Introduction
Hepatitis B virus (HBV) infection is an important
cause of chronic hepatitis and remains a significant public health
issue worldwide. An estimated 350 million individuals are
chronically infected with HBV worldwide (1,2).
Patients with chronic hepatitis have a high risk of progression to
cirrhosis and hepatocellular carcinoma (HCC), and early diagnosis
of liver cirrhosis is essential for their prognosis. The diagnosis
of liver cirrhosis is usually achieved by liver biopsy, imaging
examinations, and clinical signs. However, use of a serum biomarker
for diagnosis of liver cirrhosis is rare.
Autoantibodies comprise a novel type of serum
biomarker identified in recent years. Based on the various
environments in diseases, abnormal proteins may lead to
antigenicity, which can drive the humoral immune responses to
produce autoantibodies. The presence of serum autoantibodies has
been observed not only in autoimmune diseases, but also in
non-autoimmune diseases, such as cancer (3–5). A
recent study showed that autoantibodies against α-enolase (ENO1)
had potential diagnostic value in liver fibrosis (6). Furthermore, autoantibodies may have
various advantages as immunodiagnostic markers, as the magnified
signals of autoantibodies can be easier to detect than the
autoantigens themselves (3,7).
Our previous study, identified potential
tumor-associated antigens in hepatocellular carcinoma using serum
proteomics analysis (8). Notably,
there were elevated levels of autoantibodies in raised against some
of these autoantigens in patients with liver cirrhosis. Chronic
hepatitis patients have a high risk of progression to cirrhosis and
HCC, and early diagnosis of liver cirrhosis is essential for their
prognosis. Therefore, in the present study, our established protein
chip technology (8) was used to
evaluate the diagnostic value of these autoantibodies in liver
cirrhosis with the aim of distinguishing HBV-related liver
cirrhosis from chronic hepatitis B (CHB), and to provide the basis
for further research on autoantibody biomarkers for staging liver
fibrosis.
Materials and methods
Patients
A total of 443 participants were recruited for
clinical evaluation, comprising 89 patients with HBV-related liver
cirrhosis (65 men and 24 women, aged 27–89 years with a median age
of 46.0 years), 89 patients with CHB (61 men and 28 women, aged
23–75 years with a median age of 39.3 years), and 265 healthy
controls (166 men and 99 women, aged 20–66 years with a median age
of 45.8 years). The patients were retrospectively recruited between
2010 and 2014 from the Liver Research Center, Beijing Friendship
Hospital, Capital Medical University, Department of Minimally
Invasive Interventional Radiology, Beijing Youan Hospital, Capital
Medical University, and Department of Hepatopancreatobiliary and
Splenic Medicine, Affiliated Hospital of Medical College of Chinese
People's Armed Police Force. The healthy controls were qualified
blood donors with normal liver biochemistry, no history of liver
disease and no malignant disease.
Inclusion criteria: The diagnosis of CHB included
the presence of HBsAg ≥6 months, HBV DNA concentrations
≥103 copies/ml, and elevation of serum alanine
aminotransferase (9), without
evidence of hepatitis C virus infection or a history of alcohol
abuse. The diagnosis of liver cirrhosis met at least one of the
following three criteria: i) Histologically confirmed cirrhosis
(Ishak 5/6 or Metavir F4); ii) Presence of portal hypertension
(endoscopy showing esophageal varices, or imaging showing liver
surface nodularity, splenomegalia or hypersplenism) and liver
dysfunction (albumin <35.0 g/l, or International Normalized
Ratio >1.3); iii) chronic liver disease patients experiencing
variceal bleeding, ascites or encephalopathy. Patients with
autoimmune liver diseases, such as autoimmune hepatitis, primary
biliary cirrhosis, and other autoimmune diseases, such as systemic
lupus erythematosus, rheumatoid arthritis, and diabetes mellitus,
were excluded from the study.
All serum samples were stored at −80°C until
testing. The study protocol was approved by the Clinical Research
Ethics Committee of Beijing Friendship Hospital, Capital Medical
University (Beijing, China).
Protein microarray analysis for
clinical evaluation of candidate autoantigens
Recombinant proteins for candidate autoantigens
screened in our previous study available for enzyme-linked
immunosorbent assay were purchased when required, including
including insulin-like growth factor 2 mRNA-binding protein 2
(IMP-2), calreticulin (CRT), centromere protein F (CENPF), 60 kDa
heat shock protein (HSP60), protein disulfide-isomerase (PDIA1),
aminoacylase-1 (ACY1), α-enolase (ENO1), annexin A4 (ANXA4), Ig κ
chain C region (IGKC), Regucalcin (RGN), type II cytoskeletal 1
(K2C1), heat shock 70 kDa protein 6 (HSP A6), α-1-antitrypsin
(AIAT), fibrinogen β chain (FGB), selenium-binding protein 1(SBP1),
peroxiredoxin 3 (PRDX3), histidine triad nucleotide-binding protein
1 (HINT1), tubulin β-4B chain (TUBB4B), ATP synthase subunit β
(ATPB) and retinal dehydrogenase 1 (ALDH1A1). Two other
autoantigens, heterogeneous nuclear ribonucleoprotein A2 (hnRNP A2)
and apoptosis-inducing factor (AIF), were prepared as described
previously (8).
Preparation and detection of the protein microarray
were performed according to our previous study (8). Briefly, the screened protein antigens
were diluted to individually optimized concentrations and
robotically attached to aldehyde-activated glass slides in ordered
arrays by a computer-controlled microchip spotting instrument
(Cartesian Technologies, Irvine, CA, USA). Human IgG
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) was used as a
positive control and an internal standard for signal intensity
calibration in each test, while sample liquid and bovine serum
albumin (Sigma-Aldrich; Merck Millipore) were used as negative
controls.
The prepared antigen microarrays were blocked in PBS
containing 25% fetal bovine serum (Sigma-Aldrich; Merck Millipore)
at 37°C for 2 h and then washed with PBST [0.01 mol/l PBS (pH
7.2–7.4) with 0.05% Tween 20] for 10 sec and repeated three times.
Then the serum samples were added to the microarray at 1:5 dilution
with PBS with a 10 µl sample for each matrix and incubated at 37°C
for 30 min. The microarray was washed six times with PBST and
incubated with horseradish peroxidase-labeled rabbit anti-human IgG
(cat. no. A8792; Sigma-Aldrich; Merck Millipore) with a working
concentration of 1:8,000 for a further 30 min at 37°C and followed
by six repetitions of PBST washing. After it was dried at room
temperature, the immunoreactive spots were detected by an enhanced
chemiluminescence Western Blotting kit (Merck Millipore) using a
PLNT YQ001 scanner (Puli Knight Biotechnology Co. Ltd, Beijing,
China). The signal intensities of the spots and the background
values were measured by Array Vision 7.0 (Imaging Research, St.
Catharines, Canada).
Statistical analysis
Statistical analyses were performed with SPSS
version 19.0 (SPSS Inc., Chicago, IL, USA) and MedCalc version
10.4.7.0 (MedCalc Software, Oostende, Belgium). Receiver-operating
characteristic (ROC) curves were used to assess the sensitivity,
specificity, and area under curve (AUC) value with 95% confidence
interval (95% CI) to evaluate the diagnostic value of the serum
markers. The optimum cut-off values were determined by calculating
the Youden index, and the corresponding signal intensity number was
set as the cut-off value for positivity of individual
autoantibodies to protein antigens. The correlation between
clinical parameters and autoantibody positivities were analyzed by
the χ2 test with Yate's correction. P<0.05 was considered to
indicate a statistically significant difference.
Results
High-throughput clinical
evaluation
For the high-throughput clinical evaluation, 22
candidate protein antigens were used for protein microarrays:
IMP-2, CRT, CENPF, HSP60, PDIA1, ACY1, ENO1, ANXA4, IGKC, RGN,
K2C1, HSP A6, AIAT, FGB, SBP1, PRDX3, HINT1, TUBB4B, ATPB, ALDH1A1,
AIF and hnRNP A2. A schematic representation of the antigen array
is shown in Fig. 1A and B, and
representative scanned images of protein microarrays are shown in
Fig. 1C.
 | Figure 1.Schematic representation of the
protein microarray for high-throughput clinical evaluation. (A)
Scanned image of a representative antigen array. (B) Design of the
protein microarray. The numbered spots correspond to the following
antigens: •, IgG; 1, IGKC; 2, HSP60; 3, AIAT; 4, IMP-2; 5, FGB; 6,
HSP A6; 7, ATPB; 8, BSA; 9, ALDH1A1; 10, PDIA1; 11, ENO1; 12,
ANXA4; 13, CENPF; 14, SBP1; 15, ACY1; 16, hnRNP; 17, K2C1; 18, AIF;
19, CRT; 20, RGN; 21, PRDX3; 22, HINT1; 23, TUBB4B; 24, sample
liquid. (C) Representative microarray detection of serum samples.
Individual arrays were incubated with sera from HBV-related liver
cirrhosis patients, CHB patient and healthy controls (left to
right). IGKC, Ig κ chain C region; HSP60, 60 kDa heat shock
protein; AIAT, α-1-antitrypsin; IMP-2, insulin-like growth factor 2
mRNA-binding protein 2; FGB, fibrinogen β chain; HSP A6, heat shock
70 kDa protein 6; ATPB, ATP synthase subunit β; BSA, bovine serum
aldumin; ALDH1A1, retinal dehydrogenase 1; PDIA1, protein
disulfide-isomerase; ENO1, α-enolase; ANXA4, annexin A4; CENPF,
centromere protein F; SBP1, selenium-binding protein 1; ACY1,
aminoacylase-1; hnRNP, heterogeneous nuclear ribonucleoprotein A2;
K2C1, type II cytoskeletal 1; AIF, apoptosis-inducing factor; CRT,
calreticulin; RGN, regucalcin; PRDX3, peroxiredoxin 3; HINT1,
histidine triad nucleotide-binding protein 1; TUBB4B, tubulin β-4B
chain. |
After microarray detection with the 443 serum
samples, ROC curves were generated for all 22 protein antigens
based on the individual signal intensities. The results showed that
the autoantibodies against ACY1, HINT1, IMP-2, PRDX3, HSP A6, AIF
and RGN differed significantly (P<0.05) between HBV-related
liver cirrhosis and CHB patients, with an AUC value of >0.7,
which is a recognized standard for biomarkers that have a promising
diagnostic value in general (Fig.
2A, Table I). The levels of
autoantibodies against these protein antigens in CHB and
HBV-related liver cirrhosis showed trends toward increases. Among
them, the ACY1 autoantibody showed the highest diagnostic value for
HBV-related liver cirrhosis, with AUC value of 0.872, a sensitivity
of 77.3% and specificity of 85.0% (Table I).
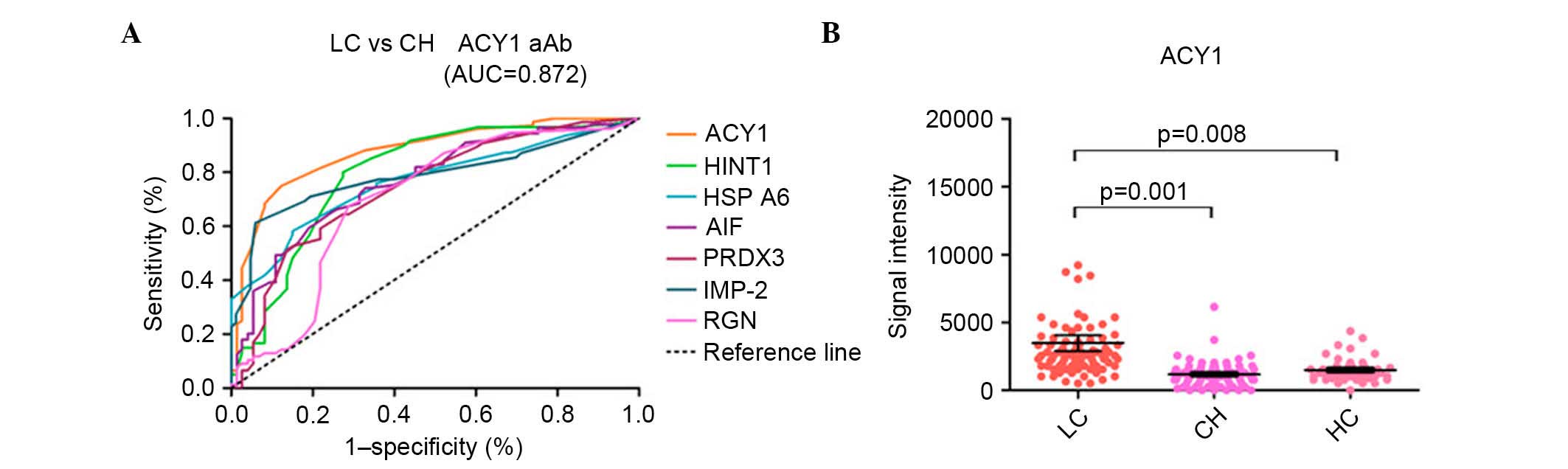 | Figure 2.ROC curves of seven candidate
autoantigens in discriminating between HBV-related liver cirrhosis
and CHB with AUC values >0.7 and representative scatter diagram
of the signal intensity of ACY1. (A) Receiver-operating
characteristic curves of the seven autoantibodies for
discriminating between HBV-related liver cirrhosis and CHB with AUC
values >0.7. (B) Scatter diagram of the signal intensity of
ACY1. The black horizontal lines indicate the mean values, and the
error bars are standard errors. LC, liver cirrhosis; CH, chronic
hepatitis; HC, healthy controls; CHB, chronic hepatitis B; HBV,
hepatitis B virus; AUC, area under the curve; ACY1, aminoacylase-1;
HINT1, histidine triad nucleotide-binding protein 1; HSP A6, heat
shock 70 kDa protein 6; AIF, apoptosis-inducing factor; PRDX3,
peroxiredoxin 3; IMP-2, insulin-like growth factor 2 mRNA-binding
protein 2; RGN, regucalcin. |
 | Table I.Diagnostic value of autoantibodies to
distinguish hepatitis B virus-related liver cirrhosis from chronic
hepatitis B. |
Table I.
Diagnostic value of autoantibodies to
distinguish hepatitis B virus-related liver cirrhosis from chronic
hepatitis B.
| Autoantibody | AUC value | 95% CI | Cut-off value | Sensitivity(%) | Specificity(%) | PPV(%) | NPV(%) | P-value |
|---|
| ACY1 | 0.872 | 0.810–0.934 | 1920 | 77.3 | 85.0 | 85.0 | 77.3 | <0.0001 |
| HINT1 | 0.794 | 0.709–0.878 | 1856 | 85.5 | 70.0 | 72.3 | 84.0 | <0.0001 |
| IMP-2 | 0.777 | 0.691–0.863 | 896 | 62.3 | 92.9 | 88.4 | 73.9 | <0.0001 |
| PRDX3 | 0.777 | 0.696–0.859 | 1728 | 54.5 | 90.0 | 85.7 | 64.3 | <0.0001 |
| HSP A6 | 0.765 | 0.682–0.848 | 1600 | 58.5 | 83.3 | 79.2 | 64.9 | <0.0001 |
| AIF | 0.756 | 0.675–0.837 | 5504 | 60.0 | 80.0 | 80.0 | 60.0 | <0.0001 |
| RGN | 0.711 | 0.617–0.804 | 640 | 86.1 | 53.3 | 68.9 | 76.2 | <0.0001 |
| hnRNP A2 | 0.669 | 0.583–0.755 | 1664 | 61.4 | 66.1 | 72.0 | 54.7 | <0.0001 |
| ATPB | 0.666 | 0.595–0.738 | 704 | 68.2 | 59.6 | 68.8 | 59.0 | <0.0001 |
| HSP60 | 0.665 | 0.580–0.750 | 4672 | 59.7 | 68.2 | 54.1 | 59.0 | <0.0001 |
| SBP1 | 0.664 | 0.571–0.756 | 704 | 74.0 | 56.7 | 67.5 | 64.2 | 0.001 |
| FGB | 0.657 | 0.564–0.751 | 1088 | 65.0 | 65.0 | 71.2 | 58.2 | 0.001 |
| IGKC | 0.654 | 0.556–0.751 | 2112 | 76.9 | 60.6 | 58.8 | 78.2 | 0.004 |
| CRT | 0.633 | 0.534–0.731 | 704 | 44.4 | 83.3 | 73.7 | 58.8 | 0.011 |
| CENPF | 0.630 | 0.540–0.721 | 1088 | 69.0 | 61.0 | 62.0 | 68.1 | 0.006 |
| PDIA1 | 0.618 | 0.524–0.712 | 704 | 54.3 | 72.7 | 64.4 | 63.6 | 0.013 |
| ANXA4 | 0.618 | 0.524–0.712 | 448 | 73.8 | 48.3 | 65.6 | 64.4 | 0.017 |
| ENO1 | 0.595 | 0.510–0.679 | 896 | 44.8 | 72.1 | 61.9 | 56.4 | 0.032 |
Mean signal intensities for ACY1
The mean signal intensities for ACY1 autoantibody in
HBV-related liver cirrhosis patients, CHB patients, and healthy
controls were 3,791, 1,186 and 1,487, respectively, with
significant differences between HBV-related liver cirrhosis and CHB
(P=0.001) or healthy controls (P=0.008) (Fig. 2B). The mean signal intensities for
the other six autoantibodies with AUC values of >0.7 for
discriminating HBV-related liver cirrhosis and CHB patients are
shown in Fig. 3.
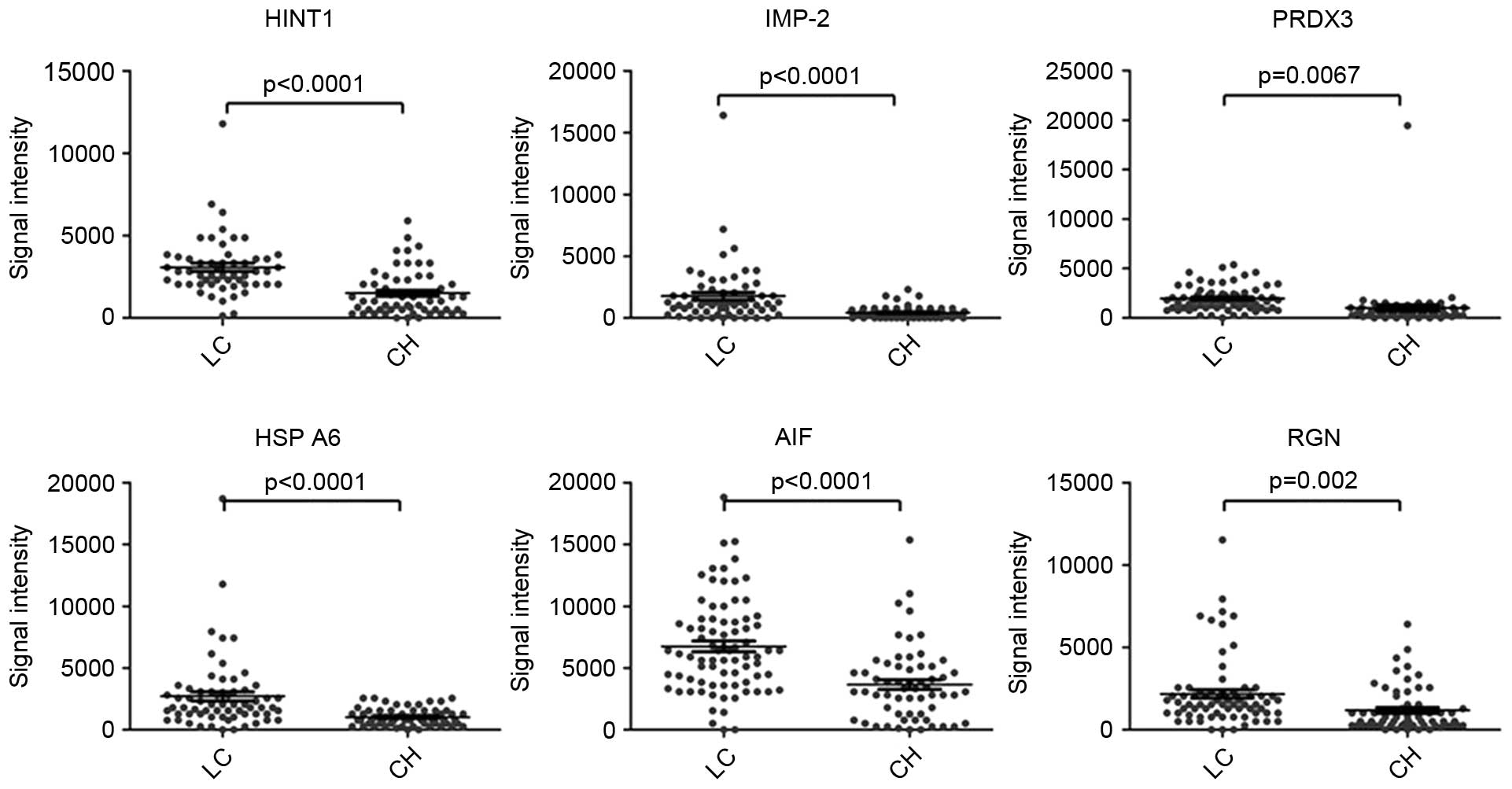 | Figure 3.Representative scatter diagrams of
the signal intensity of HINT1, IMP-2, PRDX3, HSP A6, AIF and RGN
for detection of LC and CH. The black horizontal lines indicate the
mean values, and the error bars are standard errors. LC, liver
cirrhosis; CH, chronic hepatitis; HINT1, histidine
triadnucleotide-binding protein 1; IMP-2, insulin-like growth
factor 2 mRNA-binding protein 2; PRDX3, peroxiredoxin 3; HSP A6,
heat shock 70 kDa protein 6; AIF, apoptosis induced factor; RGN,
regucalcin. |
Prevalence of autoantibody
positivity
Comparisons of the prevalence of autoantibody
positivity against ACY1 and the other six protein antigens between
HBV-related liver cirrhosis and CHB revealed significant
differences in the numbers of patients with autoantibody
positivity, with a prevalence of autoantibody positivity against
ACY1 of 77.3% for HBV-related liver cirrhosis and 15.0% for CHB
(P<0.0001; Table II). Analyses
of the associations with demographic parameters of HBV-related
liver cirrhosis and CHB patients showed that the prevalence of the
autoantibodies were not significantly correlated with gender and
age (Table III).
 | Table II.Comparisons of autoantibody
positivity among hepatitis B virus-related liver cirrhosis and CHB
patients. |
Table II.
Comparisons of autoantibody
positivity among hepatitis B virus-related liver cirrhosis and CHB
patients.
| Autoantibody | Cut-off value | LC (%) | CHB (%) | P-value |
|---|
| ACY1 | 1920 | 77.3 | 15.0 | <0.0001 |
| HINT1 | 1856 | 85.5 | 30.0 | <0.0001 |
| IMP-2 | 896 | 62.3 | 7.10 | <0.0001 |
| PRDX3 | 1728 | 54.5 | 10.0 | <0.0001 |
| HSP A6 | 1600 | 58.5 | 16.7 | <0.0001 |
| AIF | 5504 | 60.0 | 24.0 | <0.0001 |
| RGN | 640 | 86.1 | 46.7 | <0.0001 |
 | Table III.Correlation between autoantibody
positivity and demographic parameters in hepatitis B virus-related
liver cirrhosis and chronic hepatitis B. |
Table III.
Correlation between autoantibody
positivity and demographic parameters in hepatitis B virus-related
liver cirrhosis and chronic hepatitis B.
|
| Prevalence of
autoantibody positivity |
|---|
|
|
|
|---|
|
| Gender |
| Age (years) |
|
|---|
|
|
|
|
|
|
|---|
| Autoantibody | Male | Female | P-value | ≥50 | <50 | P-value |
|---|
| ACY1 |
|
|
|
|
|
|
| LC | 38/48 (79.2%) | 13/18 (72.2%) | 0.787 | 20/24 (83.3%) | 31/42 (73.8%) | 0.374 |
| CH | 5/38 (13.2%) | 4/22 (18.2%) | 0.881 | 7/41 (17.1%) | 2/19 (10.5%) | 0.786 |
| HINT1 |
|
|
|
|
|
|
| LC | 33/37 (89.2%) | 14/18 (77.8%) | 0472 | 16/20 (80.0%) | 31/35 (88.6%) | 0.638 |
| CH | 11/38 (28.9%) | 7/22 (31.8%) | 0.815 | 14/45 (31.1%) | 4/15 (26.7%) | 1.000 |
| IMP-2 |
|
|
|
|
|
|
| LC | 26/44 (59.1%) | 12/17 (70.6%) | 0.406 | 17/25 (68.0%) | 21/36 (58.3%) | 0.444 |
| CH | 4/47 (8.5%) | 1/23 (4.3%) | 0.525 | 4/55 (7.3%) | 1/15 (6.7%) | 1.000 |
| PRDX3 |
|
|
|
|
|
|
| LC | 26/46 (56.5%) | 10/20 (50.0%) | 0.625 | 10/25 (40.0%) | 26/41 (63.4%) | 0.064 |
| CH | 6/40 (15.0%) | 0/20 (0.00%) | 0.171 | 3/44 (6.80%) | 3/16 (18.8%) | 0.381 |
| HSP A6 |
|
|
|
|
|
|
| LC | 30/47 (63.8%) | 8/18 (44.4%) | 0.156 | 14/26 (53.8%) | 24/39 (61.5%) | 0.538 |
| CH | 8/39 (20.5%) | 2/21 (9.50%) | 0.468 | 7/47 (14.9%) | 3/13 (23.1%) | 0.779 |
| AIF |
|
|
|
|
|
|
| LC | 35/57 (61.4%) | 13/23 (56.5%) | 0.687 | 22/33 (66.7%) | 26/47 (55.3%) | 0.308 |
| CH | 8/38 (21.1%) | 4/22 (18.2%) | 1.000 | 10/44 (22.7%) | 2/16 (12.5%) | 0.609 |
| RGN |
|
|
|
|
|
|
| LC | 43/51 (84.3%) | 19/21 (90.5%) | 0.755 | 23/29 (79.3%) | 39/43 (90.7%) | 0.306 |
| CH | 18/38 (47.4%) | 10/22 (45.5%) | 0.886 | 20/36 (55.6%) | 8/24 (33.3%) | 0.091 |
Discussion
During the process of disease development, certain
proteins may cause antigenicity through mutation, abnormal
expression and abnormal localization, thereby triggering the immune
system to produce autoantibodies (10,11).
Since the magnified signals of autoantibodies can be easier to
detect than autoantigens themselves (3,7), the
autoantibody based serum biomarkers may exhibit higher sensitivity
than protein antigens, and may be promising biomarkers for early
diagnosis of diseases.
Current studies on serum autoantibodies are
predominantly focused on autoimmune diseases, such as systemic
lupus erythematosus, rheumatoid arthritis and autoimmune liver
diseases. In liver diseases, screening of autoantibodies is mainly
used to aid in the diagnosis of autoimmune liver diseases, such as
autoimmune hepatitis and primary biliary cirrhosis. A number of
autoimmune liver disease-related autoantibodies, such as
antinuclear antibodies and antimitochondrial antibodies have been
reported (12). However, the
underlying mechanism of the generation of these autoantibodies in
autoimmune diseases remains poorly understood. Autoantibodies have
also been observed in non-autoimmune diseases, such as cancer
(3–5). In the present study, several
autoantibodies were identified with different levels in the serum
of CHB and HBV-related liver cirrhosis patients without autoimmune
diseases.
Protein microarray technology was applied in the
present study for high throughput clinical evaluation of the
candidate protein antigens. The level of CENPF autoantibody was
detected by western blot analysis in out previous study and the
results were consistent with that of the protein microarray
(8), suggesting the robust results
obtained by the microarray assays in our study. The protein
antigens with significant difference of signal intensity between
HBV-related liver cirrhosis and CHB screened by the present study
were classified as follows: Proteins involved in construction of
the cytoskeleton and promotion of cell proliferation and
activation, such as CENPF, ANXA4, TUBB4B, K2C1 and FGB; proteins
related to metabolism and transport, such as ALDH1A1, ENO1, ATPB,
ACY1, PRDX3 and RGN; proteins involved in the immune system, such
as IGKC and HINT1, and stress-related proteins, such as HSP60,
HSPA6 and PDIA1. Among these candidate autoantigens, ENO1, ACY1 and
HINT1 were previously reported to be associated with fibrosis
(6,13,14).
As the most valuable candidate biomarker for liver
cirrhosis screened in the present study, ACY1 is associated with
protein metabolism, participates in protein degradation, and was
found to be associated with colon cancer, small cell lung cancer
and liver cancer (15–17). In a previous study, mice with renal
fibrosis were treated with the antifibrotic drug mycophenolate
mofetil, and evaluated for the protein abundance in kidney sections
(13). The results showed the
level of ACY1 was elevated, while tubulointerstitial fibrosis was
inhibited by mycophenolate mofetil. These observations indicated
that ACY1 may be involved in the process of fibrosis, but its
specific function remained unclear. In the present study, to the
best of our knowledge, it was demonstrated for the first time that
the level of ACY1 autoantibody was higher in patients with
HBV-related liver cirrhosis compared with patients with CHB.
However, further studies are required to clarify the mechanism
underlying the generation of the ACY1 autoantibody.
HINT1 is a novel tumor inhibiter identified in
recent years (18,19). It is implicated in the pathological
progression of a number of human diseases, including cancer and
schizophrenia (20). Wu et
al (14) found that rhHint1
was capable of attenuating CCl4-induced liver fibrosis
in rats by simultaneously targeting multiple pathogenic pathways,
and may have potential for development as a novel treatment for
liver fibrosis. The present study demonstrated that the level of
HINT1 autoantibody in patients with HBV-related liver cirrhosis was
higher than that in patients with CHB. However, further studies on
the mechanism underlying the generation of HINT1 autoantibody are
required.
Consistent with the study by Peng et al
(6) in which ENO1 was identified
as a potential marker for liver fibrosis, ENO1 autoantibody was
present at a significantly higher level in patients with
HBV-related liver cirrhosis compared with patients with CHB in the
present study. Enolases are a family of cytoplasmic proteins
involved in glycolytic metabolism and energy regulation in
prokaryotes and eukaryotes. ENO1 is located in the nucleus and aids
in the regulation of cell growth and differentiation by
downregulating the activity of the proto-oncogene c-myc (21). ENO1 autoantibody has been found in
autoimmune diseases, such as systemic lupus erythematosus,
rheumatoid arthritis and Crohn's disease, and also appeared in
autoimmune hepatitis with a low titer (22–24).
Peng et al (6) found that
the frequency of ENO1 autoantibodies in sera from patients at the
precirrhotic stage of liver fibrosis (21.6%, 27/125) was
significantly higher than that in sera from patients with cirrhosis
(9.1%, 5/55) and liver cancer (14.3%, 12/84), as well as that in
sera of healthy individuals (4.1%, 3/74). In the present study, it
was demonstrated that the level of the ENO1 autoantibody was higher
in patients with HBV-related cirrhosis compared with patients with
CHB (P<0.05). Therefore, ENO1 may be an autoantigen that elicits
autoimmune responses in liver fibrosis and it may be a potential
prognostic factor for liver fibrosis diagnosis.
Liver fibrosis is an important stage of the
progression from chronic hepatitis to cirrhosis and hepatocellular
carcinoma. Increasing evidence suggests that liver fibrosis is
reversible, even for advanced fibrosis (25). Early detection and accurate
predication of the degree of fibrosis are crucial for the
prevention of liver cirrhosis and carcinoma. A liver biopsy has
traditionally been used as the golden standard for liver fibrosis,
but the process is unpleasant for patients and may have severe side
effects, such as local hemorrhage and pain caused by liver
puncture, and pneumothorax in a small number of patients (26,27).
Since fibrosis not only occurs in the liver, but also in the
kidney, lung, and skin, poor specificity is a common problem of
frequently-used serum biomarkers, such as hyaluronic acid, laminin,
metal matrix proteinase, tissue inhibitor of matrix
metalloprotease-1 and cytokines (28). Although a number of studies have
shown that transient elastography performs well in the assessment
of significant and advanced fibrosis (29,30),
its reproducibility was reported to be lower in patients with
steatosis, increased body mass index, lower degrees of hepatic
fibrosis and severe ascites (31).
Therefore, a noninvasive method with high diagnostic value and
specificity is strongly required.
Few studies have addressed the value of an
autoantibody-based serum biomarker in the staging of liver
fibrosis. The data showed that autoantibodies against ACY1, HINT1,
IMP-2, PRDX3, HSPA6, AIF and RGN may be useful in the
discrimination of HBV-related liver cirrhosis and CHB. Since liver
fibrosis is the intermediate stage of the progress from chronic
hepatitis to liver cirrhosis, the screened candidate autoantibody
biomarkers may have further potential value in the diagnosis of
liver fibrosis of different stages. Another study may be conducted
in the future to evaluate the efficacy of these autoantibodies in
liver fibrosis and analyze the underlying mechanism of the
appearance of these autoantibodies.
One limitation of the present study is that all of
the cases of liver cirrhosis and hepatitis were caused by HBV
infection. Although hepatitis C virus infection and alcohol abuse
are also important causes of liver cirrhosis, the effects of these
factors were not investigated in this study. However, future
investigation into the contributions of these factors is required.
In addition, the number of participants enrolled in the present
study was insufficient, thus a large-scale study is required. In
summary, with the elevated level in the disease progression of CHB,
ACY1 autoantibody maybe a valuable serum biomarker for
discriminating HBV-associated liver cirrhosis from CHB, providing a
convenient method for the screening and early diagnosis of patients
with liver cirrhosis, and the basis for further research on
autoantibody-based biomarkers for the staging of liver
fibrosis.
Acknowledgements
The authors would like to thank Dr Jidong Jia, Dr
Hong You and Dr Hong Ma (Liver Research Center, Beijing Friendship
Hospital, Capital Medical University) for supplying clinical
materials and helpful suggestion. This study was supported by a
grant from the National Natural Science Foundation of China (grant
no. 81071973), a grant from the Scientific Research Foundation for
Returned Overseas Chinese Scholars, Bureau of Human Resources and
Social Security of Beijing, China (Key project, 2010, grant no.
20110323), and a grant from the program for National Science and
Technology Major Project (grant no. 2013ZX10002004).
Glossary
Abbreviations
Abbreviations:
|
ACY1
|
aminoacylase-1
|
|
IMP-2
|
insulin-like growth factor 2
mRNA-binding protein 2
|
|
CRT
|
calreticulin
|
|
CENPF
|
centromere protein F
|
|
HSP60
|
60 kDa heat shock protein
|
|
PDIA1
|
protein disulfide-isomerase
|
|
ENO1
|
α-enolase
|
|
ANXA4
|
annexin A4
|
|
IGKC
|
Ig κ chain C region
|
|
RGN
|
regucalcin
|
|
K2C1
|
type II cytoskeletal 1
|
|
HSPA6
|
heat shock 70 kDa protein 6
|
|
AIAT
|
α-1-antitrypsin
|
|
FGB
|
fibrinogen β chain
|
|
SBP1
|
selenium-binding protein 1
|
|
PRDX3
|
peroxiredoxin 3
|
|
HINT1
|
histidine triad nucleotide-binding
protein 1
|
|
TUBB4B
|
tubulin β-4B chain
|
|
ATPB
|
ATP synthase subunit β
|
|
ALDH1A1
|
retinal dehydrogenase 1
|
|
hnRNP A2
|
heterogeneous nuclear
ribonucleoprotein A2
|
|
AIF
|
apoptosis-inducing factor
|
References
|
1
|
European Association For The Study Of The
Liver, . EASL Clinical Practice Guidelines: Management of chronic
hepatitis B. J Hepatol. 50:227–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bini EJ and Perumalswami PV: Hepatitis B
virus infection among American patients with chronic hepatitis C
virus infection: Prevalence, racial/ethnic differences, and viral
interactions. Hepatology. 51:759–766. 2010.PubMed/NCBI
|
|
3
|
Tan HT, Low J, Lim SG and Chung MC: Serum
autoantibodies as biomarkers for early cancer detection. FEBS J.
276:6880–6904. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu YW, Peng YH, Chen B, Wu ZY, Wu JY, Shen
JH, Zheng CP, Wang SH, Guo HP, Li EM and Xu LY: Autoantibodies as
potential biomarkers for the early detection of esophageal squamous
cell carcinoma. Am J Gastroenterol. 109:36–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Werner S, Chen H, Tao S and Brenner H:
Systematic review: Serum autoantibodies in the early detection of
gastric cancer. Int J Cancer. 136:2243–2252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peng B, Huang X, Nakayasu ES, Petersen JR,
Qiu S, Almeida IC and Zhang JY: Using immunoproteomics to identify
alpha-enolase as an autoantigen in liver fibrosis. J Proteome Res.
12:1789–1796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lacombe J, Mangé A and Solassol J: Use of
autoantibodies to detect the onset of breast cancer. J Immunol Re.
2014:5749812014.
|
|
8
|
Hong Y, Long J, Li H, Chen S, Liu Q, Zhang
B, He X, Wang Y, Li H, Li Y, et al: An analysis of immunoreactive
signatures in early stage hepatocellular carcinoma. EBioMedicine.
2:438–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lok AS and McMahon BJ: Chronic hepatitis
B: Update 2009. Hepatology. 50:661–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Casiano CA, Mediavilla-Varela M and Tan
EM: Tumor-associated antigen arrays for the serological diagnosis
of cancer. Mol Cell Proteomics. 5:1745–1759. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Caron M, Choquet-Kastylevsky G and
Joubert-Caron R: Cancer immunomics using autoantibody signatures
for biomarker discovery. Mol Cell Proteomics. 6:1115–1122. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura M: Clinical significance of
autoantibodies in primary biliary cirrhosis. Semin Liver Dis.
34:334–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Petrova DT, Brehmer F, Schultze FC, Asif
AR, Gross O, Oellerich M and Brandhorst G: Differential kidney
proteome profiling in a murine model of renal fibrosis under
treatment with mycophenolate mofetil. Pathobiology. 78:162–170.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu F, Huang S, Zhu N, Liu W, Zhang Y and
He Y: Recombinant human histidine triad nucleotide-binding protein
1 attenuates liver fibrosis induced by carbon tetrachloride in
rats. Mol Med Rep. 8:1023–1038. 2013.PubMed/NCBI
|
|
15
|
Miller YE, Minna JD and Gazdar AF: Lack of
expression of aminoacylase-1 in small cell lung cancer. Evidence
for inactivation of genes encoded by chromosome 3p. J Clin Invest.
83:2120–2124. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi H, Hayes MT, Kirana C, Miller RJ,
Keating JP and Stubbs RS: Overexpression of aminoacylase 1 is
associated with colorectal cancer progression. Hum Pathol.
44:1089–1097. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei X, Li J, Xie H, Ling Q, Wang J, Lu D,
Zhou L, Xu X and Zheng S: Proteomics-based identification of the
tumor suppressor role of aminoacylase 1 in hepatocellular
carcinoma. Cancer Lett. 351:117–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Zhang Y, Su T, Santella RM and
Weinstein IB: Hint1 is a haplo-insufficient tumor suppressor in
mice. Oncogene. 25:713–721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weiske J and Huber O: The histidine triad
protein Hint1 triggers apoptosis independent of its enzymatic
activity. J Biol Chem. 281:27356–27366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dang YH, Liu ZW, Chen F, Guo K and Wang
JB: Histidine triad nucleotide-binding protein 1 and human
diseases. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 36:454–460. 2014.(In
Chinese). PubMed/NCBI
|
|
21
|
Terrier B, Degand N, Guilpain P, Servettaz
A, Guillevin L and Mouthon L: Alpha-enolase: A target of antibodies
in infectious and autoimmune diseases. Autoimmun Rev. 6:176–182.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kinloch A, Tatzer V, Wait R, Peston D,
Lundberg K, Donatien P, Moyes D, Taylor PC and Venables PJ:
Identification of citrullinated alpha-enolase as a candidate
autoantigen in rheumatoid arthritis. Arthritis Res Ther.
7:R1421–R1429. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roozendaal C, Zhao MH, Horst G, Lockwood
CM, kleibeuker JH, Limburg PC, Nelis GF and Kallenberg CG: Catalase
and alpha-enolase: Two novel granulocyte autoantigens in
inflammatory bowel disease (IBD). Clin Exp Immunol. 112:10–16.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pratesi F, Moscato S, Sabbatini A,
Chimenti D, Bombardieri S and Migliorini P: Autoantibodies specific
for alpha-enolase in systemic autoimmune disorders. J Rheumatol.
27:109–115. 2000.PubMed/NCBI
|
|
25
|
Friedman SL: Liver fibrosis-from bench to
bedside. J Hepatol. 38:(Suppl 1). S38–S53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siddique I, El-Naga HA, Madda JP, Memon A
and Hasan F: Sampling variability on percutaneous liver biopsy in
patients with chronic hepatitis C virus infection. Scand J
Gastroenterol. 38:427–432. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rockey DC, Caldwell SH, Goodman ZD, Nelson
RC and Smith AD: American Association for the Study of Liver
Diseases: Liver biopsy. Hepatology. 49:1017–1044. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu T, Wang X, Karsdal MA, Leeming DJ and
Genovese F: Molecular serum markers of liver fibrosis. Biomark
Insights. 7:105–117. 2012.PubMed/NCBI
|
|
29
|
Chon YE, Choi EH, Song KJ, Park JY, Kim do
Y, Han KH, Chon CY, Ahn SH and Kim SU: Performance of transient
elastography for the staging of liver fibrosis in patients with
chronic hepatitis B: A meta-analysis. PLoS One. 7:e449302012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Verveer C, Zondervan PE, ten Kate FJ,
Hansen BE, Janssen HL and de Knegt RJ: Evaluation of transient
elastography for fibrosis assessment compared with large biopsies
in chronic hepatitis B and C. Liver Int. 32:622–628. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fraquelli M, Rigamonti C, Casazza G, Conte
D, Donato MF, Ronchi G and Colombo M: Reproducibility of transient
elastography in the evaluation of liver fibrosis in patients with
chronic liver disease. Gut. 56:968–973. 2007. View Article : Google Scholar : PubMed/NCBI
|

















