Introduction
As one of the most effective treatments against
end-stage renal disease, renal allografts markedly improve patient
survival rates and quality of life (1–3).
With developments in transplantation immunobiology and the
application of novel immunosuppressants, numerous problems,
including acute rejection and short-term graft loss, have been
addressed, resulting in decreased mortality rates (2). However, improvements in long-term
graft survival are not commensurate. With further prolongation of
the transplantation period, renal allografts gradually lose their
function (3). This irreversible,
progressive loss is generally termed chronic renal allograft
dysfunction (CRAD) (1), which is
caused by immunological and non-immunological factors (2). As a non-immunological factor,
dyslipidemia leads to cardiovascular disease (CVD) and severely
impairs the long-term survival of grafts and patients (4–6). Of
note, 40–80% patients have been reported to exhibit hyperlipidemia
following renal transplantation (3,7).
Low-density lipoprotein (LDL) is critical in lipid
metabolism, as is its bioactive oxidized form, oxidized low-density
lipoprotein (ox-LDL). Lectin-like ox-LDL receptor-1 (LOX-1), the
major receptor of ox-LDL, is a key molecule in vascular endothelial
function disorder due to atherosclerosis, which can accelerate the
development of CRAD following renal transplantation (8–10).
In the current clinical setting, tacrolimus (FK-506) is widely used
as a selective immunosuppressor to improve the short-term survival
rates of grafts and patients. However, with further prolongation of
the transplantation period, its toxic effect aggravates CRAD and
can even increase long-term mortality rates (11). Although several reports have
focussed on this side effect, the underlying mechanism remains to
be elucidated. Current knowledge is limited to the suggestion that
lipid peroxidation and excess reactive oxygen species (ROS)
production caused by oxidative stress are crucial to this toxic
function (12).
Hyperlipidemia is closely associated with CRAD
(13). The early pathological
changes of atherosclerosis are similar to those of CRAD, and CRAD
is sometimes considered another form of atherosclerosis, induced by
immune injury and aggravated by immunological and non-immunological
factors. Metabolic syndrome is common in transplant patients, and
it is largely attributed to the use of immunosuppressant agents,
such as calcinurin inhibitors and prednisone. FK-506 is associated
with a lower incidence of hypertension and hypercholesterolaemia
than cyclosporine. However, 10–30% of FK-506 users still develop
hypercholesterolemia (14,15), and the higher the tacrolimus trough
level, the higher the rate of hypercholesterolemia (16). FK-506 is the most widely used
calcineurin inhibitor in transplantation. It is associated with a
complicated side effect profile, including post-transplant diabetes
mellitus, dyslipidemia and toxicity; which eventually result in
allograft fibrosis. However, the exact mechanism underlying this
effect is unclear. Therefore, the present study aimed to
investigate whether renal fibrosis caused by FK506 occurs via its
effects on high lipid levels; this effect was mimicked by
administering a high fat diet or inducing high ox-LDL levels.
The present study used in vitro and in
vivo models, which were exposed to FK506 and high lipid levels
to observe the expression levels of ox-LDL, LOX-1 and certain
chronic fibrosis-associated genes, including transforming growth
factor-β (TGF-β) and connective tissue growth factor (CTGF), to
determine the effects of FK506 and high lipid levels on CRAD.
Materials and methods
Cells and animals
The mouse proximal renal tubular epithelial cell
strain, NRK-52E, was purchased from the American Type Culture
Collection (Manassas, VA, USA). Healthy male Sprague-Dawley rats
6–8 weeks weighing 180–220 g were provided by the Experimental
Center of Sichuan University (Sichuan, China). Animals were fed and
maintained at 20–22°C in a 12 h light/dark cycle, with access to
standard chow and water ad libitum. Animal experimentation
was performed according to the Ethical Committee of Sichuan
University.
Cell experiments
Thawed NRK-52E cells were sustained in 60-mm dishes
with high-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco,
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum, 4 mmol/l glutamine, 100 µg/ml streptomycin
and 100 IU/ml penicillin at 37°C in 5% CO2. The culture medium was
refreshed 2–3 days later and the cells were passaged on day 4–5 at
a ratio of 1:3. The cells were then cultured in different treatment
groups, as follows: 80 µg/ml ox-LDL; 50 µg/ml FK506; 80 µg/ml
ox-LDL+50 µg/ml FK506; and vehicle, respectively. The expression of
LOX-1 was determined using immunofluorescence. The levels of ROS
and hydrogen peroxide were analyzed using ROS and hydrogen peroxide
test kits (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China), according to the manufacturer's protocol. Total RNA was
isolated using the SV Total RNA isolation system (Promega
Corporation, Madison, WI, USA), according to the manufacturer's
protocol. mRNA levels were determined using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
proteins levels were determined using western blotting. In
addition, three fluorescent-labeled small interfering (si)RNAs
(Guangdong Ruibo Biotechnology Co., Ltd., Guangdong, China) were
designed specifically against LOX-1, and were transfected into the
NRK-52E cells.
Animal experiments
A total of 60 Sprague-Dawley rats were divided
randomly into four treatment groups, which included a high-fat
group, FK506 group, high-fat+FK506 group and control group. The
FK506 rats were fed with common chow supplemented with 0.15
mg/kg/day FK506. The rats in the high-fat+FK506 group were fed a
high-fat diet (which consists of 40% lard, 5% milk powder, 5%
peanut flour, 5% sesame powder and 40% general fodder) in addition
to supplementation with 0.15 mg/kg/day FK506. Access to water and
food were unrestricted in all experiments. The rats were
anaesthetized with an intraperitoneal injection of 10% chloral
hydrate (35 mg/kg). The eyeballs of five randomly-selected rats in
each group at weeks 2, 4 and 8 were extracted to collect blood
samples (1.5 ml). Then the serum levels of total cholesterol,
triglycerides, LDL cholesterol and ox-LDL were analyzed. Following
blood lipid analyses, the randomly selected rats were sacrificed by
decapitation prior to kidney isolation and the remaining unselected
rats were sacrificed by high level of CO2. The isolated
kidney was divided in half, one of which was maintained in liquid
nitrogen for further analyses, including RT-qPCR analysis and
western blotting, whereas the other was fixed in 4% formalin for
24–48 h for pathological analysis and immunohistochemistry.
Immunofluorescence
The expression of LOX-1 was determined using
immunofluorescence. Briefly, cells were cultured on cover slips in
6-well plates. When the cells were grown to >80% confluence, the
cells were fixed in 4% paraformaldehyde for 10 min. The cells were
then washed with Tris-buffered saline with 0.1% Tween (TBST) twice
and blocked in 1.5% bovine serum albumin (Beyotime Institute of
Biotechnology, Shanghai, China)/TBST for 1 h at room temperature.
Then cells were incubated with primary antibodies against LOX-1
(1:100; cat no. DY1564; Santa Cruz Biotechnology Inc., Dallas, TX,
USA) overnight at 4°C. After washing in TBST 3 times, cells were
incubated with fluorescein isothiocyanate-conjugated goat
anti-rabbit immunoglobulin G (1:100; cat no. 2729S; Santa Cruz
Biotechnology Inc.) for 1 h and stained with DAPI for 5 min. The
images were viewed and recorded by Olympus BX40 and SPOT Flex 4.6
Prelim/4.6.0.0. (Diagnostic Instruments, Thermo Fisher Scientific,
Inc.).
ROS and H2O2
measurement
The levels of ROS and H2O2 in
NRK-52E cells were analyzed using ROS and
H2O2 test kits (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China), according to the
manufacturer's protocol. The Superoxide Anion Assay kit (BYJC0022,
Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was
used to simulate the reaction between xanthine and xanthine oxidase
in organisms. It produces the superoxide anion radical
O2•, which results in a purple coloring when Griess
reagent is added. This was measured at optical density (OD)550
using a spectrophotometer (SmartSpec™ 3000; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The influence of the checked samples to
O2 can be calculated using VitC as the reference. The
complex, which was produced by H2O2 and the
molybdic acid, was examined by measuring OD405 utilizing the
Hydrogen Peroxide Assay kit (K265-200, Nanjing Jiancheng
Bioengineering Institute, Nanjing, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using the SV Total RNA
isolation system (Promega Corporation, Madison, WI, USA), according
to the manufacturer's protocol. To compare LOX-1, TGF-β1 and CTGF
mRNA expression in different experimental groups, RNA samples were
isolated from cells or kidney tissue using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). First strand cDNA
synthesis was conducted using the cDNA synthesis kit (Toyobo Co.,
Ltd., Osaka, Japan). qPCR was performed with SYBR Green PCR Master
mix on the CFX96 Touch PCR system (Applied Biosystems, Thermo
Fisher Scientific, Inc.). The reaction consisted of 5 µl of 2X SYBR
Green mix, 1 µl cDNA, 1 µl of each primer at a concentration of 10
µmol/ml and 3 µl of ddH2O mixed gently in each well of
the Applied Biosystems MicroAmp Optical 96-Well Reaction Plate.
Reactions were performed under the following conditions: Initial
denaturation at 95°C for 30 sec (one cycle), followed by
denaturation at 95°C for 15 sec and annealing/extension at 60°C for
30 sec (40 cycles), with a final melt curve cycle of 5 sec at 65°C.
The endogenous Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
gene was used as the internal control for promoter screening. The
relative RNA expression was calculated with the comparative Cq
method, which was normalized to the internal references (17). Data were analyzed using CFX Manager
Software v3.1 Upgrader (Applied Biosystems, Thermo Fisher
Scientific, Inc.). The primers used were as follows: Forward:
5′-GAACGCTTCAGAGGAGTCCA-3′ and reverse: 5′-AGGCAATTCTCCCGACTTTT-3′
for LOX-1; forward: 5′-TGAGTGGCTGTCTTTTGACG-3′ and reverse:
5′-TTCTCTGTGGAGCTGAAGCA-3′ for TGF-β1; forward:
5′-AAGACACATTTGGCCCTGAC-3′ and reverse primer
5′-CCACAGAACTTAGCCCGGTA-3′ for CTGF; and forward:
5′-ACCACAGTCCATGCCATCAC-3′ and reverse: 5′-TCCACCACCCTGTTGCTGTA-3′
for GAPDH.
Western blot analysis
Levels of LOX-1 and TGF-β1 proteins in cells and
tissues were determined using western blotting. Cells or kidney
tissue were lysed with radioimmunoprecipitation assay buffer in (50
mM Tris base, 1.0 mM EDTA, 150 mM NaCl, 0.1% SDS, 1% Triton X-100,
1% sodium deoxycholate and 1 mM PMSF). The protein sample was
separated by 10% SDS-PAGE electrophoresis, and then transferred
from the gel onto the polyvinylidene difluoride membrane. After
blocking with 5% nonfat milk in TBST for 1 h at 37°C, the membrane
was incubated with the following primary antibodies: Goat
anti-LOX-1 polyclonal antibody (1:1;000; cat no. DY1564; Santa Cruz
Biotechnology Inc.) and mouse anti-TGF-β1 monoclonal antibody
(1:1,000; cat. no. MAB240; R&D Systems, Minneapolis, MN, USA)
and rabbit monoclonal anti-GAPDH antibody (1:5,000; cat. no.
ab181602, Abcam, Cambridge, CA, USA) at 4°C overnight. The next
day, membranes were washed in phosphate-buffered saline (PBS) with
0.1% Tween-20 for 15 min, three times. The membranes were then
incubated with horseradish peroxidase (HRP)-conjugated donkey
anti-goat (1:1,000; cat. no. A0181) and goat anti-mouse (1:1,000;
cat. no. A0216) secondary antibodies (Beyotime Institute of
Biotechnology) at 37°C for 1 h. Detection was performed with
enhanced chemiluminescence (Amersham Biosciences UK Ltd., Little
Chalfont, UK) and bands were quantified using ImageJ v2.1.4.7
(National Institutes of Health, Bethesda, MD, USA). GAPDH was used
as a control and was detected using anti-GAPDH antibodies (1:5,000;
cat no. ab181602; Abcam).
Small interfering (si)RNAs against
LOX-1 in NRK-52e cells
Three fluorescent-labeled siRNAs (Guangdong Ruibo
Biotechnology Co., Ltd., Guangdong, China) were designed
specifically against LOX-1, and were transfected into the NRK-52E
cells (sequences are listed in Table
I) Briefly, 2.5–3×104 cells per well were plated in
24-well plates. The following day, 50 pmol FAM-siRNA was combined
with 1.5 µl Lipofectamine 2000 transfection reagent (Invitrogen,
Thermo Fisher Scientific, Inc.) plus 100 µl of Opt-MEM. The control
RNA duplex was used to ensure that parallel experiments had equal
amounts of RNA. After 6 h of culture at 37°C, fluorescence
expression was observed under a fluorescence microscope in 4 fields
per well and repeat in 3 different wells. Transfection efficiency
was calculated using flow cytometry (Bio-Rad Labotatories, Inc.) to
determine the transfection efficiency according to the
manufacturer's instructions using the following equation: %
Transfection efficiency = (number of cells stained with fluorescent
positive control dye/total number of cells per field) × 100. All
experiments were conducted with 3 parallel duplicates.
 | Table I.siRNA sequences used in the present
study. |
Table I.
siRNA sequences used in the present
study.
| Name | Serial number | Target
sequence | siRNA sequence |
|---|
| SiLOX-1_001 | siB0822282236 |
CTGGAAGCTAAACGAGAAA | Sense:
5′-CUGGAAGCUAAACGAGAAA dTdT-3′ |
| (NM_133306) |
|
| Antisense: 3′-dTd
TGACCUUCGAUUUGCUCUUU-5′ |
| SiLOX-1_002 | siB0822282305 |
GCAGAATCAGAACCTCCAA | Sense:
5′-GCAGAAUCAGAACCUCCAA dTdT-3′ |
| (NM_133306) |
|
| Antisense: 3′-dTdT
CGUCUUAGUCUUGGAGGUU-5′ |
| SiLOX-1_003 | siB0822282327 |
GGAGAATTGCCTATCTTTA | Sense:
5′-GGAGAAUUGCCUAUCUUUA dTdT-3′ |
| (NM_133306) |
|
| Antisense: 3′-dTdT
CCUCUUAACGGAUAGAAAU-5′ |
Pathological analysis
The kidney tissues were fixed in 4% formaldehyde and
embedded in paraffin. They were cut into 4-µm slices and stained
with hematoxylin and eosin and Masson's trichrome stain. For
Masson's trichrome stain, the collagen stained green, muscle fibers
stained red and blood cells stained orange. Images were viewed
under a light microscope and analyzed using the Leica DMR+Q550
system (Leica Microsystems GmbH, Wetzlar, Germany).
Immunological analysis
LOX-1, TGF-β1 and CTGF proteins were detected and
localized by immunohistochemical staining. Kidney tissue sections
(4 µm) were deparaffinized in xylene and dehydrated with graduated
ethanol solutions and antigen retrieval was performed. Briefly, 3%
H2O2 was used to block the endogenous
peroxidase activity for 20 min at room temperature away from light.
The tissues were then incubated with the following primary
antibodies for 18 h at 4°C: Anti-LOX-1 (1:200, Sigma-Aldrich, St.
Louis, MO, USA), anti-TGF-β1 (1:400; Wuhan Boster Biological
Technology, Ltd., Wuhan, China), and anti-CTGF (sc-1493; 1:200
Santa Cruz Biotechnology Inc.). The slides were then washed with
PBS, and incubated with DAB. The images were gathered through Nikon
spot cool CCD (Nikon, Tokyo, Japan) and the staining results were
analyzed by the multimedia color pathological image analysis system
(Mais2000-P3; LEIKE SI China Electric appliance Co., Ltd., Hubei,
China).
Statistical analysis
For analysis of the immunohistochemical expression
of each protein, ROS level, lipid level and kidney damage between
all treatment groups, the nonparametric Kruskal-Wallis (analysis of
variance) test was used. Analysis between specific pairs of
treatment groups was compared using the Mann-Whitney unpaired
t-test. For comparisons before and after treatment, a two-tailed
paired t-test was used. Statistical software SPSS version 17.0
(SPSS Software, Inc., Chicago, IL, USA) was used for statistical
analysis. P<0.05, was considered to indicate a statistically
significant difference.
Results
Changes in cell morphology and levels
of LOX-1 in NRK-52E cells following treatment with FK506 and
ox-LDL
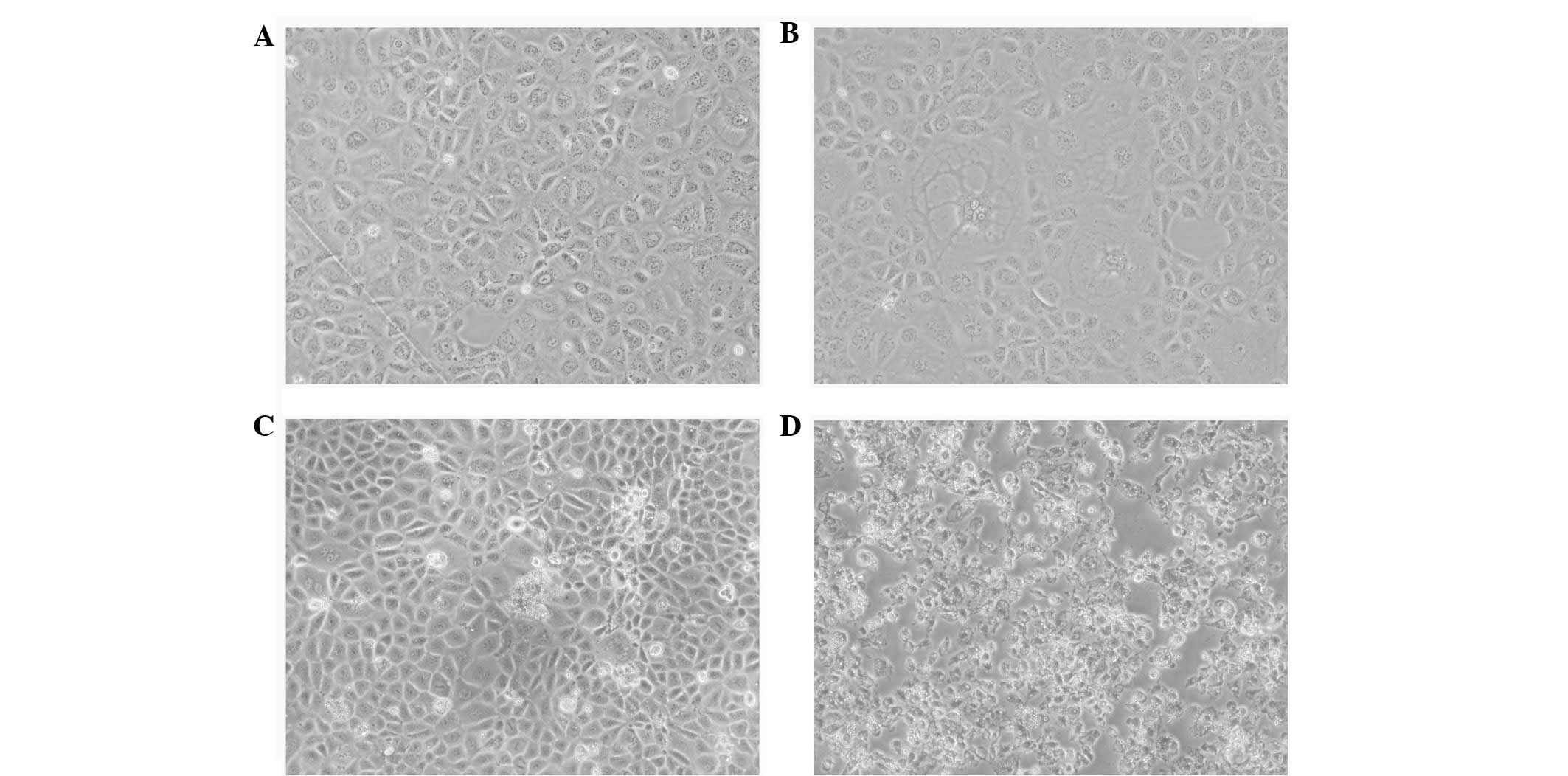
At 48 h post-ox-LDL treatment, a number of cells
showed vacuolized cytoplasms and enlarged extracellular spaces,
whereas cells in the FK506 group showed marked changes, with ground
bodies, enlarged extracellular spaces and an increased proportion
of floating dead cells. In addition to the majority of the
dual-treated cells exhibiting the morphology of the other two
groups, these cells also appeared aggregated and fragmented
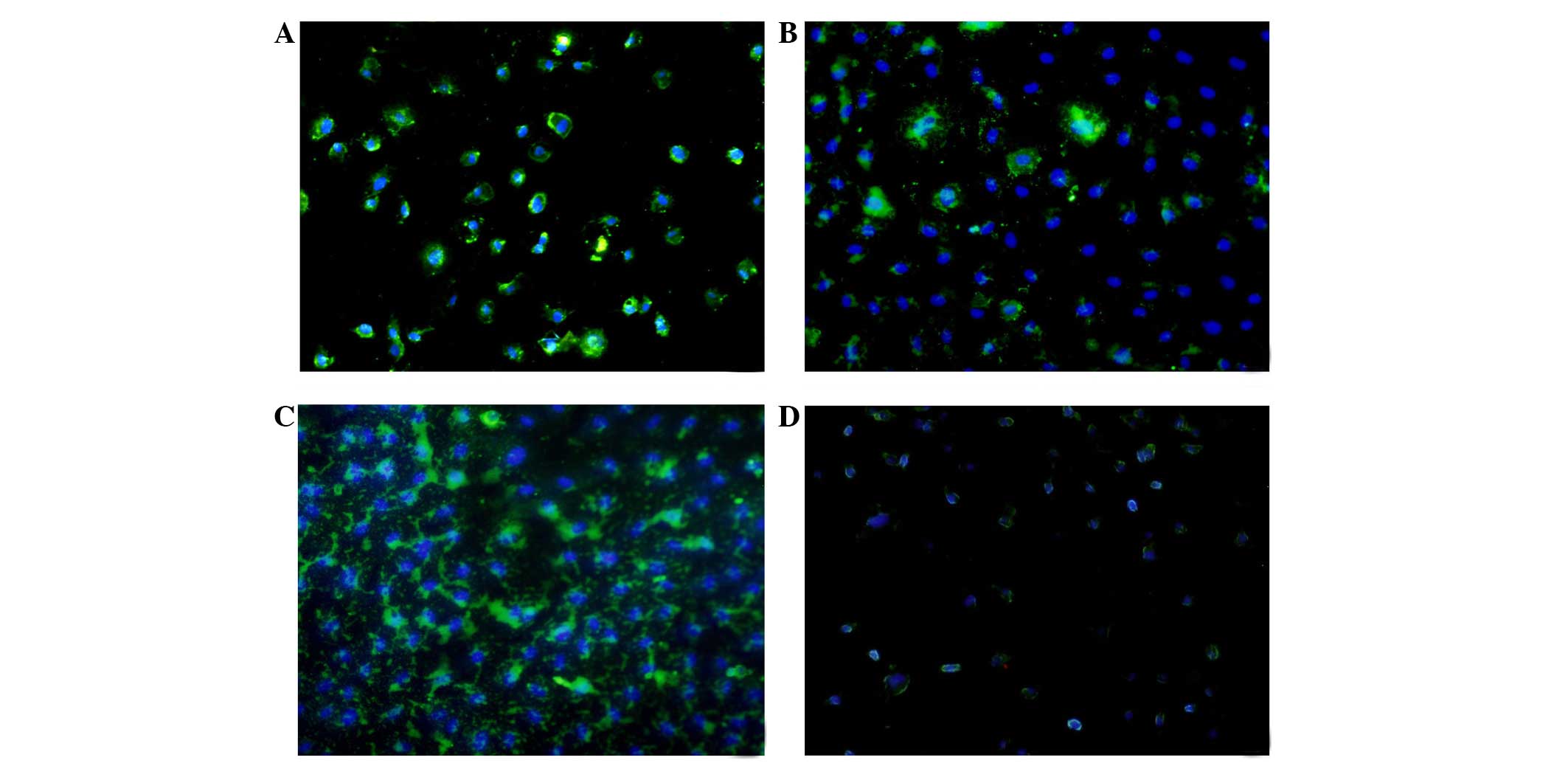
(Fig. 1). The immunofluorescence
results showed that LOX-1 was expressed 24 h following
administration in all groups, and reached the highest level at 48 h
(Fig. 2). The expression level of
LOX-1 in the ox-LDL group was 1.9-fold higher, compared with that
in control group, whereas the level in the FK506 group was 1.4-fold
higher, and that in the dual-treated group was 2.3-fold higher,
compared with the control group. The latter was highest among all
groups (P<0.01)
Levels of ROS and hydrogen peroxide
increase in NRK-52E cells following treatment with FK506 and
ox-LDL
The cells in all treatment groups were assessed
using the ROS and hydrogen peroxide test kits at 24, 48 and 72 h.
The levels of ROS and hydrogen peroxide increased at 24 h, and
reached the highest levels at 48 h, prior to decreasing thereafter.
The levels of ROS and hydrogen peroxide in the three treated groups
were higher, compared with those in the control group, and the
levels in the dual-treated group were the highest overall
(P<0.01).
Levels of LOX-1, TGF-β1 and CTGF in
NRK-52E cells following treatment with FK506 and ox-LDL
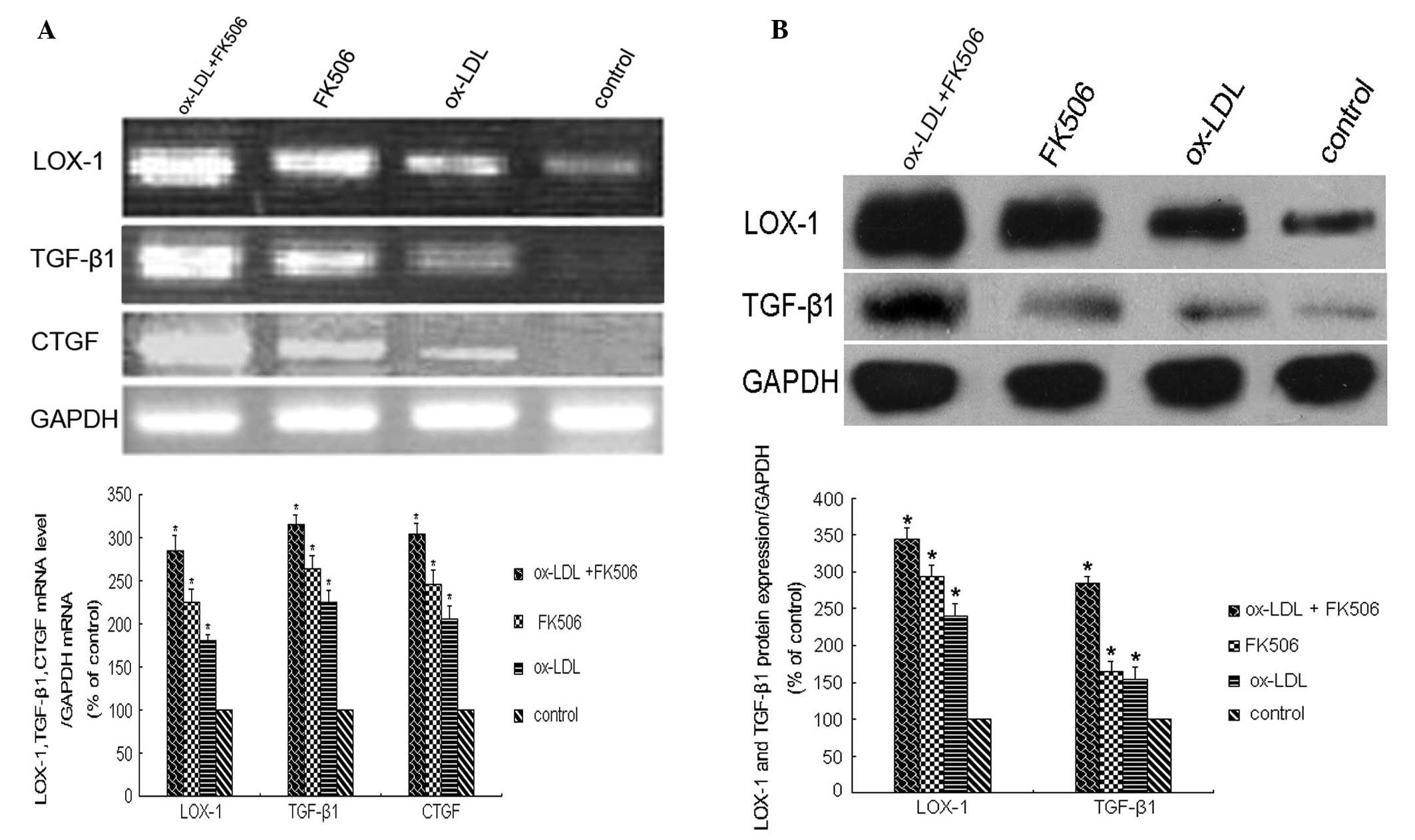
At 48 h post-treatment, the mRNA levels of
LOX-1, TGF-β1 and CTGF, determined using
RT-qPCR, were significantly increased (P<0.01), and were highest
in the dual-treated group (P<0.01; Fig. 3A). The results of the western
blotting to examine the protein levels of LOX-1 and TGF-β1 were
similar, with the levels of LOX-1 and TGF-β1 increasing
significantly following treatment (P<0.01). The highest levels
of these proteins were observed in the dual-treated cells
(P<0.01; Fig. 3B).
Decreased LOX-1 decreases the
expression of TGF-β1
In the present study, NRK-52E cells were transfected
with three FAM-labeled siRNAs against LOX-1, and the levels of
TGF-β1 in these cells were determined using flow cytometry 48 h
following transfection. The decrease in LOX-1 markedly reduced the
levels of TGF-β1. The levels of TGF-β1 were significantly decreased
following transfection with any of the siRNAs (P<0.01), with
siLOX-1_001 transfection resulting in the most marked
reduction.
Changes in serum lipid and ox-LDL
levels in rats following treatment with FK506 and a high-fat
diet
After 2 weeks on a high-fat diet combined with
FK506, the serum levels of total cholesterol, triglycerides and LDL
cholesterol in the rats were significantly increased (P<0.01).
This increase was enhanced at weeks 4 and 8, whereas the level of
high-density lipoprotein cholesterol were significantly decreased
(P<0.01). In all the treated rats, those in the high-fat
diet+FK506 had the highest serum lipid levels (P<0.01). The
increase in serum levels of ox-LDL, determined using ELISA, were
significant in all the treated rats at 2 weeks (P<0.01), and
this was more marked at 4 and 8 weeks (P<0.01). These levels
were highest in the rats in the high-fat diet+FK506 group.
Significant differences were observed between the three groups
(P<0.05).
Levels of ROS and rat kidney
fibrogenesis increase following treatment with FK506 and a high-fat
diet
The levels of ROS and hydrogen peroxide in the rat
kidneys increased in a time-dependent manner, and the highest
levels were observed in rats in the high-fat diet+FK506 group
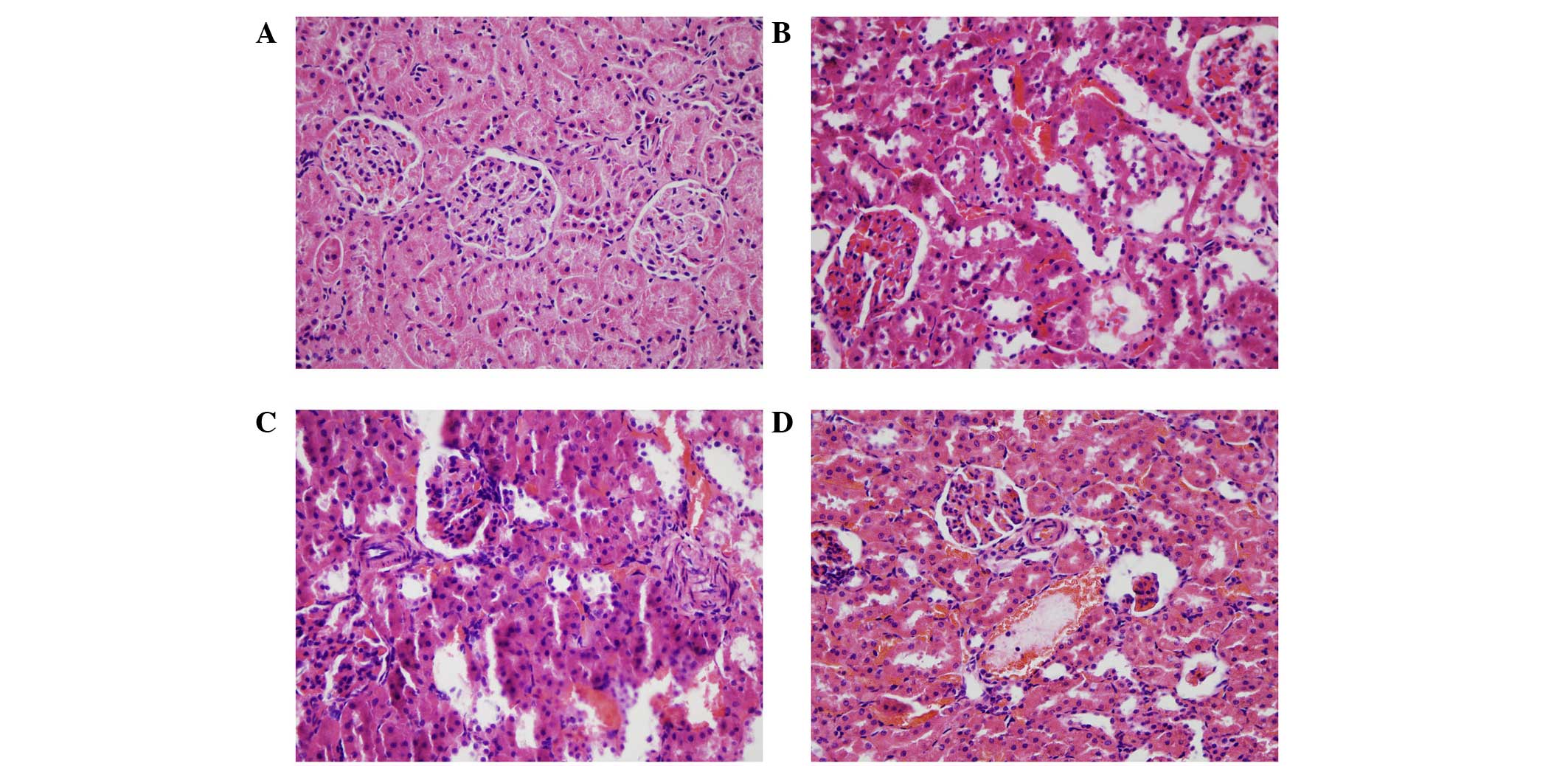
(P<0.01). The rat kidneys were isolated for H&E and Masson's
trichrome staining at 2, 4 and 8 weeks. The results of the H&E
staining showed that, in the kidneys treated for 2 weeks, the renal
tubules showed marginal swelling, with hemorrhaging of the
tubulointerstitial substance and glomeruli. At 4 weeks, the renal
tubule swelling became more marked, whereas the degree of
hemorrhage declined and hyperplasia was observed. At 8 weeks,
necrosis and atrophy were present in certain tubules and glomeruli,
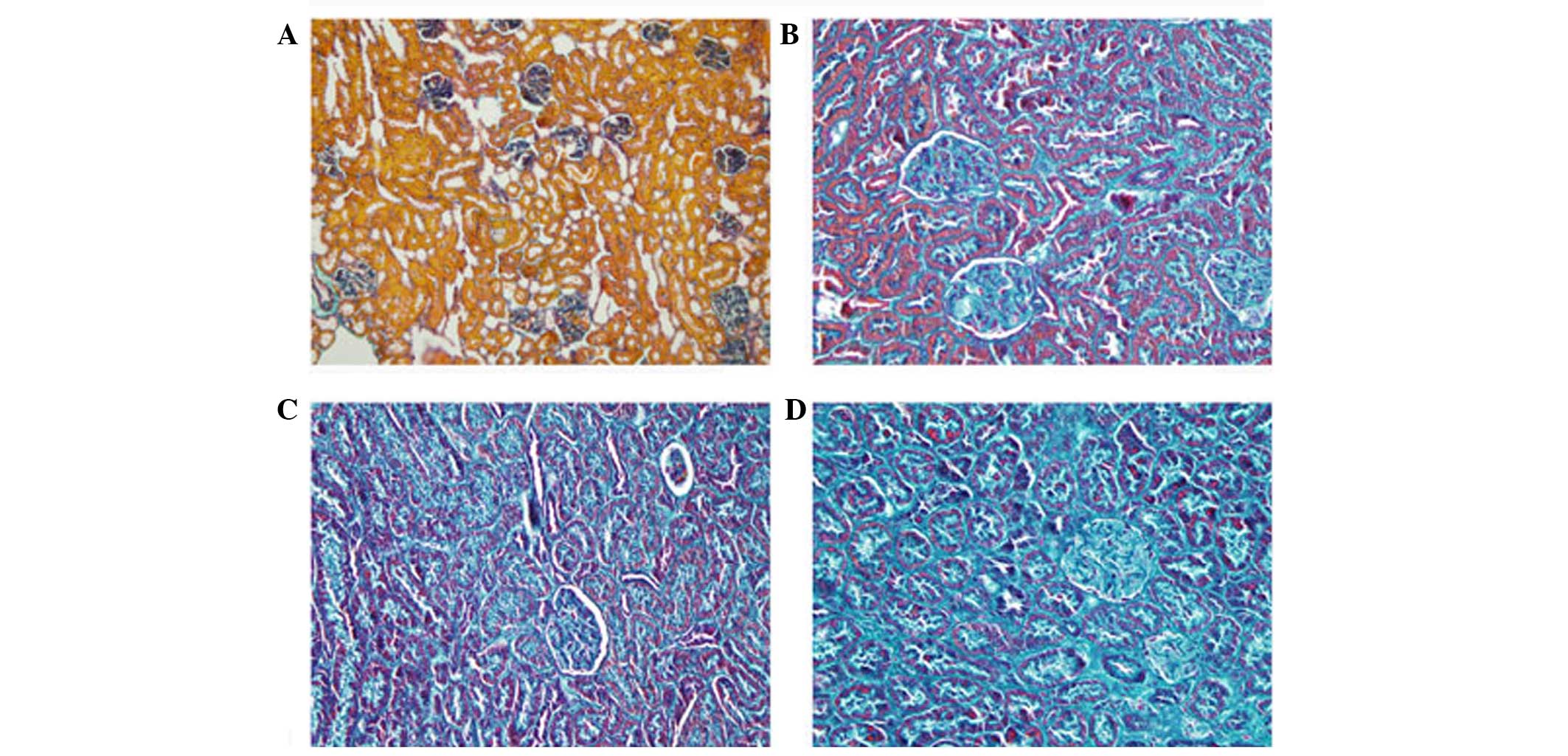
and bleeding of the tubulointerstitial substance remained (Fig. 4). In the Masson's trichrome
staining, green tubular and tubulointerstitial fibrogenesis was
observed, which increased over time (Fig. 5). Compared with the controls, the
degrees of fibrogenesis at weeks 2, 4 and 8 increased by 2.1-, 3.2-
and 5.3-fold, respectively (P<0.01). The increase in the degree
of fibrogenesis at 8 weeks was significantly higher, compared with
the increases at 2 and 4 weeks (P<0.05).
Levels of LOX-1, TGF-β1 and CTGF are
increased in rats following treatment with FK506 and a high-fat
diet
The results of the immunohistochemical analyses
showed that LOX-1 was expressed at low levels in the glomeruli and
tubules following 2 weeks of treatment, which increased over time,
and was highest in the rats in the high-fat diet+FK506 rats
(Fig. 6). Compared with the
control, the levels of LOX-1 in the rats of the high-fat diet+FK506
group were 1.5-, 2.0- and 3.1-fold higher at 2, 4 and 8 weeks,
respectively (P<0.01). The levels of TGF-β1 and CTGF were also
highest in this group of rats (Figs.
7 and 8). By contrast, the
highest level of TGF-β1 was observed at 4 weeks, which subsequently
decreased. Compared with the controls, these levels were increased
by 1.5-, 1.9- and 1.3-fold at 2, 4 and 8 weeks, respectively
(P<0.01). Similar to LOX-1, the levels of CTGF began to increase
in the tubules at 2 weeks, and continued to increase. At 8 weeks,
CTGF was also expressed in areas of the glomeruli. The levels of
CTGF increased 1.4-, 1.6- and 2-fold at 2, 4 and 8 weeks,
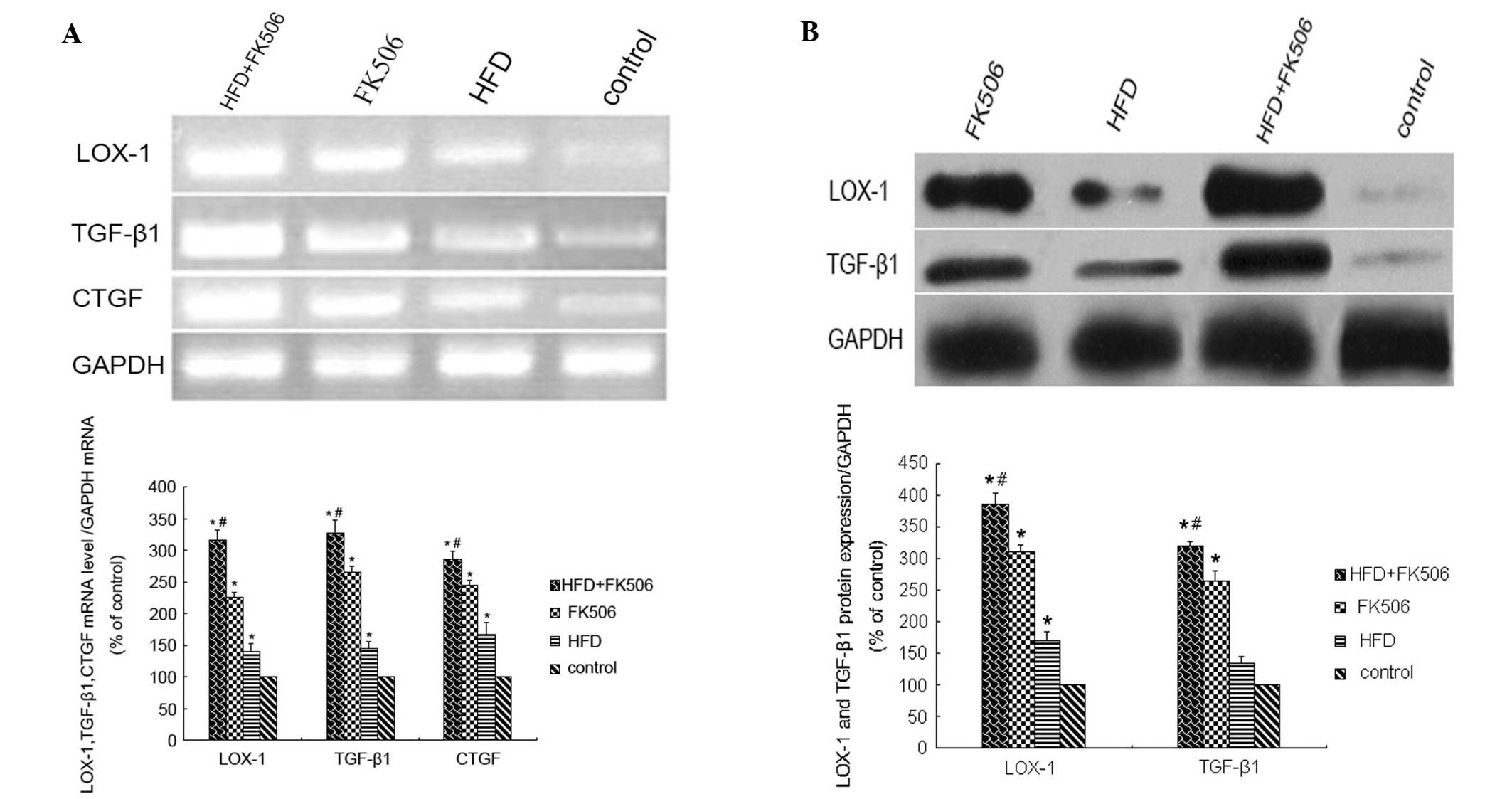
respectively (P<0.01). At week 4, the mRNA levels of
LOX-1, TGF-β1 and CTGF were significantly
increased (P<0.01), and were highest in the rats of the high-fat
diet+FK506 group (P<0.01), and significantly different between
the three treatment groups (P<0.05; Fig. 9A). The protein levels of LOX-1 and
TGF-β1 were also significantly increased (P<0.01), which were
highest in the rats of the high-fat diet+FK506 group (P<0.01),
and significantly different between the three treatment groups
(P<0.05; Fig. 9B).
Discussion
CRAD refers to renal hypofunction, which develops ≥3
months following renal transplantation and leads to clinical
findings of progressive serum creatinine increase, proteinuria and
hypertension, and histological changes of vascular intimal
hyperplasia, tubular atrophy, interstitial fibrosis and chronic
transplant glomerulopathy (8–13).
Differential diagnoses that require exclusion include acute
rejection, renal diseases and renal toxicity (18). As one of the typical
characteristics of CRAD is interstitial fibrosis, and the early
pathological changes of atherosclerosis are so similar, CRAD is
sometimes considered to be another form of atherosclerosis, which
is induced by immune injury and aggravated by immunological and
non-immunological factors (13).
Hyperlipidemia and dyslipidemia are known risk
factors for Bichat's tunic injuries, artery stenosis and
atherosclerosis (19). Of note,
40–80% patients are reported to have hyperlipidemia following renal
transplantation (3,7). In a previous statistical study of 42
patients with CRAD, their blood lipid levels were found to be
significantly higher, compared with those of other renal transplant
patients without CRAD symptoms (20). In a follow-up clinical study of 28
patients with CRAD, diagnosed with kidney puncture biopsy, the Baff
grades of the patients were positively correlated with renal
function and blood lipid levels (21). Therefore, it is suggested that
hyperlipidemia and dyslipidemia are important in the development of
CRAD as non-immunological factors.
LDL and its bioactive oxidized form, ox-LDL, are
essential in lipid metabolism. LOX-1, the major receptor of ox-LDL,
can accelerate the development of CRAD following renal
transplantation (8–10). LOX-1 was first identified as the
receptor of ox-LDL in 1997 by Sawamura (22). Studies have revealed that, in
certain pathological circumstances, including hyperlipidemia and
atherosclerosis, LOX-1 is expressed at high levels not only in
aortas, carotid arteries, thoracic aortas, coronary arteries and
the venous plexus, but also in macrophages, smooth muscle cells,
fibroblasts and platelets (23–25).
LOX-1 is able to bind to and assist in the digestion of ox-LDL,
which is recognized by other signaling molecules and induces the
dysfunction or apoptosis of vascular endothelial cells (26). LOX-1 is expressed at high levels in
atherosclerotic plaques and atherosclerotic plaque-derived cells,
and its expression can be induced by specific proinflammatory or
pro-atherosclerotic cytokines (23,24).
Therefore, LOX-1 is crucial to the development of several CVDs,
including atherosclerosis and hypertension (10).
Post-transplantation dyslipidemia can be achieved by
a number of factors, and the application of immunosuppressors,
including FK506, are likely to be the most important (16). At present, the exact mechanism
underlying FK506 toxicity remains to be fully elucidated, although
lipid peroxidation and excess ROS production due to oxidative
stress are known to be important in this process (12,16).
The results of the present study were consistent with this, which
showed that high levels of ROS and hydrogen peroxide were produced
following treatment, which was particularly true of ox-LDL
following FK506 combination treatment. In a previous study, Apanay
et al used different doses of Cs11A to treat transplant
patients for 2 months, and found that the levels of ox-LDL
increased significantly at high and normal doses of Cs11A, compared
with low doses (27). Similar
results were obtained in the present study, indicating that the
effect of FK506 on the oxidative stress response was enhanced by
high-fat exposure and vice versa. The high levels of ox-LDL
resulting from this enhancement may cause irreversible damage to
the blood vessel walls of transplanted grafts.
The importance of TGF-β1 and its downstream gene,
CTGF, is well established. TGF-β1 is overexpressed in early
fibrosis, and induces the expression of CTGF to maintain fibrosis,
which is associated with CRAD (28,29).
Following exposure to a high-fat diet, increased levels of ox-LDL
and LOX-1 can promote the expression of TGF-β1 and CTGF (30). In the present study, H&E and
Masson's trichrome staining showed kidney injury and the
enhancement of fibrosis following exposure to a high-fat diet and
FK506 treatment, which worsened over time. The expression levels
determined in vivo and in vitro provided additional
evidence that high-fat exposure and FK506 were able to increase the
expression levels of TGF-β1 and CTGF, with the most marked fibrosis
developing following combined high-fat diet+FK506 treatment. The
above results suggested that fibrosis genes can function to maximum
extent under such circumstance and cause marked damage to renal
grafts. Furthermore, the results of the present study demonstrated
that siRNA against LOX-1 inhibited the synthesis of TGF-β1,
demonstrating that LOX-1 was capable of regulating TGF-β1. Ox-LDL
can promote levels of fibrinogen and increase the risk of allograft
fibrosis through a synergistic mechanism (31). These findings may provide further
information to assist in the therapy of post-renal transplant
fibrosis.
As common complications of renal transplantation,
hyperlipidemia and dyslipidemia severely impair long-term survival
rates of patients. At present, increasing evidence supports the
hypothesis that immunosuppressants, particularly glucocorticoids or
cyclosporin, are critical in dyslipidemia, resulting in CVD and
CRAD following transplantation (17,32,33).
However, the underlying mechanism remains to be fully elucidated.
How ox-LDL and LOX-1 function in these processes also remains to be
fully elucidated, as they are involved in multiple signaling
pathways. Therefore, further investigations are required to
determine the mechanism of immunosuppressant-induced hyperlipidemia
or dyslipidemia resulting in CRAD. This may assist in the
development of future therapies to reduce the occurrence of CVD or
CRAD following renal transplantation, and prolong renal transplant
graft and patient survival rates.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 2012sz0026), the Technology
Support Plan of Sichuan Province (grant no. 2014SZ0210) and the
Scientific Research Projects of Sichuan Health Bureau (grant no. YN
20140030).
References
|
1
|
Womer KL, Vella JP and Sayegh MH: Chronic
allograft dysfunction: Mechanisms and new approaches to therapy.
Semin Nephrol. 20:126–147. 2000.PubMed/NCBI
|
|
2
|
Hernandez-Fuentes MP and Lechler RI:
Chronic graft loss. Immunological and non-immunological factors.
Contrib Nephrol. 146:54–64. 2005.PubMed/NCBI
|
|
3
|
Divakar D, Bailey RR, Frampton CM, George
PM, Walmsley TA and Murphy J: Hyperlipidemia in stable renal
transplant recipients. Nephron. 59:423–428. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roodnat JI, Mulder PG, Zietse R,
Rischen-Vos J, van Riemsdijk IC, IJzermans JN and Weimar W:
Cholesterol as an independent predictor of outcome after renal
transplantation. Transplantation. 69:1704–1710. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Serón D, Moreso F, Ramón JM, Hueso M,
Condom E, Fulladosa X, Bover J, Gil-Vernet S, Castelao AM, Alsina J
and Grinyó JM: Protocol renal allograft biopsies and the design of
clinical trials aimed to prevent or treat chronic allograft
nephropathy. Transplantation. 69:1849–1855. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wissing KM, Abramowicz D, Broeders N and
Vereerstraeten P: Hypercholesterolemia is associated with increased
kidney graft loss caused by chronic rejection in male patients with
previous acute rejection. Transplantation. 70:464–472. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friemann S, Feuring E, Padberg W and Ernst
W: Improvement of nephrotoxicity, hypertension, and lipid
metabolism after conversion of kidney transplant recipients from
cyclosporine to tacrolimus. Transplant Proc. 30:1240–1242. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vela CG, Cristol JP, Descomps B and Mourad
G: Prospective study of lipid disorders in FK506-versus
cyclosporine-treated renal transplant patients. Transplant Proc.
32:3982000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruan XZ, Varghese Z, Fernando R and
Moorhead JF: Cytokine regulation of low-density lipoprotein
receptor gene transcription in human mesangial cells. Nephrol Dial
Transplant. 13:1391–1397. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen M, Masaki T and Sawamura T: LOX-1,
the receptor for oxidized low-density lipoprotein identified from
endothelial cells: Implications in endothelial dysfunction and
atherosclerosis. Pharmacol Ther. 95:89–100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shihab FS, Bennett WM, Tanner AM and Andoh
TF: Mechanism of fibrosis in experimental tacrolimus
nephrotoxicity. Transplantation. 64:1829–1837. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khanna AK and Pieper GM: NADPH oxidase
subunits (NOX-1, p22phox, Rac-1) and tacrolimus-induced
nephrotoxicity in a rat renal transplant model. Nephrol Dial
Transplant. 22:376–385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobashigawa JA and Kasiske BL:
Hyperlipidemia in solid organ transplantation. Transplantation.
63:331–338. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheung CY, Wong KM, Chan HW, Liu YL, Chan
YH, Wong HS, Chak WL, Cho KS, Chau KF and Li CS: Paired kidney
analysis of tacrolimus and cyclosporine microemulsion-based therapy
in Chinese cadaveric renal transplant recipients. Transpl Int.
19:657–666. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ekberg H, Bernasconi C, Tedesco-Silva H,
Vitko S, Hugo C, Demirbas A, Acevedo RR, Grinyó J, Frei U,
Vanrenterghem Y, et al: Calcineurin inhibitor minimization in the
Symphony study: Observational results 3 years after
transplantation. Am J Transplant. 9:1876–1885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li HY, Li B, Wei YG, Yan LN, Wen TF, Zhao
JC, Xu MQ, Wang WT, Ma YK and Yang JY: Higher tacrolimus blood
concentration is related to hyperlipidemia in living donor liver
transplantation recipients. Dig Dis Sci. 57:204–209. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Racusen LC, Halloran PF and Solez K: Banff
2003 meeting report: New diagnostic insights and standards. Am J
Transplant. 4:1562–1566. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mango R, Clementi F, Borgiani P, Forleo
GB, Federici M, Contino G, Giardina E, Garza L, Fahdi IE, Lauro R,
et al: Association of single nucleotide polymorphisms in the
oxidised LDL receptor 1 (OLR1) gene in patients with acute
myocardial infarction. J Med Genet. 40:933–936. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dimény E, Fellström B, Larsson E, Tufveson
G and Lithell H: Hyperlipoproteinemia in renal transplant
recipients: Is there a linkage with chronic vascular rejection?
Transplant Proc. 25:2065–2066. 1993.PubMed/NCBI
|
|
21
|
Dimény E, Wahlberg J, Lithell H and
Fellström B: Hyperlipidaemia in renal transplantation-risk factor
for long-term graft outcome. Eur J Clin Invest. 25:574–583. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sawamura T, Kume N, Aoyama T, Moriwaki H,
Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T and Masaki
T: An endothelial receptor for oxidized low-density lipoprotein.
Nature. 386:73–77. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen M, Kakutani M, Minami M, Kataoka H,
Kume N, Narumiya S, Kita T, Masaki T and Sawamura T: Increased
expression of lectin-like oxidized low density lipoprotein
receptor-1 in initial atherosclerotic lesions of Watanabe heritable
hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol.
20:1107–1115. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kataoka H, Kume N, Miyamoto S, Minami M,
Moriwaki H, Murase T, Sawamura T, Masaki T, Hashimoto N and Kita T:
Expression of lectinlike oxidized low-density lipoprotein
receptor-1 in human atherosclerotic lesions. Circulation.
99:3110–3117. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Draude G, Hrboticky N and Lorenz RL: The
expression of the lectin-like oxidized low-density lipoprotein
receptor (LOX-1) on human vascular smooth muscle cells and
monocytes and its down-regulation by lovastatin. Biochem Pharmacol.
57:383–386. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cominacini L, Rigoni A, Pasini AF, Garbin
U, Davoli A, Campagnola M, Pastorino AM, Lo Cascio V and Sawamura
T: The binding of oxidized low density lipoprotein (ox-LDL) to
ox-LDL receptor-1 reduces the intracellular concentration of nitric
oxide in endothelial cells through an increased production of
superoxide. J Biol Chem. 276:13750–13755. 2001.PubMed/NCBI
|
|
27
|
Apanay DC, Neylan JF, Ragab MS and Sgoutas
DS: Cyclosporine increases the oxidizability of low-density
lipoproteins in renal transplant recipients. Transplantation.
58:663–669. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leask A: Transcriptional profiling of the
scleroderma fibroblast reveals a potential role for connective
tissue growth factor (CTGF) in pathological fibrosis. Keio J Med.
53:74–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng O, Thuillier R, Sampson E, Schultz
G, Ruiz P, Zhang X, Yuen PS and Mannon RB: Connective tissue growth
factor is a biomarker and mediator of kidney allograft fibrosis. Am
J Transplant. 6:2292–2306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sohn M, Tan Y, Wang B, Klein RL,
Trojanowska M and Jaffa AA: Mechanisms of low-density
lipoprotein-induced expression of connective tissue growth factor
in human aortic endothelial cells. Am J Physiol Heart Circ Physiol.
290:H1624–H1634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin S, Mathis AS, Rosenblatt J, Minko T,
Friedman GS, Gioia K, Serur DS and Knipp GT: Insights into
cyclosporine A-induced atherosclerotic risk in transplant
recipients: Macrophage scavenger receptor regulation.
Transplantation. 77:497–504. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Massy ZA: Hyperlipidemia and
cardiovascular disease after organ transplantation.
Transplantation. 72:S13–S15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Varghese Z, Fernando RL, Turakhia G,
Psimenou E, Fernando ON, Sweny P, Powis SH and Moorhead JF:
Calcineurin inhibitors enhance low-density lipoprotein oxidation in
transplant patients. Kidney Int Suppl. 71:S137–S140. 1999.
View Article : Google Scholar : PubMed/NCBI
|