time and
replaced with necrotic myocardium by 56 days after MI (Fig. 1D).
MI-induced neuronal damage
The PVNH and PVNT were identified by CV staining
(Fig. 2). The staining pattern
observed in the MI-operated group was comparable with the sham
group (Fig. 2B, C, H and I). F-J B
fluorescence staining was used to detect neurodegenerating
structures and F-J B+ cells were not detected in the
PVNH (Fig. 2D–F) and PVNT
(Fig. 2J–L) of the sham- or
MI-operated groups.
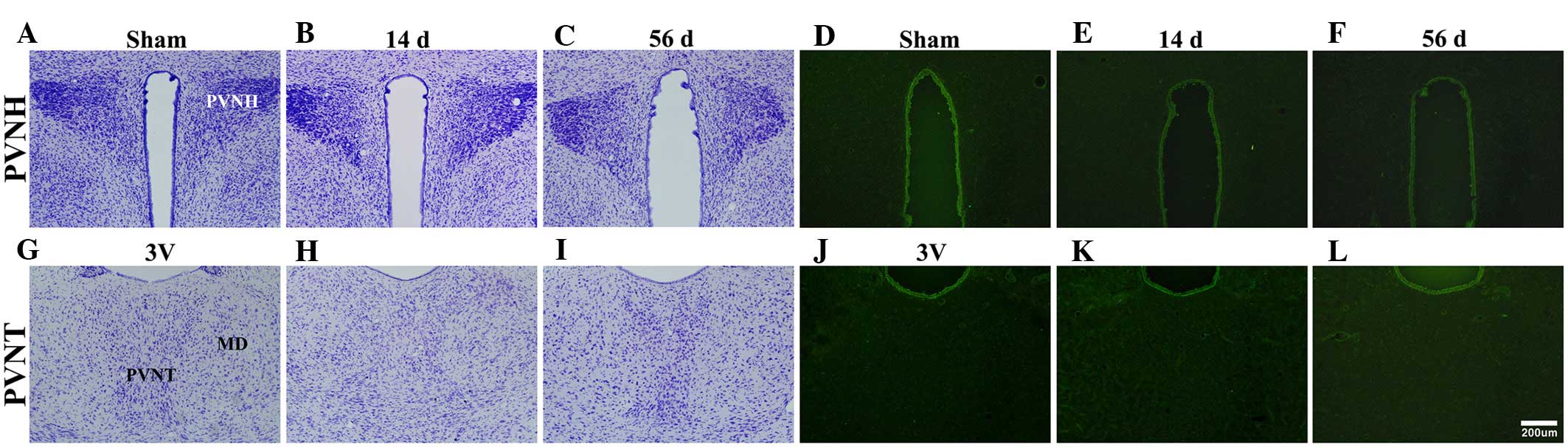 | Figure 2Crystal violet and F-J B
histofluorescence staining of the PVNH and PVNT. Crystal violet
staining of PVNH in (A) sham, and (B) 14 d and (C) 56 d myocardial
infarction-operated groups. F-J B histofluorescence of PVNH in (D)
sham, and (E) 14 d and (F) 56 d myocardial infarction-operated
groups. Crystal violet staining of PVNT in (G) sham, and (H) 14 d
and (I) 56 d myocardial infarction-operated groups. F-J B
histofluorescence of PVNT in (J) sham, and (K) 14 d and (L) 56 d
myocardial infarction-operated groups. F-J B+ cells were
not found in all groups. Scale bar, 200 μm. d, day; PVNH,
paraventricular nucleus of the hypothalamus; PVNT, paraventricular
nucleus of the thalamus; F-J B, Fluoro-Jade B; 3V, 3rd ventricle;
MD, mediodorsal thalamic nucleus. |
c-Fos immunoreactivity following MI
In the sham-operated group, c-Fos immunoreactivity
was infrequently observed in the PVNH and PVNT (Fig. 3). By contrast, the number of
c-Fos+ cells in the nuclei significantly increased from
3 days onwards following coronary artery ligation compared with the
sham group (P<0.05; Figs. 3 and
4A). In the PVNH, the mean number
of c-Fos+ cells peaked at 3 days after MI
(140.5±7.2/mm2 per section; Figs. 3B and 4). c-Fos+ cells subsequently
decreased over time (Figs. 3C–F
and 4). In the PVNT,
c-Fos+ cells were gradually increased and their mean
number was significantly greater at 3 days after MI compared with
the sham group (P<0.05; Fig.
4B) and peaked at 14 days after MI (159.7±16.4/mm2
per section; Figs. 3H–L and
4B). c-Fos+ cells were
infrequently observed in PVNH and PVNT 56 days after MI (Figs. 3F, L and 4).
 | Figure 3c-Fos immunohistochemistry in the
PVNH and PVNT. Staining was performed in the PVNH of (A) sham and
MI-operated groups at (B) 3 d, (C) 7 d, (D) 14 d, (E) 28 d and (F)
56 d. Staining was performed in the PVNT of (G) sham and
MI-operated groups at (H) 3 d, (I) 7 d, (J) 14 d, (K) 28 d and (L)
56 d. In the sham-operated group, c-Fos immunoreactivity was
infrequently detected. The arrows indicate the c-Fos immunoreactive
cells, the number of c-Fos immunoreactive cells peaked in the PVNH
and PVNT at 3 and 14 days, respectively, subsequent to MI. Scale
bar, 100 μm. d, day; PVNH, paraventricular nucleus of the
hypothalamus; PVNT, paraventricular nucleus of the thalamus; MI,
myocardial infarction; c-Fos, proto-oncogene c-Fos. |
c-Fos protein expression levels increase
following MI
Western blot analysis determined that the changes in
c-Fos protein expression levels in PVNH and PVNT following coronary
artery ligation (Fig. 5) were
similar to the immunohistochemistry findings. c-Fos protein
expression levels in the PVNH peaked at the 3 days after MI, and
were significantly increased at 3, 7 and 14 days compared with the
sham group (P<0.05; Fig. 5B).
The protein expression levels subsequently decreased gradually over
time following MI. In the PVNT, c-Fos protein expression levels
were significantly increased at 7 days after MI compared with the
sham group (P<0.05; Fig. 5C)
and peaked at 14 days, a decrease in c-Fos expression was
subsequently was observed. The c-Fos protein levels in the group
observed 56 days after MI were comparable with the sham-operated
group (Fig. 5).
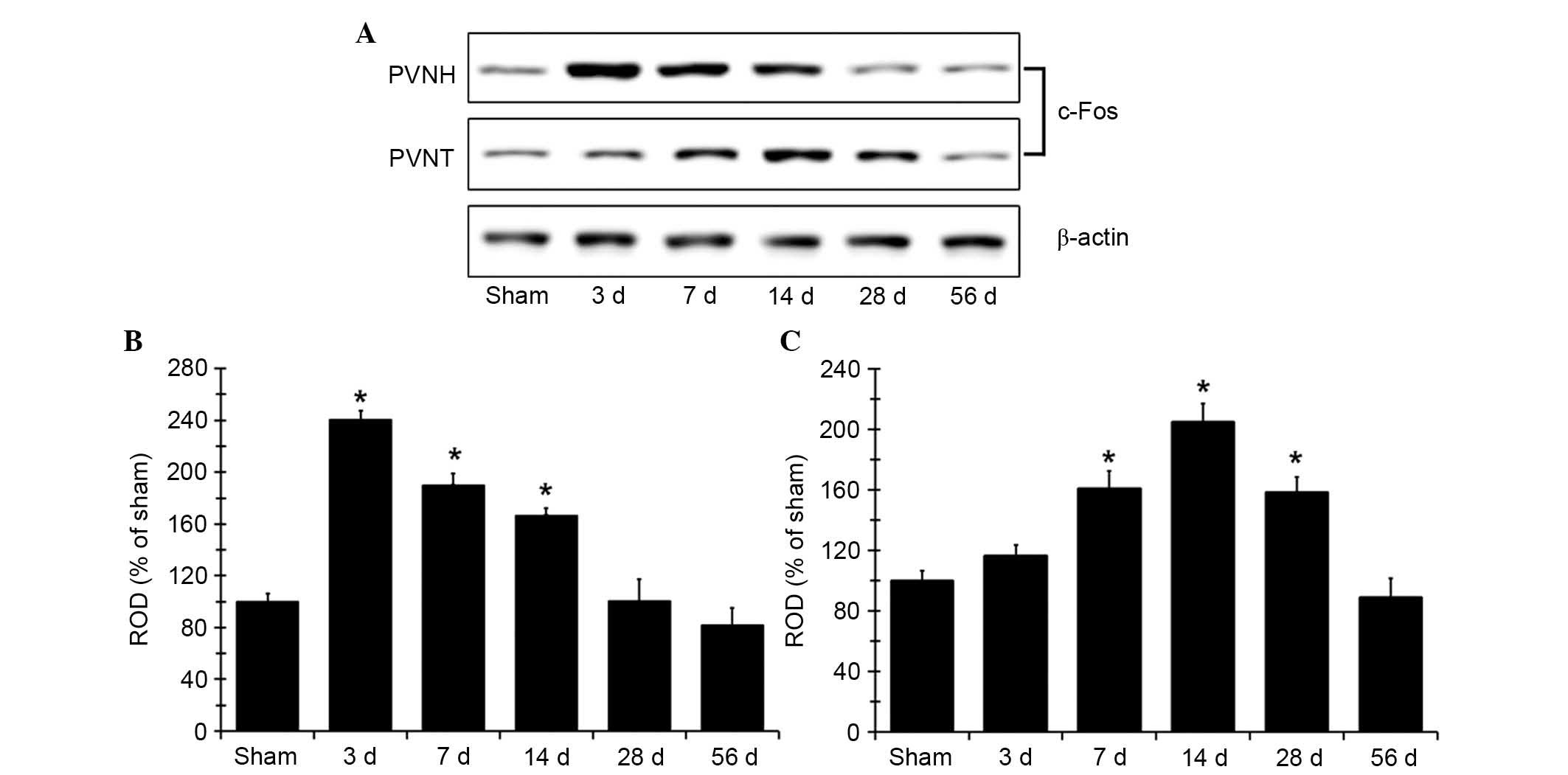 | Figure 5c-Fos expression in PVNH and PVNT.
(A) Western blot analysis of c-Fos protein in the PVNH and PVNT of
the sham- and MI-operated rats in sham, 3, 7, 14, 28 and 56 days
after MI. c-Fos protein expression is represented as ROD of target
immunoblot band to the sham band in the (B) PVNH and (C) PVNT (n=7
per group). *P<0.05 vs. sham group. Data are
presented as the mean ± standard error. MI, myocardial infarction;
PVNH, paraventricular nucleus of the hypothalamus; PVNT,
paraventricular nucleus of the thalamus; c-Fos, proto-oncogene
c-Fos; d, day; ROD, relative optical density. |
Discussion
c-Fos is a good biological marker for detecting
pathogenesis in the central nervous system. Few studies regarding
change in MI-induced c-Fos in the brain have been reported
(11,20), and the change of c-Fos in the PVNH
and PVNT following MI remains to be elucidated. The average infarct
size was ~44% of the left ventricle circumference following MI.
Neuronal damage was not detected in PVNH and PVNT following MI.
c-Fos+ cells were not detected in the sham-group.
However, the number of c-Fos+ cells in the PVNH and PVNT
was increased following the induction of MI and peaked at 3 and 14
days, respectively. c-Fos+ cells were not detected in
the PVNH and PVNT at 56 days after MI induction. Therefore, MI
significantly induces c-Fos immunoreactivity and increases c-Fos
protein expression levels in the PVNH and PVNT. The increase of
c-Fos expression levels may be associated with increased cerebral
stress that occurs following MI.
Limiting the blood supply to the left anterior
descending coronary artery has been previously used to induce MI in
experimental animals, which may model human heart failure (21). Infarcts of similar magnitude have
been reported to be associated with reduced cardiac function
(15,22). The present study did not identify a
significant difference between the size of the infarcts at any time
following the induction of MI.
Previous studies have demonstrated that MI induces
neuronal damage in several regions of the brain, including the
hippocampal CA1 region and amygdala (5,23–25).
However, neuronal damage was not detected in the PVNH and PVNT
using CV and F-J B histofluorescence staining in the present study.
Therefore, it is possible that neurons in the PVNH and PVNT may be
less susceptible to MI-induced damage.
The PVNH contains sub-populations of neurons that
may be activated by various stresses and physiological changes
(26). Additionally, it is the
primary site for the activation of vasopressin-synthesizing neurons
in humans and rats following MI (3). Therefore, the PVNH may be involved in
altering volume reflex, which has been previously observed in MI
(11). The PVNT is also important
for the arousal/attention response and modulating the process of
stress-associated information following MI (27). PVNT is activated following various
stresses, including immobilization, pain and fear (28–31).
c-Fos is associated with the neuronal activation
underlying learning and memory processes in the central nervous
system (CNS) (32,33). Additionally, it has been reported
that c-Fos is useful for investigating the neuronal plasticity
required for spatial memory processes (33–35).
A previous study demonstrated that pain and immobilization stress
increased the number of neurons exhibiting Fos-like
immunoreactivity in several regions of the brain, including the
PVNH (36). However, the
association between c-Fos expression and neuronal activation in the
CNS following MI has not been fully elucidated. It was previously
reported in a rat model of the MI, that the number of
c-Fos+ cells was increased in the hypothalamus
containing PVNH (11).
Additionally, changes in the number of c-Fos+ cells in
the PVNH occurred at 2 and 4 weeks following MI compared with the
sham group in a mouse model (20).
The present study determined that the number of c-Fos+
cells in the PVNH peaked 3 days after MI induction, and
subsequently gradually decreased and were comparable with sham
controls at 56 days after MI. By contrast with previous findings
(11,20), the present study observed that the
number of c-Fos+ cells in the PVNH peaked at an early
time-point (3 days) after MI.
However, it was determined that the number of
c-Fos+ cells in the PVNT was significantly increased
compared with the sham group until 28 days after MI and
subsequently decreased with time and were comparable with the sham
group at 56 days after MI. To the best of our knowledge, the
present study was the first to determine that c-Fos remains
expressed in the PVNT to a late time-point following MI. This
indicates that the increase of c-Fos+ cells in the PVNT
may be associated with the stress response following MI.
In conclusion, the present study did not detect
MI-induced neuronal damage in the PVNH and PVNT. Whereas, MI
induced different time-dependent c-Fos expression patterns in the
PVNH and PVNT. This suggests that an increased number of
c-Fos+ cells in the PVNH and PVNT may be associated with
MI-induced stress.
Acknowledgments
The present study was supported by Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant no. NRF-2014R1
A1A2057263), the Gangwon Cardiovascular Health Research Institute
and a 2014 research grant from Kangwon National University (grant
no. 120140271).
References
|
1
|
Van de Werf F, Bax J, Betriu A,
Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber
K, Kastrati A, et al: ESC Committee for Practice Guidelines (CPG):
Management of acute myocardial infarction in patients presenting
with persistent ST-segment elevation: The Task Force on the
Management of ST-Segment Elevation Acute Myocardial Infarction of
the European Society of Cardiology. Eur Heart J. 29:2909–2945.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim MS and Kim JJ: Heart and brain
interconnection - clinical implications of changes in brain
function during heart failure. Circ J. 79:942–947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hodsman GP, Kohzuki M, Howes LG, Sumithran
E, Tsunoda K and Johnston CI: Neurohumoral responses to chronic
myocardial infarction in rats. Circulation. 78:376–381. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frahm C, Haupt C and Witte OW: GABA
neurons survive focal ischemic injury. Neuroscience. 127:341–346.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wann BP, Boucher M, Kaloustian S, Nim S,
Godbout R and Rousseau G: Apoptosis detected in the amygdala
following myocardial infarction in the rat. Biol Psychiatry.
59:430–433. 2006. View Article : Google Scholar
|
|
6
|
Schrier RW and Abraham WT: Hormones and
hemodynamics in heart failure. N Engl J Med. 341:577–585. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patel KP, Zhang PL and Krukoff TL:
Alterations in brain hexokinase activity associated with heart
failure in rats. Am J Physiol. 265:R923–R928. 1993.PubMed/NCBI
|
|
8
|
Sole MJ, Hussain MN and Lixfeld W:
Activation of brain catecholaminergic neurons by cardiac vagal
afferents during acute myocardial ischemia in the rat. Circ Res.
47:166–172. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sole MJ, Hussain MN, Versteeg DH, de Kloet
ER, Adams D and Lixfeld W: The identification of specific brain
nuclei in which catecholamine turnover is increased by left
ventricular receptors during acute myocardial infarction in the
rat. Brain Res. 235:315–325. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sole MJ, Versteeg DH, de Kloet ER, Hussain
N and Lixfeld W: The identification of specific serotonergic nuclei
inhibited by cardiac vagal afferents during acute myocardial
ischemia in the rat. Brain Res. 265:55–61. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patel KP, Zhang K, Kenney MJ, Weiss M and
Mayhan WG: Neuronal expression of Fos protein in the hypothalamus
of rats with heart failure. Brain Res. 865:27–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoffman GE, Smith MS and Verbalis JG:
c-Fos and related immediate early gene products as markers of
activity in neuroendocrine systems. Front Neuroendocrinol.
14:173–213. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sagar SM, Sharp FR and Curran T:
Expression of c-fos protein in brain: Metabolic mapping at the
cellular level. Science. 240:1328–1331. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rockhold RW, Acuff CG and Clower BR:
Excitotoxic lesions of the paraventricular hypothalamus: Metabolic
and cardiac effects. Neuropharmacology. 29:663–673. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmet I, Tae HJ, Brines M, Cerami A,
Lakatta EG and Talan MI: Chronic administration of small
nonerythropoietic peptide sequence of erythropoietin effectively
ameliorates the progression of postmyocardial infarction-dilated
cardiomyopathy. J Pharmacol Exp Ther. 345:446–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang IK, Yoo KY, Han TH, Lee CH, Choi JH,
Yi SS, Lee SY, Ryu PD, Yoon YS and Won MH: Enhanced cell
proliferation and neuroblast differentiation in the rat hippocampal
dentate gyrus following myocardial infarction. Neurosci Lett.
450:275–280. 2009. View Article : Google Scholar
|
|
17
|
Lee CH, Hwang IK, Choi JH, Yoo KY, Han TH,
Park OK, Lee SY, Ryu PD and Won MH: Calcium binding proteins
immunoreactivity in the rat basolateral amygdala following
myocardial infarction. Cell Mol Neurobiol. 30:333–338. 2010.
View Article : Google Scholar
|
|
18
|
Lee CH, Park JH, Cho JH, Ahn JH, Yan BC,
Lee JC, Shin MC, Cheon SH, Cho YS, Cho JH, et al: Changes and
expressions of Redd1 in neurons and glial cells in the gerbil
hippocampus proper following transient global cerebral ischemia. J
Neurol Sci. 344:43–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JC, Kim IH, Cho GS, Park JH, Ahn JH,
Yan BC, Kwon HM, Kim YM, Cheon SH, Cho JH, et al: Ischemic
preconditioning-induced neuroprotection against transient cerebral
ischemic damage via attenuating ubiquitin aggregation. J Neurol
Sci. 336:74–82. 2014. View Article : Google Scholar
|
|
20
|
Lindley TE, Doobay MF, Sharma RV and
Davisson RL: Superoxide is involved in the central nervous system
activation and sympathoexcitation of myocardial infarction-induced
heart failure. Circ Res. 94:402–409. 2004. View Article : Google Scholar
|
|
21
|
Sun Y, Zhang JQ, Zhang J and Lamparter S:
Cardiac remodeling by fibrous tissue after infarction in rats. J
Lab Clin Med. 135:316–323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fletcher PJ, Pfeffer JM, Pfeffer MA and
Braunwald E: Left ventricular diastolic pressure-volume relations
in rats with healed myocardial infarction. Effects on systolic
function. Circ Res. 49:618–626. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaloustian S, Wann BP, Bah TM, Girard SA,
Apostolakis A, Ishak S, Mathieu S, Ryvlin P, Godbout R and Rousseau
G: Apoptosis time course in the limbic system after myocardial
infarction in the rat. Brain Res. 1216:87–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boucher M, Wann BP, Kaloustian S, Cardinal
R, Godbout R and Rousseau G: Reduction of apoptosis in the amygdala
by an A2A adenosine receptor agonist following
myocardial infarction. Apoptosis. 11:1067–1074. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arseneault-Bréard J, Rondeau I, Gilbert K,
Girard SA, Tompkins TA, Godbout R and Rousseau G: Combination of
Lactobacillus helveticus R0052 and Bifidobacterium longum R0175
reduces post-myocardial infarction depression symptoms and restores
intestinal permeability in a rat model. Br J Nutr. 107:1793–1799.
2012. View Article : Google Scholar
|
|
26
|
Nillni EA: Regulation of the hypothalamic
thyrotropin releasing hormone (TRH) neuron by neuronal and
peripheral inputs. Front Neuroendocrinol. 31:134–156. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li S and Kirouac GJ: Projections from the
paraventricular nucleus of the thalamus to the forebrain, with
special emphasis on the extended amygdala. J Comp Neurol.
506:263–287. 2008. View Article : Google Scholar
|
|
28
|
Fernandes GA, Perks P, Cox NK, Lightman
SL, Ingram CD and Shanks N: Habituation and cross-sensitization of
stress-induced hypothalamic-pituitary-adrenal activity: Effect of
lesions in the paraventricular nucleus of the thalamus or bed
nuclei of the stria terminalis. J Neuroendocrinol. 14:593–602.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Penzo MA, Robert V, Tucciarone J, De
Bundel D, Wang M, Van Aelst L, Darvas M, Parada LF, Palmiter RD, He
M, et al: The paraventricular thalamus controls a central amygdala
fear circuit. Nature. 519:455–459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spencer SJ, Fox JC and Day TA: Thalamic
paraventricular nucleus lesions facilitate central amygdala
neuronal responses to acute psychological stress. Brain Res.
997:234–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Otake K, Kin K and Nakamura Y: Fos
expression in afferents to the rat midline thalamus following
immobilization stress. Neurosci Res. 43:269–282. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Radulovic J, Kammermeier J and Spiess J:
Relationship between fos production and classical fear
conditioning: Effects of novelty, latent inhibition, and
unconditioned stimulus preexposure. J Neurosci. 18:7452–7461.
1998.PubMed/NCBI
|
|
33
|
Tischmeyer W and Grimm R: Activation of
immediate early genes and memory formation. Cell Mol Life Sci.
55:564–574. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vanelzakker MB, Zoladz PR, Thompson VM,
Park CR, Halonen JD, Spencer RL and Diamond DM: Influence of
Pre-Training Predator Stress on the Expression of c-fos mRNA in the
Hippocampus, Amygdala, and Striatum Following Long-Term Spatial
Memory Retrieval. Front Behav Neurosci. 5:302011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vann SD, Brown MW and Aggleton JP: Fos
expression in the rostral thalamic nuclei and associated cortical
regions in response to different spatial memory tests.
Neuroscience. 101:983–991. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Senba E, Matsunaga K, Tohyama M and
Noguchi K: Stress-induced c-fos expression in the rat brain:
Activation mechanism of sympathetic pathway. Brain Res Bull.
31:329–344. 1993. View Article : Google Scholar : PubMed/NCBI
|